Climate-Based Modeling and Prediction of Rice Gall Midge Populations Using Count Time Series and Machine Learning Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Models
2.2.1. Integer-Valued Generalized Autoregressive Conditional Heteroscedastic (INGARCH) Model
- (a)
- conditioned on is Poisson distributed
- (b)
- The conditional mean satisfies
2.2.2. Support Vector Regression (SVR)
2.2.3. Artificial Neural Network (ANN)
2.3. Comparison Criteria
2.4. Diebold–Merino Test
3. Results
3.1. Correlation Analysis
3.2. Stepwise Regression Analysis
3.3. INGARCHX Model
3.4. SVRX Model
3.5. ANNX Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramaswamy, C.; Jatileskono, T. Inter-country comparison of insect and disease losses. In Rice Research in Asia: Progress and Priorities; Evenson, R.E., Herdt, R.W., Hussain, M., Eds.; CABI Publications: Wallingford, UK, 1996; pp. 305–316. [Google Scholar]
- Bentur, J.S.; Pasalu, I.C.; Sarma, N.P.; Prasada Rao, U.; Misra, B. Gall Midge Resistance in Rice: Current Status in India and Future Strategies-DRR Research Paper Series No. 1/2003; Directorate of Rice Research: Hyderabad, India, 2003. [Google Scholar]
- Nacro, S.; Heinrichs, E.A.; Dakouo, D. Estimation of rice yield losses due to the African rice gall midge, Orseoliaoryzivora Harris and Gagne. Int. J. Pest Manag. 1996, 42, 331–334. [Google Scholar] [CrossRef][Green Version]
- Mathur, K.C.; Rajamani, S. Orseolia and rice: Cecidogenousinteractions. Proc. Anim. Sci. 1984, 93, 283–292. [Google Scholar] [CrossRef]
- Chelliah, A.; Bentur, J.S.; Prakasa Rao, P.S. Approaches to rice management-achievements and opportunities. Oryza 1989, 26, 12–26. [Google Scholar]
- Rajamani, S.; Pasalu, I.C.; Mathur, K.C.; Sain, M. Biology and ecology of rice gall midge, Orseoliaoryzae (Wood-Mason). In New Approaches to Gall Midge Resistance in Rice, Proceedings of the International Workshop, Hyderabad, India 22–24 November 1998; Bennett, J., Bentur, J.S., Pasalu, I.C., Krishnaiah, K., Eds.; International Rice Research Institute: Hyderabad, India, 2004; pp. 7–16. [Google Scholar]
- Samui, R.P.; Chattopadhyay, N.; Sabale, J.P. Weather based forewarning of gall midge attack on rice and operational crop protection using weather information at Pattambi. Mausam 2004, 55, 329–338. [Google Scholar]
- Sinha, D.K.; Atray, I.; Agarrwal, R.; Bentur, J.S.; Nair, S. Genomics of the Asian rice gall midge and its interactions with rice. Curr. Opin. Insect Sci. 2017, 19, 76–81. [Google Scholar] [CrossRef]
- Fokianos, K.; Rahbek, A.; Tjøstheim, D. Poisson autoregression. J. Am. Stat. Assoc. 2009, 104, 1430–1439. [Google Scholar] [CrossRef]
- Zhu, F. Modeling time series of counts with COM-Poisson INGARCH models. Math. Comput. Model. 2012, 56, 191–203. [Google Scholar] [CrossRef]
- Weiß, C.H. Modelling time series of counts with overdispersion. Stat. Methods Appt. 2009, 18, 507–519. [Google Scholar] [CrossRef]
- Weiß, C.H. The INARCH(1) model for overdispersed time series of counts. Commun. Stat. Simul. Comput. 2010, 39, 1269–1291. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, D. Diagnostic checking integer-valued ARCH(p) models using conditional residual autocorrelations. Comput. Stat. Data Anal. 2010, 54, 496–508. [Google Scholar] [CrossRef]
- Liboschik, T.; Kerschke, P.; Fokianos, K.; Fried, R. Modelling interventions in INGARCH processes. Int. J. Comput Math. 2014, 93, 640–657. [Google Scholar] [CrossRef]
- Tanawi, I.N.; Vito, V.; Sarwinda, D.; Tasman, H.; Hertono, G.F. Support Vector Regression for Predicting the Number of Dengue Incidents in DKI Jakarta. Procedia Comput. Sci. 2021, 179, 747–753. [Google Scholar] [CrossRef]
- Kim, M. Network traffic prediction based on INGARCH model. Wirel. Netw. 2020, 26, 6189–6202. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yoo, S.J.; Gu, Y.H.; Lim, J.H.; Han, D.; Baik, S.W. Crop Pests Prediction Method Using Regression and Machine Learning Technology: Survey. IERI Procedia 2014, 6, 52–56. [Google Scholar] [CrossRef]
- Alam, W.; Ray, M.; Kumar, R.R.; Sinha, K.; Rathod, S.; Singh, K.N. Improved ARIMAX modal based on ANN and SVM approaches for forecasting rice yield using weather variables. Indian J. Agric. Sci. 2018, 88, 1909–1913. [Google Scholar]
- Alam, W.; Sinha, K.; Kumar, R.R.; Ray, M.; Rathod, S.; Singh, K.N.; Arya, P. Hybrid linear time series approach for long term forecasting of crop yield. Indian J. Agric. Sci. 2018, 88, 1275–1279. [Google Scholar]
- Rathod, S.; Mishra, G.C. Statistical Models for Forecasting Mango and Banana Yield of Karnataka. India J. Agric. Sci. Technol. 2018, 20, 803–816. [Google Scholar]
- Rathod, S.; Singh, K.N.; Patil, S.G.; Naik, R.H.; Ray, M.; Meena, V.S. Modeling and forecasting of oilseed production of India through artificial intelligence techniques. Indian J. Agric. Sci. 2018, 88, 22–27. [Google Scholar]
- Amaratunga, V.; Wickramasinghe, L.; Perera, A.; Jayasinghe, J.; Rathnayake, U. Artificial Neural Network to Estimate the Paddy Yield Prediction Using Climatic Data. Math. Probl. Eng. 2020, 2020, 8627824. [Google Scholar] [CrossRef]
- Su, Y.X.; Xu, H.; Yan, L.J. Support vector machine-based open crop model (SBOCM): Case of rice production in China. Saudi J. Biol. Sci. 2017, 24, 537–547. [Google Scholar] [CrossRef]
- Ma, C.; Liang, Y.; Lyu, X. Weather Analysis to Predict Rice Pest Using Neural Network and D-S Evidential Theory. In Proceedings of the 2019 International Conference on Cyber-Enabled Distributed Computing and Knowledge Discovery (CyberC), Guilin, China, 17–19 October 2019; pp. 277–283. [Google Scholar] [CrossRef]
- Paul, R.K.; Vennila, S.; Bhat, M.N.; Yadav, S.K.; Sharma, V.K.; Nisar, S.; Panwar, S. Prediction of early blight severity in tomato (Solanumlycopersicum) by machine learning technique. Indian J. Agric. Sci. 2019, 89, 1921–1927. [Google Scholar]
- Huang, T.; Yamg, R.; Huang, W.; Huang, Y.; Qiao, X. Detecting sugarcane borer diseases using support vector machine. Inf. Process. Agric. 2018, 5, 74–82. [Google Scholar] [CrossRef]
- O’Hara, R.B.; Kotze, D.J. Do not log-transform count data. Meth. Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef]
- St-Pierre, A.P.; Shikon, V.; Schneider, D.C. Count data in biology-Data transformation or model reformation? Ecol. Evol. 2018, 8, 3077–3085. [Google Scholar] [CrossRef]
- Vennila, S.; Bagri, M.; Tomar, A.; Rao, M.S.; Sarao, P.S.; Sharma, S.; Jalgaonkar, V.; Kumar, M.K.P.; Suresh, S.; Mathirajan, V.G.; et al. Future of Rice Yellow Stem Borer Scirpophaga incertulas (Walker) Under Changing Climate. Natl. Acad. Sci. Lett. 2019, 42, 309–313. [Google Scholar] [CrossRef]
- Rajpoot, S.K.S.; Giri, S.P.; Yadav, S.K.; Singh, R.A.N.; Parkash, N. Sustainable Management of Rice Insect Pests Chinsurah Light Trap at Uttar Pradesh. Int. J. Curr. Micr. Appl. Sci. 2020, 10, 158–167. [Google Scholar]
- Ogah, E.; Nwilene, F.; Ukwungwu, M.; Omoloye, A.; Agunbiade, T. Population dynamics of the African rice gall midge Orseolia oryzivora (Diptera: Cecidomyiidae) and its parasitoids in the forest and southern Guinea savanna zones of Nigeria. Int. J. Trop. Insect Sci. 2009, 29, 86–92. [Google Scholar] [CrossRef]
- SAS Software, Version 9.3; SAS Institute: Cary, NC, USA, 2011.
- Kedem, B.; Fokianos, K. Regression Models for Time Series Analysis; Wiley Series in Probability and Statistics; Wiley-Interscience: Hoboken, NJ, USA, 2002; ISBN 0-471-36355-3. [Google Scholar]
- Heinen, A. Modelling Time Series Count Data: An Autoregressive Conditional Poisson Model; MPRA Paper 8113; University Library of Munich: Munich, Germany, 2003. [Google Scholar] [CrossRef]
- Ferland, R.; Latour, A.; Oraichi, D. Integer-valued GARCH process. J. Time Ser. Anal. 2006, 27, 923–942. [Google Scholar] [CrossRef]
- Christou, V.; Fokianos, K. Quasi-Likelihood Inference for Negative Binomial Time Series Models. J. Time Ser. Anal. 2014, 35, 55–78. [Google Scholar] [CrossRef]
- Fokianos, K. Some Recent Progress in Count Time Ser. Statistics 2011, 45, 49–58. [Google Scholar] [CrossRef]
- Liboschik, T.; Fried, R.; Fokianos, K.; Probst, P. tscount: An R Package for Analysis of Count Time Series Following Generalized Linear Models; R Package Version 1.4.3. 2020. Available online: https://CRAN.R-project.org/package=tscount (accessed on 11 October 2021).
- Vapnik, V.N. The Nature of Statistical Learning Theory; Springer: New York, NY, USA, 1995; Available online: https://link.springer.com/book/10.1007%2F978-1-4757-2440-0 (accessed on 11 October 2021).
- Zhang, G.P. Time-series forecasting using a hybrid ARIMA and neural network model. Neurocomputing 2003, 50, 159–175. [Google Scholar] [CrossRef]
- Diebold, F.X.; Mariano, R.S. Comparing predictive accuracy. J. Bus. Econ. Stat. 1995, 13, 253–263. [Google Scholar]
- Kumari, P.; Mishra, G.C.; Srivastava, C.P. Forecasting of productivity and pod damage by Helicoverpa armigera using artificial neural network model in pigeonpea (Cajanus Cajan). Int. J. Agric. Environ. Biotechnol. 2013, 6, 335–340. [Google Scholar]
- Kumari, P.; Mishra, G.C.; Srivastava, C.P. Time series forecasting of losses due to pod borer, pod fly and productivity of pigeonpea (Cajanus cajan) for North West Plain Zone (NWPZ) by using artificial neural network (ANN). Int. J. Agric. Stat. Sci. 2014, 10, 15–21. [Google Scholar]
- Chitikela, G.; Admala, M.; Ramalingareddy, V.K.; Bandumula, N.; Ondrasek, G.; Sundaram, R.M.; Rathod, S. Artificial-Intelligence-Based Time-Series Intervention Models to Assess the Impact of the COVID-19 Pandemic on Tomato Supply and Prices in Hyderabad, India. Agronomy 2021, 11, 1878. [Google Scholar] [CrossRef]
- Giovanelli, C.; Sierla, S.; Ichise, R.; Vyatkin, V. Exploiting artificial neural networks for the prediction of ancillary energy market prices. Energies 2018, 11, 1906. [Google Scholar] [CrossRef]
- Piekutowska, M.; Niedbała, G.; Piskier, T.; Lenartowicz, T.; Pilarski, K.; Wojciechowski, T.; Pilarska, A.A.; Czechowska-Kosacka, A. The Application of Multiple Linear Regression and Artificial Neural Network Models for Yield Prediction of Very Early Potato Cultivars before Harvest. Agronomy 2021, 11, 885. [Google Scholar] [CrossRef]
- Liu, L.-W.; Hsieh, S.-H.; Lin, S.-J.; Wang, Y.-M.; Lin, W.-S. Rice Blast (Magnaportheoryzae) Occurrence Prediction and the Key Factor Sensitivity Analysis by Machine Learning. Agronomy 2021, 11, 771. [Google Scholar] [CrossRef]
- Haider, S.A.; Naqvi, S.R.; Akram, T.; Umar, G.A.; Shahzad, A.; Sial, M.R.; Khaliq, S.; Kamran, M. LSTM Neural Network Based Forecasting Model for Wheat Production in Pakistan. Agronomy 2019, 9, 72. [Google Scholar] [CrossRef]

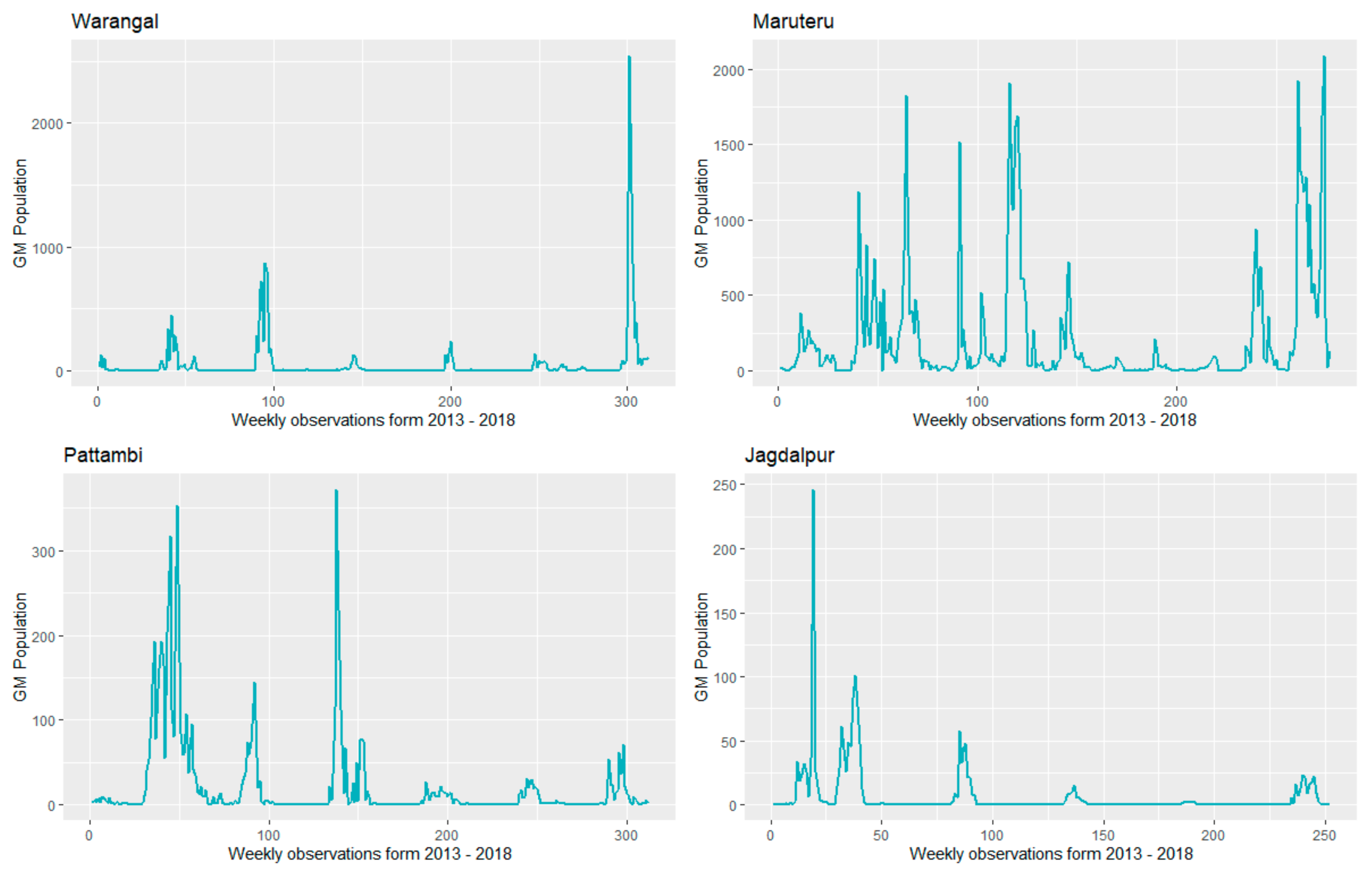

| Location | Statistics | Population | MAXT | MINT | RF | RHM | RHE | SSH |
|---|---|---|---|---|---|---|---|---|
| Warangal | Mean | 42 | 32.32 | 20.05 | 9.96 | 86.97 | 55.93 | 6.55 |
| S.E. | 7.29 | 0.27 | 0.27 | 1.53 | 0.18 | 0.55 | 0.14 | |
| Skewness | 4.8 | 1.07 | −0.34 | 4.44 | −1.94 | 0.2 | −0.76 | |
| Kurtosis | 24.68 | 0.23 | −1.06 | 22.63 | 10.82 | −0.68 | −0.18 | |
| Minimum | 0 | 25.71 | 11.29 | 0 | 62.29 | 33 | 0.31 | |
| Maximum | 875 | 45.93 | 31 | 204.7 | 93.14 | 80.14 | 11.11 | |
| CV (%) | 303.53 | 14.52 | 23.38 | 271.23 | 3.74 | 17.51 | 36.73 | |
| Maruteru | Mean | 215 | 31.03 | 24.28 | 14.27 | 86.37 | 73.68 | 6 |
| S.E. | 23.24 | 0.15 | 0.19 | 1.94 | 0.21 | 0.23 | 0.21 | |
| Skewness | 2.7 | 0.78 | −0.35 | 4.11 | −0.46 | 0 | 4.07 | |
| Kurtosis | 7.4 | 1 | −0.57 | 22.73 | 0.21 | 1.15 | 32.53 | |
| Minimum | 0 | 24.86 | 16.17 | 0 | 75.43 | 60.71 | 0.04 | |
| Maximum | 2088 | 39.71 | 33.57 | 284.6 | 93.71 | 85.71 | 34.65 | |
| CV (%) | 179.76 | 7.83 | 12.74 | 225.77 | 4.08 | 5.2 | 57.08 | |
| Pattambi | Mean | 22 | 32.53 | 23.17 | 26.24 | 88.39 | 58.82 | 5.95 |
| S.E. | 2.98 | 0.15 | 0.11 | 3.13 | 0.36 | 0.85 | 0.13 | |
| Skewness | 3.9 | −0.03 | 0.75 | 3.04 | −1.23 | −0.07 | −0.55 | |
| Kurtosis | 17.66 | −0.46 | 3.36 | 10.13 | 1.58 | −0.76 | −0.64 | |
| Minimum | 0 | 24.89 | 18.11 | 0 | 58.29 | 17.43 | 0.19 | |
| Maximum | 372 | 39.09 | 32.54 | 340.5 | 96.86 | 94 | 9.7 | |
| CV (%) | 238.91 | 7.97 | 8.63 | 210.76 | 7.27 | 25.61 | 39.18 | |
| Jagdalpur | Mean | 6 | 30.56 | 18.64 | 13.45 | 89.51 | 40.49 | 5.39 |
| S.E. | 1.3 | 0.24 | 0.35 | 1.96 | 0.48 | 1.55 | 0.16 | |
| Skewness | 7.43 | 0.23 | −0.65 | 3.88 | −1.79 | −0.01 | −0.33 | |
| Kurtosis | 75.43 | 1.42 | −0.85 | 17.01 | 3.02 | −0.98 | −1.03 | |
| Minimum | 0 | 17.84 | 6.57 | 0 | 57.57 | 1.91 | 0.03 | |
| Maximum | 246 | 41.36 | 27.8 | 200.9 | 97.86 | 91 | 9.87 | |
| CV (%) | 330.76 | 12.3 | 29.83 | 231.81 | 8.44 | 60.8 | 48.04 |
| Location | Gall Midge | MAXT | MINT | RF | RHM | RHE | |
|---|---|---|---|---|---|---|---|
| Warangal | MAXT | −0.091 (0.1077) | |||||
| MINT | −0.055 (0.3254) | 0.59 <0.0001 | |||||
| RF | −0.053 (0.3483) | −0.18 (0.0013) | 0.19 (0.0009) | ||||
| RHM | 0.151 (0.0072) | −0.16 (0.0054) | 0.008 (0.8874) | 0.194 (0.0006) | |||
| RHE | 0.136 (0.0156) | −0.114 (0.0450) | 0.56 (<0.0001) | 0.41 (<0.0001) | 0.32 (<0.0001) | ||
| SSH | 0.126 (0.0256) | 0.43 (<0.0001) | 0.011 (0.8404) | −0.48 (<0.0001) | −0.18 (0.0011) | −0.39 (<0.0001) | |
| Maruteru | MAXT | 0.0234 (0.6977) | |||||
| MINT | −0.271 (0.653) | 0.685 <0.0001 | |||||
| RF | −0.0647 (0.283) | −0.041 (0.0497) | 0.173 (0.0038) | ||||
| RHM | 0.092 (0.126) | 0.250 (<0.0001) | −0.396 (<0.0001) | 0.101 (0.0916) | |||
| RHE | −0.0173 (0.774) | 0.316 (<0.0001) | 0.169 (0.0046) | 0.419 (<0.0001) | 0.054 (0.369) | ||
| SSH | 0.0404 (0.503) | 0.0798 (<0.189) | −0.329 (<0.0001) | −0.276 (<0.0001) | 0.0904 (0.1331) | −0.424 (<0.0001) | |
| Pattambi | MAXT | −0.206 (0.0002) | |||||
| MINT | 0.023 (0.6851) | −0.074 0.192 | |||||
| RF | −0.0101 (0.8585) | −0.4443 (<0.0001) | 0.095 (0.0909) | ||||
| RHM | 0.126 (0.0255) | −0.521 (<0.0001) | 0.211 (0.0002) | 0.388 (<0.0001) | |||
| RHE | 0.612 (0.2442) | −0.759 (<0.0001) | 0.251 (0.0002) | 0.526 (<0.0001) | 0.732 (<0.0001) | ||
| SSH | 0.005 (0.9261) | 0.689 (<0.0001) | 0.188 (0.0008) | −0.580 (<0.0001) | −0.569 (<0.0001) | −0.809 (<0.0001) | |
| Jagdalpur | MAXT | −0.0664 (0.2934) | |||||
| MINT | −0.0064 (0.9195) | 0.4088 <0.0001 | |||||
| RF | −0.0213 (0.7364) | −0.1367 (0.0299) | 0.3368 (<0.0001) | ||||
| RHM | 0.1570 (0.0126) | −0.6879 (<0.0001) | −0.4109 (<0.0001) | 0.0996 (0.1148) | |||
| RHE | 0.1506 (0.0167) | −0.2337 (0.0002) | 0.3658 (<0.0001) | 0.4056 (<0.0001) | 0.1831 (0.0035) | ||
| SSH | 0.1058 (0.0937) | 0.2182 (0.0005) | −0.5653 (<0.0001) | −0.3894 (<0.0001) | −0.1245 (0.0482) | −0.4686 (<0.0001) |
| Centre | Variable | Estimate | S.E. | F Value | Pr > F | R2 | Model R2 |
|---|---|---|---|---|---|---|---|
| Warangal | Intercept | −290.71 | 91.65 | 10.06 | 0.0017 | 0.0854 | |
| MINT | −12.14 | 3.15 | 14.88 | 0.0001 | 0.0136 | ||
| RHE | 7.80 | 1.63 | 22.84 | <0.0001 | 0.0412 | ||
| SSH | 22.86 | 5.49 | 17.34 | <0.0001 | 0.0854 | ||
| Maruteru | Intercept | −955.99 | 745.79 | 1.64 | 0.2010 | 0.092 | |
| RHM | 13.79 | 8.62 | 2.56 | 0.0110 | 0.092 | ||
| Pattambi | Intercept | −175.75 | 80.77 | 4.73 | 0.0303 | 0.1010 | |
| MAXT | −8.705 | 1.723 | 25.50 | <0.0001 | 0.0427 | ||
| RHM | 1.534 | 0.652 | 5.54 | 0.0193 | 0.0844 | ||
| RHE | −0.677 | 0.432 | 2.46 | 0.1177 | 0.0938 | ||
| SSH | 5.661 | 2.121 | 7.12 | 0.0080 | 0.1010 | ||
| Jagdalpur | Intercept | −97.48 | 25.14 | 15.04 | 0.0001 | 0.1062 | |
| MINT | 0.89 | 0.35 | 6.57 | 0.0110 | 0.0247 | ||
| RHM | 0.73 | 0.21 | 11.73 | 0.0007 | 0.0160 | ||
| RHE | 0.15 | 0.06 | 6.51 | 0.0113 | 0.0420 | ||
| SSH | 2.88 | 0.67 | 18.27 | <0.0001 | 0.0236 |
| Centre | Parameters | Estimate | S.E. | Z Value | p | Box-Pierce Non-Correlation Test | |
|---|---|---|---|---|---|---|---|
| Original | Residuals | ||||||
| Warangal | Intercept | 3.63 × 10−5 | 44.48 | 8.16 × 10−7 | 0.9999 | = 166.61 p ≤ 0.0001 | = 14.24 p = 0.00016 |
| beta_1 | 0.46 | 0.19 | 2..42 | 0.0191 | |||
| beta_52 | 0.14 | 0.12 | 1.17 | 0.2604 | |||
| MAXT | 2.25 × 10−8 | 0.64 | 3.52 × 10−8 | 0.9999 | |||
| MINT | 7.63 × 10−7 | 0.78 | 9.78 × 10−7 | 0.9999 | |||
| RF | 6.70 × 10−8 | 0.08 | 8.38 × 10−7 | 0.9999 | |||
| RHM | 7.90 × 10−8 | 0.49 | 1.61 × 10−7 | 0.9999 | |||
| RHE | 0.23 | 0.34 | 0.6.76 | 0.5084 | |||
| SSH | 0.024119 | 0.87 | 0.0277 | 0.9778 | |||
| Over dispersion Parameter | 7.32 | ||||||
| Maruteru | Intercept | 0.0003 | 412.4300 | 7.3 × 10−7 | 0.9999 | = 138.96 p ≤ 0.0001 | = 7.5346 p = 0.00605 |
| beta_1 | 0.8519 | 0.2369 | 3.600 | 0.0003 | |||
| MAXT | 1.48 × 10−5 | 6.8011 | 2.2 × 10−6 | 0.9999 | |||
| MINT | 2.35 × 10−5 | 4.7364 | 5.0 × 10−6 | 0.9999 | |||
| RF | 0.2519 | 0.4708 | 0.54 | 0.5926 | |||
| RHM | 0.1507 | 2.5737 | 0.059 | 0.9533 | |||
| RHE | 1.19 × 10−9 | 3.2431 | 3.7 × 10−10 | 0.9999 | |||
| SSH | 2.3553 | 4.3648 | 0.54 | 0.5895 | |||
| Over dispersion Parameter | 3.30 | ||||||
| Pattambi | Intercept | 0.0010 | 8.1951 | 0.0001 | 0.9999 | = 190.88 p ≤ 0.0001 | = 0.109 p = 0.7403 |
| beta_1 | 0.7997 | 0.1950 | 4.1014 | <0.0001 | |||
| beta_52 | 0.0095 | 0.0159 | 0.5970 | 0.5505 | |||
| MAXT | 2.30 × 10−12 | 0.2797 | 8.22 × 10−12 | 0.999 | |||
| MINT | 0.0007 | 0.1898 | 0.0036 | 0.9972 | |||
| RF | 0.0015 | 0.0075 | 0.1957 | 0.8448 | |||
| RHM | 6.88 × 10−6 | 0.0581 | 0.0001 | 0.9999 | |||
| RHE | 0.0274 | 0.0435 | 0.6325 | 0.5271 | |||
| SSH | 3.99 × 10−8 | 0.2681 | 1.49 × 10−7 | 0.999 | |||
| Over dispersion Parameter | 2.23 | ||||||
| Jagdalpur | Intercept | 4.47 × 10−5 | 3.3598 | 1.33 × 10−5 | 0.9999 | = 61.29 p ≤ 0.0001 | = 6.713 p = 0.0095 |
| beta_1 | 0.29454 | 0.1820 | 1.62 | 0.1056 | |||
| MAXT | 2.34 × 10−12 | 0.0327 | 7.16 × 10−11 | 0.9999 | |||
| MINT | 0.0032 | 0.0424 | 0.0755 | 0.9404 | |||
| RF | 0.0178 | 0.0207 | 0.86 | 0.3891 | |||
| RHM | 4.81 × 10−7 | 0.0236 | 2.04 × 10−5 | 0.9999 | |||
| RHE | 0.0228 | 0.0089 | 2.56 | 0.0103 | |||
| SSH | 1.3 × 10−5 | 0.0870 | 1.49 × 10−4 | 0.9999 | |||
| Over dispersion Parameter | 5.47 | ||||||
| Warangal | Maruteru | Pattambi | Jagdalpur | |
|---|---|---|---|---|
| SVRX Model | ||||
| Kernel function | RBF | RBF | RBF | RBF |
| No. of Support Vectors | 139 | 191 | 169 | 107 |
| Cost | 1 | 1 | 1 | 1 |
| Gamma | 0.16 | 0.166 | 0.17 | 0.170 |
| Epsilon | 0.1 | 0.1 | 0.1 | 0.1 |
| Cross validation error | 0.024 | 0.015 | 0.037 | 0.033 |
| Box-Pierce non-correlation test for residuals | 141.82 (p < 0.001) | 123.92 (p < 0.001) | 167.16 (p < 0.001) | 37.006 (p < 0.001) |
| ANNX Model | ||||
| Input lag | 4 | 5 | 8 | 3 |
| Dependent/output variable | 1 | 1 | 1 | 1 |
| Hidden layer | 1 | 1 | 1 | 1 |
| Hidden nodes | 6 | 6 | 10 | 10 |
| Exogenous variables | 6 | 6 | 6 | 6 |
| Model | 10:6S:1L | 11:6S:1L | 10:10S:1L | 9:10S:1L |
| Total number of parameters | 73 | 79 | 161 | 111 |
| Network type | Feed Forward | |||
| Activation function I:H | Sigmoidal | |||
| Activation function H:O | Identity | |||
| Box-Pierce non-correlation test for residuals | 0.36 (p = 0.55) | 1.003 × 10−6 (p = 0.992) | 1.997 (p = 0.157) | 1.761 (p = 0.184) |
| Criteria/Model | INGARCHX | SVRX | ANNX | ||
|---|---|---|---|---|---|
| Warangal | Training Set | MSE | 8696.20 | 14,291 | 572.58 |
| RMSE | 93.25 | 119.54 | 23.92 | ||
| Testing Set | MSE | 6972.96 | 7223.7 | 1135.4 | |
| RMSE | 83.5 | 85 | 33.69 | ||
| Maruteru | Training Set | MSE | 64,874.99 | 123,352.3 | 7867.69 |
| RMSE | 254.70 | 351.21 | 88.77 | ||
| Testing Set | MSE | 957,371.5 | 1,113,217 | 408,197 | |
| RMSE | 978.45 | 1055.09 | 638.9 | ||
| Pattambi | Training Set | MSE | 1094.41 | 2594.43 | 11.49 |
| RMSE | 33.08 | 50.93 | 3.38 | ||
| Testing Set | MSE | 4.88 | 39.39 | 1.18 | |
| RMSE | 2.21 | 6.27 | 1.37 | ||
| Jagdalpur | Training Set | MSE | 354.28 | 356.00 | 17.14 |
| RMSE | 18.82 | 18.84 | 4.14 | ||
| Testing Set | MSE | 1.9 | 12.4 | 0.42 | |
| RMSE | 1.38 | 3.52 | 0.64 |
| Centre | Data Type | M1, M2 | M1, M3 | M2, M3 |
|---|---|---|---|---|
| Warangal | Training Set | −2.2724 (0.02377) | 3.1103 (0.00204) | 3.0902 (0.00218) |
| Testing Set | −0.69073 (0.5205) | 3.5875 (0.01575) | 4.3453 (0.00739) | |
| Maruteru | Training Set | −3.1459 (0.00184) | 3.9768 (<0.0001) | 4.4649 (<0.0001) |
| Testing Set | −1.6994 (0.15) | 1.6566 (0.1585) | 1.6902 (0.1518) | |
| Pattambi | Training Set | −3.0771 (0.0022) | 3.3392 (0.0009) | 3.6736 (0.0002) |
| Testing Set | −2.5075 (0.0539) | 1.5823 (0.1029) | 2.9792 (0.0308) | |
| Jagdalpur | Training Set | −0.0429 (0.9658) | 1.5301 (0.1273) | 1.5736 (0.1169) |
| Testing Set | −2.4567 (0.0574) | 2.2006 (0.0790) | 2.9514 (0.0318) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathod, S.; Yerram, S.; Arya, P.; Katti, G.; Rani, J.; Padmakumari, A.P.; Somasekhar, N.; Padmavathi, C.; Ondrasek, G.; Amudan, S.; et al. Climate-Based Modeling and Prediction of Rice Gall Midge Populations Using Count Time Series and Machine Learning Approaches. Agronomy 2022, 12, 22. https://doi.org/10.3390/agronomy12010022
Rathod S, Yerram S, Arya P, Katti G, Rani J, Padmakumari AP, Somasekhar N, Padmavathi C, Ondrasek G, Amudan S, et al. Climate-Based Modeling and Prediction of Rice Gall Midge Populations Using Count Time Series and Machine Learning Approaches. Agronomy. 2022; 12(1):22. https://doi.org/10.3390/agronomy12010022
Chicago/Turabian StyleRathod, Santosha, Sridhar Yerram, Prawin Arya, Gururaj Katti, Jhansi Rani, Ayyagari Phani Padmakumari, Nethi Somasekhar, Chintalapati Padmavathi, Gabrijel Ondrasek, Srinivasan Amudan, and et al. 2022. "Climate-Based Modeling and Prediction of Rice Gall Midge Populations Using Count Time Series and Machine Learning Approaches" Agronomy 12, no. 1: 22. https://doi.org/10.3390/agronomy12010022
APA StyleRathod, S., Yerram, S., Arya, P., Katti, G., Rani, J., Padmakumari, A. P., Somasekhar, N., Padmavathi, C., Ondrasek, G., Amudan, S., Malathi, S., Rao, N. M., Karthikeyan, K., Mandawi, N., Muthuraman, P., & Sundaram, R. M. (2022). Climate-Based Modeling and Prediction of Rice Gall Midge Populations Using Count Time Series and Machine Learning Approaches. Agronomy, 12(1), 22. https://doi.org/10.3390/agronomy12010022







