Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Culture Conditions
2.2. Chlorophyll Quantification
2.3. Determination of Proline and Lipid Peroxidation
2.4. Determination of Total Antioxidant Activity and Phenolic Compounds
2.5. Extraction and Quantification of SA
2.6. Enzymatic Assays
2.7. Agronomic and Fruit Quality Determinations
2.8. Statistical Analysis
3. Results
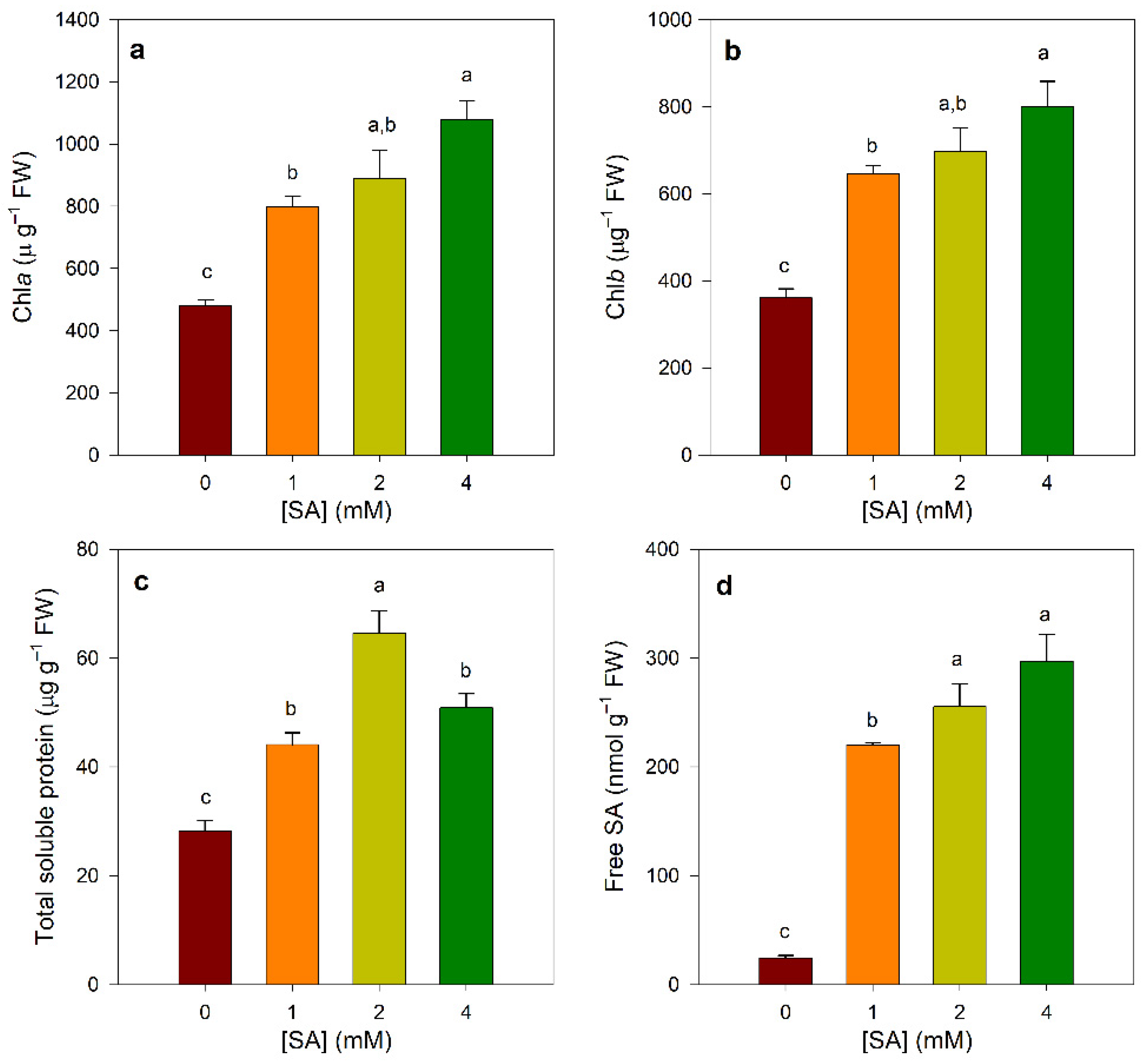
3.1. Effect of SA Treatments on Chlorophylls, Protein, and SA Contents
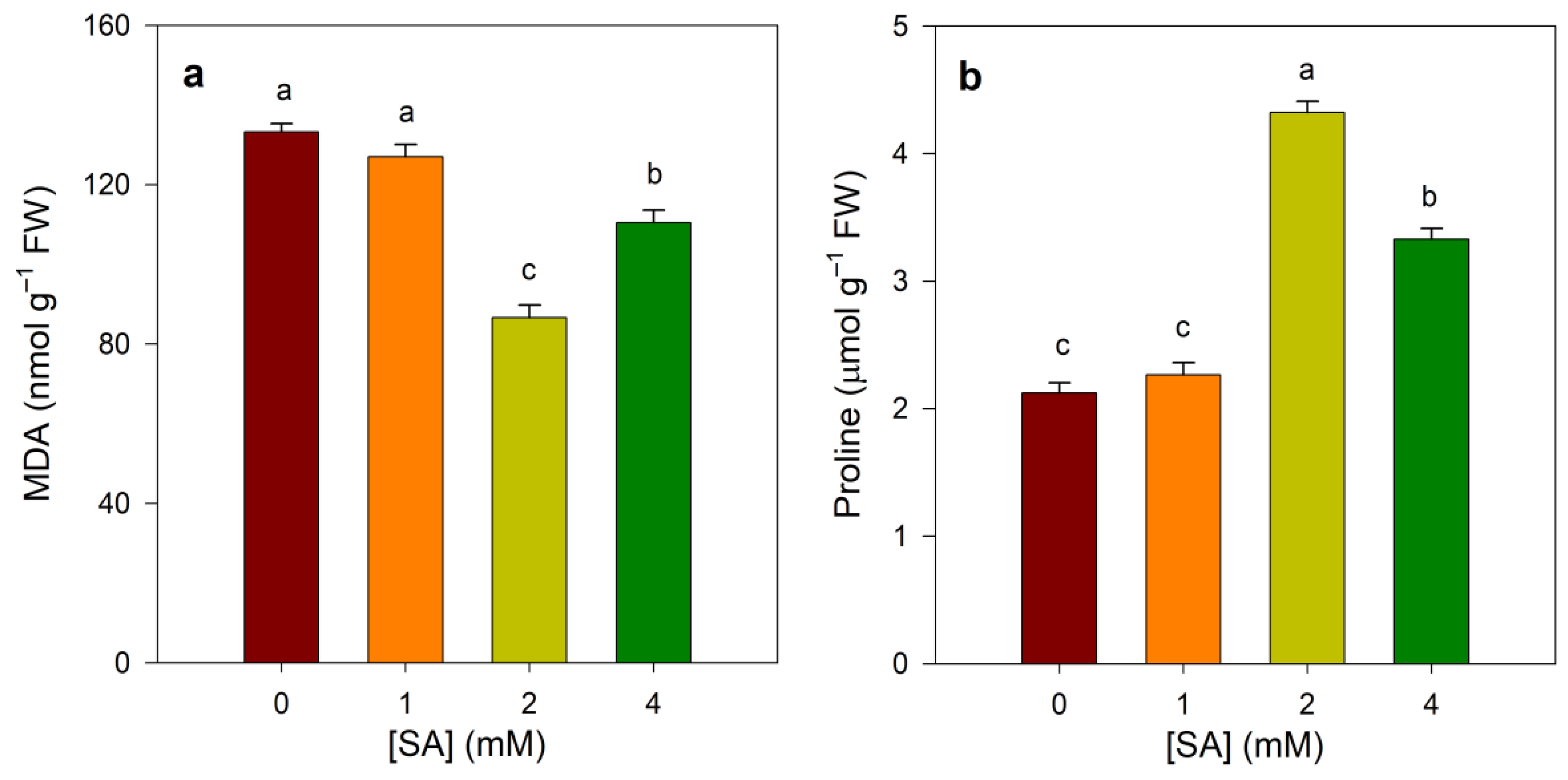
3.2. Effect of SA Treatments on Proline and MDA Contents
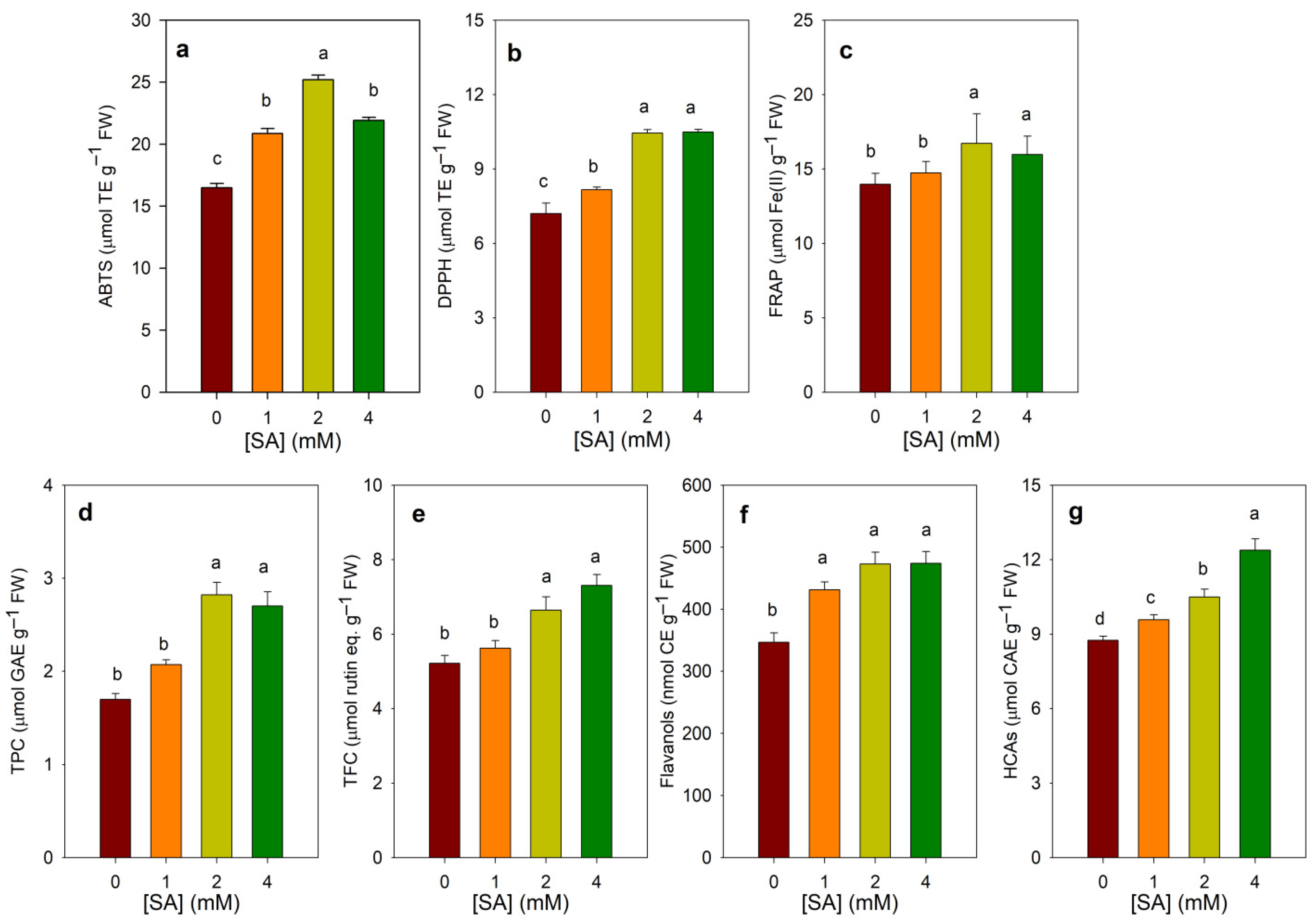
3.3. Effect of SA Treatments on Total Antioxidant Capacity and Phenolic Contents
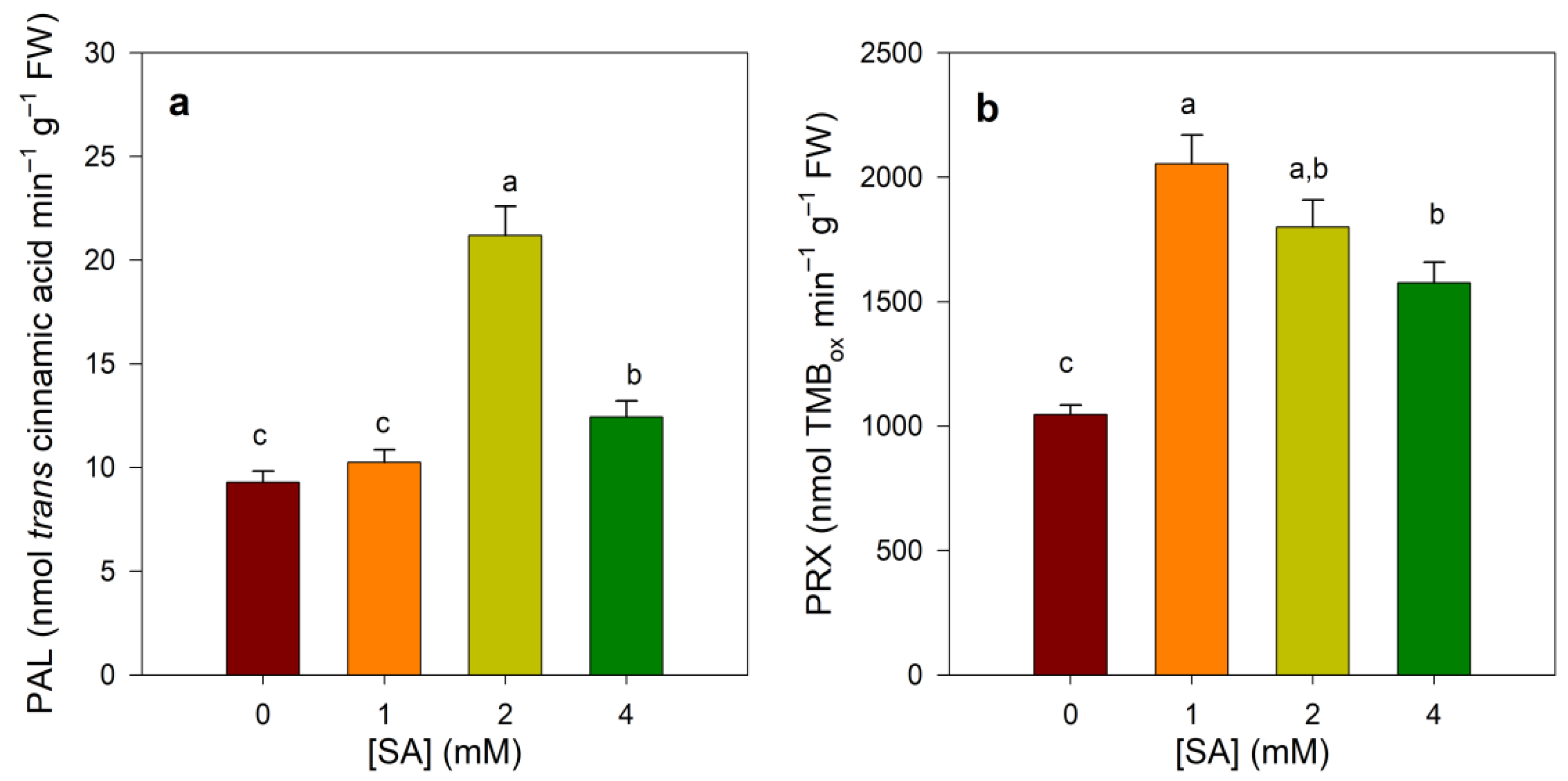
3.4. Effect of SA Treatments on PAL and Prx Activities
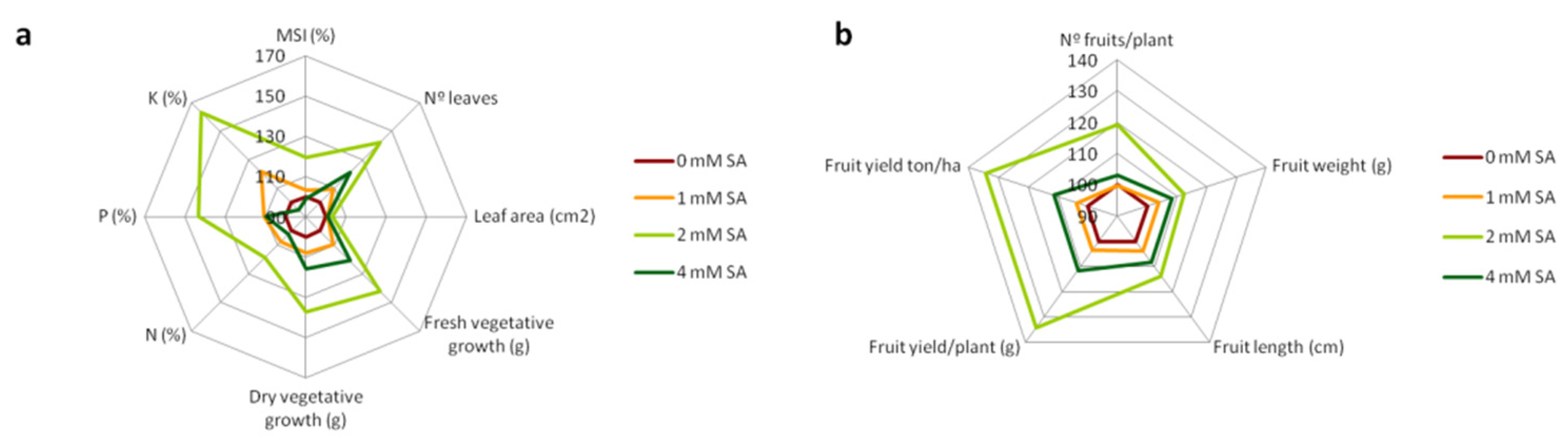
3.5. Effect of SA Treatments on Agronomic Traits
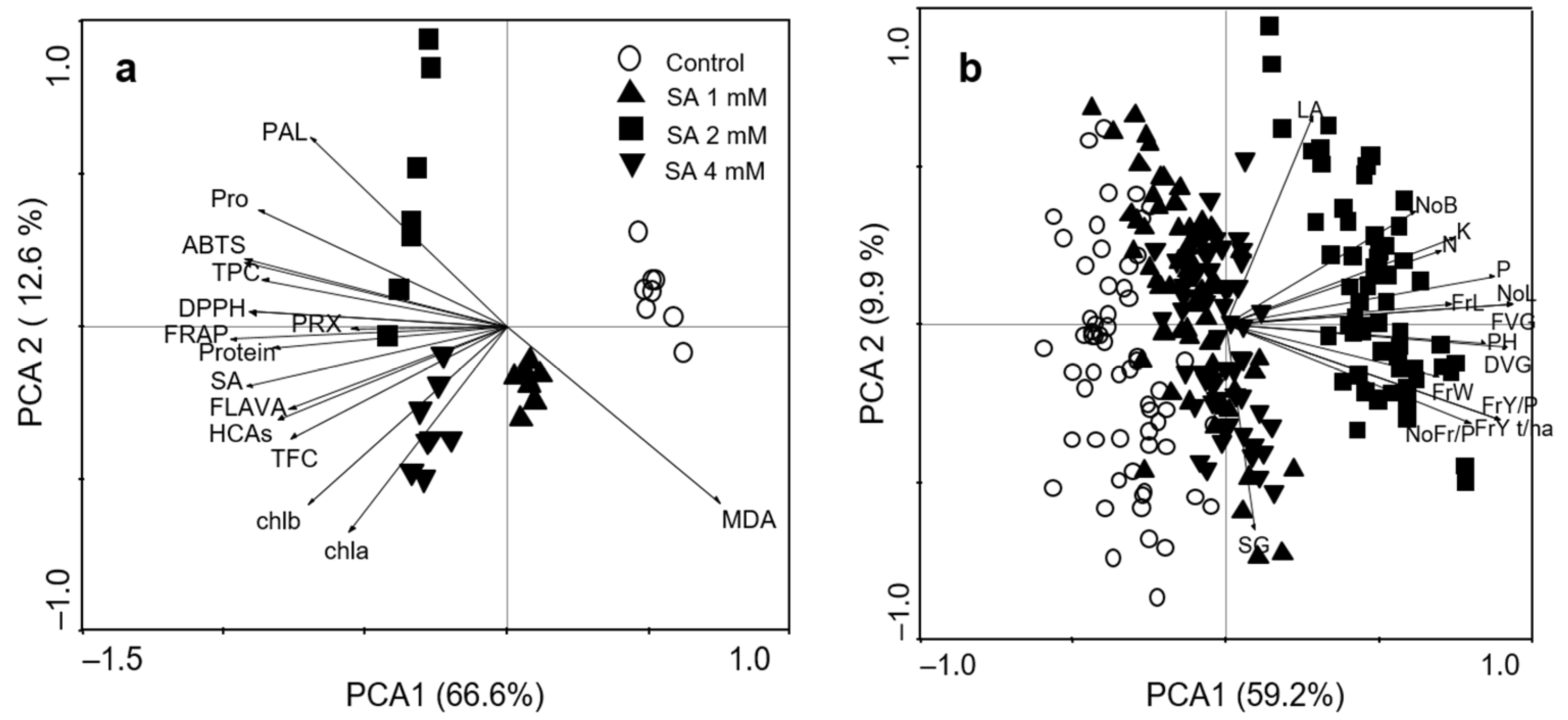
3.6. Principal Component Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drewnowski, A. 50 Foods for Healthier People and a Healthier Planet; World Wildlife Foundation, Knorr Foods, 2019. Available online: https://www.wwf.org.uk/sites/default/files/2019-02/Knorr_Future_50_Report_FINAL_Online.pdf (accessed on 20 December 2021).
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef]
- Lamont, W.J. Okra—A versatile vegetable crop. Horttechnology 1999, 9, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.T. Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature-based review. Phyther. Res. 2019, 33, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Camciuc, M.; Deplagne, M.; Vilarem, G.; Gaset, A. Okra—Abelmoschus esculentus L. (Moench.) a crop with economic potential for set aside acreage in France. Ind. Crops Prod. 1998, 7, 257–264. [Google Scholar] [CrossRef]
- Moosavi, S.A.; Aghaalikhani, M.; Ghobadian, B.; Fayyazi, E. Okra: A potential future bioenergy crop in Iran. Renew. Sustain. Energy Rev. 2018, 93, 517–524. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database: 2018; FAO: Rome, Italy, 2018. [Google Scholar]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [Green Version]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Clinton, M.; Qi, G.; Wang, D.; Liu, F.; Qing Fu, Z. Reprogramming and remodeling: Transcriptional and epigenetic regulation of salicylic acid-mediated plant defense. J. Exp. Bot. 2020, 71, 5256–5268. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Dong, Y.; Xu, L.; Kong, J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014, 73, 67–78. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, J.; Brunner, S.; Horváth, E.; Bela, K.; Ködmön, P.; Riyazuddin, R.; Gallé, Á.; Hurton, Á.; Papdi, C.; Szabados, L.; et al. Exogenously applied salicylic acid maintains redox homeostasis in salt-stressed Arabidopsis gr1 mutants expressing cytosolic roGFP1. Plant Growth Regul. 2018, 86, 181–194. [Google Scholar] [CrossRef]
- Kiddle, G.A.; Doughty, K.J.; Wallsgrove, R.M. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J. Exp. Bot. 1994, 45, 1343–1346. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Moreno, J.M.; Calderón, A.A.; Ferrer, M.A. Changes in phenolic metabolism in salicylic acid-treated shoots of Cistus heterophyllus. Plant Cell Tissue Organ Cult. 2013, 113, 417–427. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Pérez, A.; Calderón, A.A.; Ferrer, M.A. Pb-induced responses in Zygophyllum fabago plants are organ-dependent and modulated by salicylic acid. Plant Physiol. Biochem. 2014, 84, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Arora, I.; Sharma, M.; Sun, L.Y.; Tollefsbol, T.O. The Epigenetic Link between Polyphenols, Aging and Age-Related Diseases. Genes 2020, 11, 1094. [Google Scholar] [CrossRef]
- MALR Egyptian Ministry of Agriculture and Land Reclamation. Agricultural Statistics Bulletin. Part Two Summer and Autumn Crops 2018/2019; Department of Economic Affairs: Cairo, Egypt, 2020. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Hassan, A.A. Okra Production. Extension Bulletin, Care International- Egypt, USAID. 2005. Available online: https://www.mediafire.com/file/39120zxxm28v2yr/2157.pdf/file (accessed on 20 December 2021). (In Arabic).
- López-Orenes, A.; Bueso, M.C.; Conesa, H.; Calderón, A.A.; Ferrer, M.A. Seasonal ionomic and metabolic changes in Aleppo pines growing on mine tailings under Mediterranean semi-arid climate. Sci. Total Environ. 2018, 637–638, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- López-Arnaldos, T.; Muñoz, R.; Ferrer, M.A.; Calderón, A.A. Changes in phenol content during strawberry (Fragaria x ananassa, cv. Chandler) callus culture. Physiol. Plant. 2001, 113, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.E.; Huang, L.; Preston, G.M.; Naylor, M.; Carr, J.P.; Li, Y.; Singer, A.C.; Whiteley, A.S.; Wang, H. Quantitative in situ assay of salicylic acid in tobacco leaves using a genetically modified biosensor strain of Acinetobacter sp. ADP1. Plant J. 2006, 46, 1073–1083. [Google Scholar] [CrossRef]
- Huang, W.E.; Wang, H.; Zheng, H.; Huang, L.; Singer, A.C.; Thompson, I.; Whiteley, A.S. Chromosomally located gene fusions constructed in Acinetobacter sp ADP1 for the detection of salicylate. Environ. Microbiol. 2005, 7, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Defraia, C.T.; Schmelz, E.A.; Mou, Z. A rapid biosensor-based method for quantification of free and glucose-conjugated salicylic acid. Plant Methods 2008, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; ISBN 0935584870. [Google Scholar]
- López-Orenes, A.; Bueso, M.C.; Conesa, H.M.; Calderón, A.A.; Ferrer, M.A. Seasonal changes in antioxidative/oxidative profile of mining and non-mining populations of Syrian beancaper as determined by soil conditions. Sci. Total Environ. 2017, 575, 437–447. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Stevens, J.; Senaratna, T.; Sivasithamparam, K. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): Associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic Acid Influences Net Photosynthetic Rate, Carboxylation Efficiency, Nitrate Reductase Activity, and Seed Yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Szalai, G.; Horgosi, S.; Soós, V.; Majláth, I.; Balázs, E.; Janda, T. Salicylic acid treatment of pea seeds induces its de novo synthesis. J. Plant Physiol. 2011, 168, 213–219. [Google Scholar] [CrossRef]
- Niederl, S.; Kirsch, T.; Riederer, M.; Schreiber, L. Co-Permeability of 3H-Labeled Water and14C-Labeled Organic Acids across Isolated Plant Cuticles1. Plant Physiol. 1998, 116, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and Extracellular Journey of the Phytohormone Salicylic Acid. Front. Plant Sci. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Gondor, O.K.; Janda, T.; Soós, V.; Pál, M.; Majláth, I.; Adak, M.K.; Balázs, E.; Szalai, G. Salicylic Acid Induction of Flavonoid Biosynthesis Pathways in Wheat Varies by Treatment. Front. Plant Sci. 2016, 7, 1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Wang, W.; Ruan, C.; Deng, L.; Yao, S.; Zeng, K. Involvement of CsWRKY70 in salicylic acid-induced citrus fruit resistance against Penicillium digitatum. Hortic. Res. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. B 2011, 9, e0156. [Google Scholar] [CrossRef] [Green Version]
- Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bidabadi, S.S.; Mahmood, M.; Baninasab, B.; Ghobadi, C. Influence of salicylic acid on morphological and physiological responses of banana (Musa acuminata cv. “Berangan”, AAA) shoot tips to in vitro water stress induced by polyethylene glycol. Plant Omics 2012, 5, 33–39. [Google Scholar]
- Shetty, K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: A review. Process Biochem. 2004, 39, 789–804. [Google Scholar] [CrossRef]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon Fluxes between Primary Metabolism and Phenolic Pathway in Plant Tissues under Stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.L.B.; Vieira, L.G.E.; Ribas, A.F.; Moro, A.L.; Neris, D.M.; Pacheco, A.C. Proline accumulation induces the production of total phenolics in transgenic tobacco plants under water deficit without increasing the G6PDH activity. Theor. Exp. Plant Physiol. 2018, 30, 251–260. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.-M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef]
- Wen, P.-F.; Chen, J.-Y.; Kong, W.-F.; Pan, Q.-H.; Wan, S.-B.; Huang, W.-D. Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 2005, 169, 928–934. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Growth enhancement and salt tolerance of Safflower (Carthamus tinctorius L.), by salicylic acid. Curr. Plant Biol. 2018, 13, 16–22. [Google Scholar] [CrossRef]
- Chavoushi, M.; Najafi, F.; Salimi, A.; Angaji, S.A. Effect of salicylic acid and sodium nitroprusside on growth parameters, photosynthetic pigments and secondary metabolites of safflower under drought stress. Sci. Hortic. 2020, 259, 108823. [Google Scholar] [CrossRef]
- Yamasaki, H.; Sakihama, Y.; Ikehara, N. Flavonoid-Peroxidase Reaction as a Detoxification Mechanism of Plant Cells against H2O2. Plant Physiol. 1997, 115, 1405–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, S.M.; López-Orenes, A.; Ferrer, M.A.; Calderón, A.A. Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady). Agronomy 2022, 12, 168. https://doi.org/10.3390/agronomy12010168
Youssef SM, López-Orenes A, Ferrer MA, Calderón AA. Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady). Agronomy. 2022; 12(1):168. https://doi.org/10.3390/agronomy12010168
Chicago/Turabian StyleYoussef, Sabry M., Antonio López-Orenes, María A. Ferrer, and Antonio A. Calderón. 2022. "Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady)" Agronomy 12, no. 1: 168. https://doi.org/10.3390/agronomy12010168
APA StyleYoussef, S. M., López-Orenes, A., Ferrer, M. A., & Calderón, A. A. (2022). Salicylic-Acid-Regulated Antioxidant Capacity Contributes to Growth Improvement of Okra (Abelmoschus esculentus cv. Red Balady). Agronomy, 12(1), 168. https://doi.org/10.3390/agronomy12010168





