Remediation of Pesticides by Microalgae as Feasible Approach in Agriculture: Bibliometric Strategies
Abstract
:1. Introduction
2. Application of Pesticides in Agriculture
3. Environmental Impacts of Pesticides in Agriculture
4. Mechanism of Action by Microalgae Remediation
4.1. Biosorption
4.2. Bioaccumulation
4.3. Biodegradation
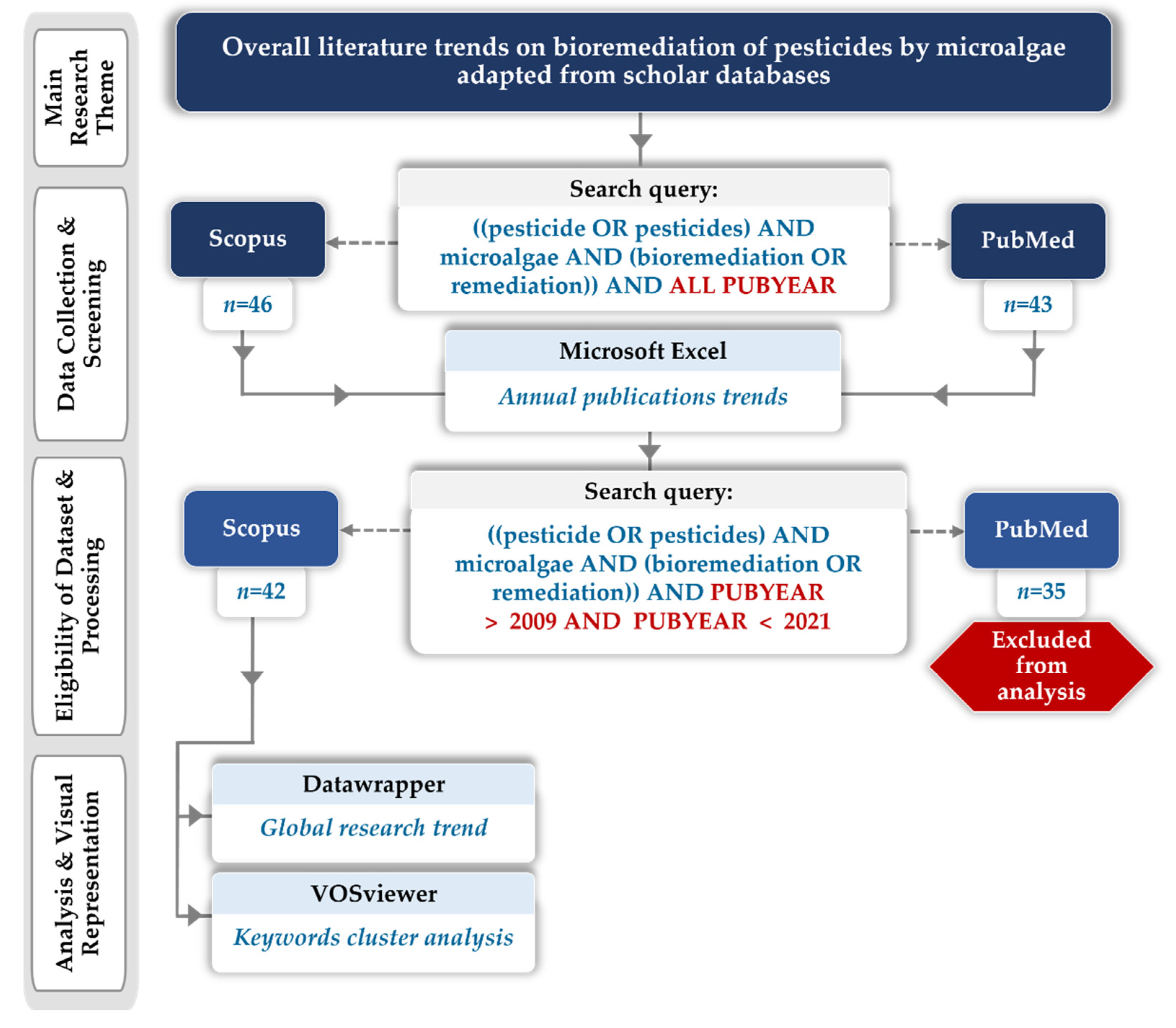
5. Microalgae as Biological Remediating Agent for Pesticides: Bibliometric Trend Analysis
5.1. Bibliometric Data and Methodology
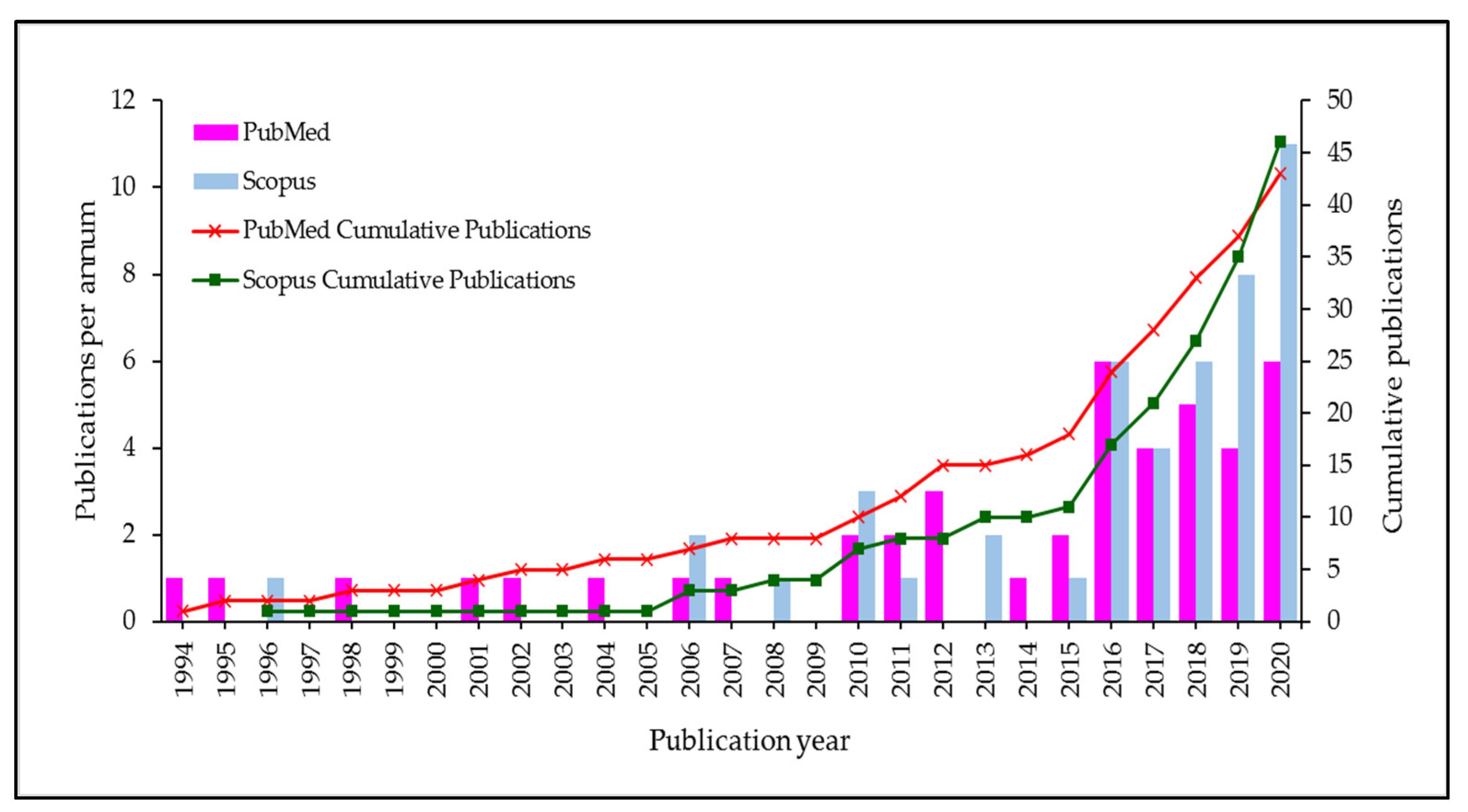
5.2. Bibliometric Evolution Analysis
5.3. Global Publications Contribution
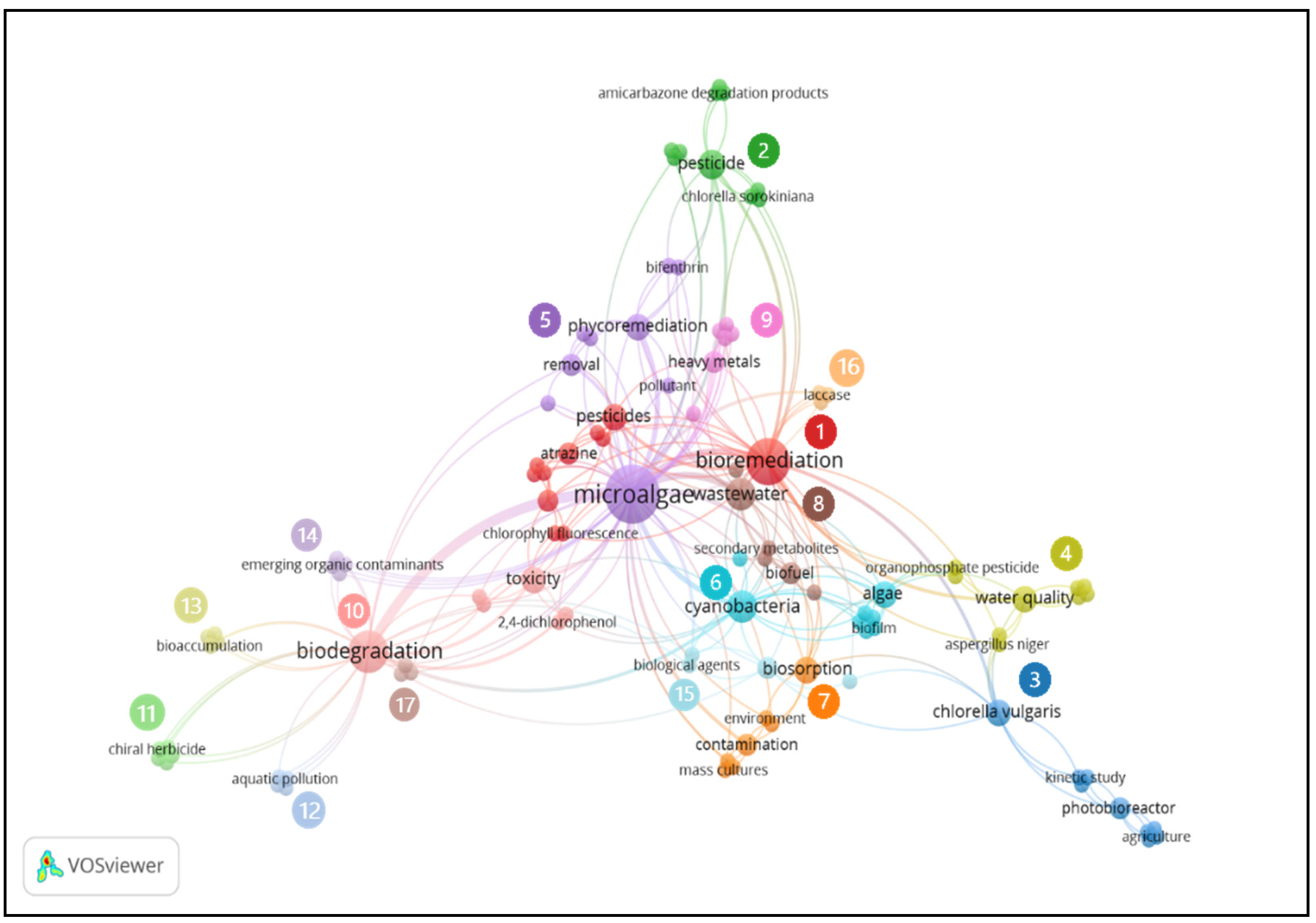
5.4. Author’s Keywords Cluster Analysis and Literature Review
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLellan, J.; Gupta, S.K.; Kumar, M. Feasibility of Using Bacterial-Microalgal Consortium for the Bioremediation of Organic Pesticides: Application Constraints and Future Prospects. In Application of Microalgae in Wastewater Treatment Volume 1: Domestic and Industrial Wastewater Treatment; Gupta, S.K., Bux, F., Eds.; Springer: Cham, Switzerland, 2019; pp. 341–362. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Avila, R.; Peris, A.; Eljarrat, E.; Vicent, T.; Blánquez, P. Biodegradation of Hydrophobic Pesticides by Microalgae: Transformation Products and Impact on Algae Biochemical Methane Potential. Sci. Total Environ. 2021, 754, 142114. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef]
- Sarath Chandran, C.; Thomas, S.; Unni, M.R. Pesticides: Classification, Detection, and Degradation. In Organic Farming; Sarath Chandran, C., Thomas, S., Unni, M.R., Eds.; Springer: Cham, Switzerland, 2019; pp. 71–87. [Google Scholar] [CrossRef]
- Conway, G.R.; Barbie, E.B. After the Green Revolution: Sustainable and Equitable Agricultural Development. Futures 1988, 20, 651–670. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Sharma, R. Microorganism as a Tool of Bioremediation Technology for Cleaning Environment: A Review. Proc. Int. Acad. Ecol. Environ. Sci. 2014, 4, 1–6. [Google Scholar]
- Prabha, R.; Singh, D.P.; Verma, M.K. Microbial Interactions and Perspectives for Bioremediation of Pesticides in the Soils. Plant-Microbe Interact. Agro-Ecol. Perspect 2017, 2, 649–671. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Microalgal Bioremediation of Emerging Contaminants—Opportunities and Challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef]
- Hammed, A.M.; Prajapati, S.K.; Simsek, S.; Simsek, H. Growth Regime and Environmental Remediation of Microalgae. Algae 2016, 31, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of Water Containing Pesticides By Microalgae: Mechanisms, Methods, and Prospects For Future Research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Herbert, S.J. Feeding China’s Growing Needs for Grain. Nature 2010, 465, 420. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). The Impact of Disasters and Crises on Agriculture and Food Security; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Roser, M.; Pesticides—Our World in Data. Food and Agriculture Organization of the United Nations (FAO). Available online: https://ourworldindata.org/pesticides (accessed on 24 August 2021).
- Maksymiv, I. Pesticides: Benefits and Hazards. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Au, A.M. Pesticides and Herbicides|Types, Uses, and Determination of Herbicides. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 4483–4487. [Google Scholar] [CrossRef]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of Climate Change Factors on Weeds and Herbicide Efficacy. Adv. Agron. 2016, 135, 107–146. [Google Scholar] [CrossRef]
- Song, Y. Insight into the Mode of Action of 2,4-Dichlorophenoxyacetic Acid (2,4-D) as an Herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, K.B. Study of Herbicidal Effect of 2,4-D on Growth and Cellular Metabolites in Cyanobacterium Synechococcus aeruginosus from Rice Fields. J. Algal Biomass Util. 2016, 7, 1–3. [Google Scholar]
- Pazmiño, D.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Archilla-Ruiz, A.; del Río, L.A.; Sandalio, L.M. Differential Response of Young and Adult Leaves to Herbicide 2,4-Dichlorophenoxyacetic Acid in Pea Plants: Role of Reactive Oxygen Species. Plant. Cell Environ. 2011, 34, 1874–1889. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ding, Y.; Chang, J.; Sun, X.; Zhang, L.; Wei, Q.; Cheng, Y.; Chen, L.; Xu, J.; Deng, X. Comprehensive Insights on How 2,4-Dichlorophenoxyacetic Acid Retards Senescence in Post-Harvest Citrus Fruits Using Transcriptomic and Proteomic Approaches. J. Exp. Bot. 2014, 65, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Lal, D.; Tripathi, V.K.; Kumar, S.; Nayyer, A. Effect of Pre-Harvest Application of Gibberellic Acid, NAA, and Calcium Nitrate on Fruit Drop, Maturity and Storage Quality of Kinnow Mandarin. Res. Environ. Life Sci. 2015, 8, 561–564. [Google Scholar]
- Koger, C.H.; Burke, I.C.; Miller, D.K.; Kendig, J.A.; Reddy, K.N.; Wilcut, J.W. MSMA Antagonizes Glyphosate and Glufosinate Efficacy on Broadleaf and Grass Weeds. Weed Technol. 2007, 21, 159–165. [Google Scholar] [CrossRef]
- Hossard, L.; Philibert, A.; Bertrand, M.; Colnenne-David, C.; Debaeke, P.; Munier-Jolain, N.; Jeuffroy, M.H.; Richard, G.; Makowski, D. Effects of Halving Pesticide Use on Wheat Production. Sci. Rep. 2014, 4, 4405. [Google Scholar] [CrossRef] [Green Version]
- Waryszak, P.; Lenz, T.L.; Leishman, M.R.; Downey, P.O. Herbicide Effectiveness in Controlling Invasive Plants under Elevated CO2: Sufficient Evidence to Rethink Weeds Management. J. Environ. Manag. 2018, 226, 400–407. [Google Scholar] [CrossRef]
- Cowie, B.W.; Venter, N.; Witkowski, E.T.; Byrne, M.J. Implications of Elevated Carbon Dioxide on the Susceptibility of the Globally Invasive Weed, Parthenium hysterophorus, to Glyphosate Herbicide. Pest Manag. Sci. 2020, 76, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Leishman, M.R.; Downey, P.O. Exotic C4 Grasses Have Increased Tolerance to Glyphosate under Elevated Carbon Dioxide. Weed Sci. 2011, 59, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Jabran, K.; Doğan, M.N. High Carbon Dioxide Concentration and Elevated Temperature Impact the Growth of Weeds but Do Not Change the Efficacy of Glyphosate. Pest Manag. Sci. 2018, 74, 766–771. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Wang, H.; Chauhan, B.S.; Adkins, S.W. Effect of Elevated Carbon Dioxide Concentration on Growth, Productivity and Glyphosate Response of Parthenium Weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2019, 75, 2934–2941. [Google Scholar] [CrossRef]
- Iqbal, N.; Manalil, S.; Chauhan, B.S.; Adkins, S. Effect of Different Climate Change Variables on the Ecology and Management of Sesbania cannabina through Glyphosate. Plants 2021, 10, 910. [Google Scholar] [CrossRef]
- Matzrafi, M.; Brunharo, C.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased Temperatures and Elevated CO2 Levels Reduce the Sensitivity of Conyza canadensis and Chenopodium album to Glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef]
- Ziska, L.H. Climate Change and the Herbicide Paradigm: Visiting the Future. Agronomy 2020, 10, 1953. [Google Scholar] [CrossRef]
- US EPA. Brief Overview about Individual Pesticides. Available online: https://www.epa.gov/ingredients-used-pesticide-products/brief-overviews-about-individual-pesticides (accessed on 25 August 2021).
- Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2,4-D Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2008; Available online: http://npic.orst.edu/factsheets/archive/2,4-DTech.html (accessed on 25 August 2021).
- Henderson, A.M.; Gervais, J.A.; Luukinen, B.; Buhl, K.; Stone, D. Glyphosate General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2010; Available online: http://npic.orst.edu/factsheets/glyphogen.html (accessed on 25 August 2021).
- Minnesota Department of Agriculture. Dicamba—General Information. Available online: https://www.mda.state.mn.us/dicamba-general-information (accessed on 25 August 2021).
- Bunch, T.R.; Gervais, J.A.; Buhl, K.; Stone, D. Dicamba Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2012; Available online: http://npic.orst.edu/factsheets/archive/dicamba_tech.html (accessed on 25 August 2021).
- Klementova, S.; Keltnerova, L. Triazine Herbicides in the Environment. In Herbicides, Physiology of Action, and Safety; InTech: London, UK, 2015; pp. 71–96. [Google Scholar] [CrossRef] [Green Version]
- Watts, M. Paraquat. Available online: http://wssroc.agron.ntu.edu.tw/note/Paraquat.pdf (accessed on 27 August 2021).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. Malathion General Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2009; Available online: http://npic.orst.edu/factsheets/malagen.html (accessed on 26 August 2021).
- Davis, M.K.; Boone, J.S.; Moran, J.E.; Tyler, J.W.; Chambers, J.E. Assessing Intermittent Pesticide Exposure from Flea Control Collars Containing the Organophosphorus Insecticide Tetrachlorvinphos. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Tiwari, M.; Prakash, O.; Singh, M. A Current Review of Cypermethrin-Induced Neurotoxicity and Nigrostriatal Dopaminergic Neurodegeneration. Curr. Neuropharmacol. 2012, 10, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Gervais, J.A.; Luukinen, B.; Buhl, K.; Stone, D. Imidacloprid Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2010; Available online: http://npic.orst.edu/factsheets/archive/imidacloprid.html (accessed on 26 August 2021).
- Minnesota Department of Agriculture. Mancozeb Fungicide. Available online: https://www.mda.state.mn.us/mancozeb-fungicide (accessed on 25 August 2021).
- Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N. Impact of Pesticides on Soil Microbiological Parameters and Possible Bioremediation Strategies. Indian J. Microbiol. 2008, 48, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Latchoumycandane, C.; Jenardhanan, P.; Mathur, P.P. Environmental Impact on Gametogenesis and Embryogenesis: An Overview. In Encyclopedia of Reproduction; Elsevier: Amsterdam, The Netherlands, 2018; pp. 446–451. [Google Scholar] [CrossRef]
- Chekroun, B.; Ben Chekroun, K.; Baghour, M. The Role of Algae in Phytoremediation of Heavy Metals: A Review. J. Mater. Environ. Sci. 2013, 4, 873–880. [Google Scholar]
- Kumar, S.; Joshi, P.C.; Nath, P.; Singh, V.K. Impacts of Insecticides on Pollinators of Different Food Plants. Entomol. Ornithol. Herpetol. 2018, 7, 211. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Singhvi, R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. Int. J. Agric. Environ. Biotechnol. 2017, 10, 675. [Google Scholar] [CrossRef]
- McDonald, T.A.; Holland, N.T.; Skibola, C.; Duramad, P.; Smith, M.T. Hypothesis: Phenol and Hydroquinone Derived Mainly from Diet and Gastrointestinal Flora Activity Are Causal Factors in Leukemia. Leukemia 2001, 15, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Prashar, P.; Shah, S. Impact of Fertilizers and Pesticides on Soil Microflora in Agriculture. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2016; pp. 331–361. [Google Scholar] [CrossRef]
- Gunstone, T.; Cornelisse, T.; Kendra, K.; Aditi, D.; Donley, N. Pesticides and Soil Invertebrates: A Hazard Assessment. Front. Environ. Sci. 2021, 9, 122. [Google Scholar] [CrossRef]
- Lo, C.-C. Effect of Pesticides on Soil Microbial Community. J. Environ. Sci. Health Part B 2010, 45, 348–359. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D. Pesticides Effect on Soil Microbial Ecology and Enzyme Activity—An Overview. J. Appl. Nat. Sci. 2016, 8, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Ripper, W.E.; Greenslade, R.M.; Lickerish, L.A. Combined Chemical and Biological Control of Insects by Means of a Systemic Insecticide. Nature 1949, 163, 787–789. [Google Scholar] [CrossRef]
- Philippat, C.; Barkoski, J.; Tancredi, D.J.; Elms, B.; Barr, D.B.; Ozonoff, S.; Bennett, D.H.; Hertz-Picciotto, I. Prenatal Exposure to Organophosphate Pesticides and Risk of Autism Spectrum Disorders and Other Non-Typical Development at 3 Years in a High-Risk Cohort. Int. J. Hyg. Environ. Health 2018, 221, 548–555. [Google Scholar] [CrossRef]
- Yadav, H.; Singh Sankhla, M.; Kumar, R. Pesticides-Induced Carcinogenic & Neurotoxic Effect on Human. Forensic Res. Criminol. Int. J. 2019, 7, 243–245. [Google Scholar] [CrossRef]
- Rocha, G.M.; Grisolia, C.K. Why Pesticides with Mutagenic, Carcinogenic and Reproductive Risks are Registered in Brazil. Dev. World Bioeth. 2019, 19, 148–154. [Google Scholar] [CrossRef]
- Mustafa, S.; Bhatti, N.; Maqbool, M. Microalgae Biosorption, Bioaccumulation and Biodegradation Efficiency for the Remediation of Wastewater and Carbon Dioxide Mitigation: Prospects, Challenges and Opportunities. J. Water Process Eng. 2021, 41, 2214–7144. [Google Scholar] [CrossRef]
- Hussein, M.H.; Abdullah, A.M.; Badr El Din, N.I.; Mishaqa, E.S.I. Biosorption Potential of the Microchlorophyte Chlorella vulgaris for Some Pesticides. J. Fertil. Pestic. 2017, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of Lipid and Glycogen Accumulations Under Different Growth Conditions in the Sulfothermophilic Red Alga Galdieria sulphuraria. Bioresour. Technol. 2016, 200, 861–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Xiao, H.; He, N.; Sun, D.; Duan, S. Biosorption and Biodegradation of the Environmental Hormone Nonylphenol By Four Marine Microalgae. Sci. Rep. 2019, 9, 5277. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current Perspectives on Concept, Definition and Application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Sethunathan, N.; Megharaj, M.; Chen, Z.L.; Williams, B.D.; Lewis, G.; Naidu, R. Algal Degradation of a Known Endocrine Disrupting Insecticide, α-Endosulfan, and Its Metabolite, Endosulfan Sulfate, in Liquid Medium and Soil. J. Agric. Food Chem. 2004, 52, 3030–3035. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Rasoul-Amini, S.; Fotooh-Abadi, E. The Biotransformation, Biodegradation, and Bioremediation of Organic Compounds by Microalgae. J. Phycol. 2011, 47, 969–980. [Google Scholar] [CrossRef]
- Weis, L.; de Cassia de Souza Schneider, R.; Hoeltz, M.; Rieger, A.; Tostes, S.; Lobo, E.A. Potential for Bifenthrin Removal Using Microalgae from a Natural Source. Water Sci. Technol. 2020, 82, 1131–1141. [Google Scholar] [CrossRef]
- Gregory, W.W.; Reed, J.K.; Priester, L.E. Accumulation of Parathion and DDT by Some Algae and Protozoa. J. Protozool. 1969, 16, 69–71. [Google Scholar] [CrossRef]
- Kurade, M.B.; Kim, J.R.; Govindwar, S.P.; Jeon, B.H. Insights into Microalgae Mediated Biodegradation of Diazinon by Chlorella vulgaris: Microalgal Tolerance to Xenobiotic Pollutants and Metabolism. Algal Res. 2016, 20, 126–134. [Google Scholar] [CrossRef]
- Velásquez, L.; Dussan, J. Biosorption and Bioaccumulation of Heavy Metals on Dead and Living Biomass of Bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef]
- Dosnon-Olette, R.; Trotel-Aziz, P.; Couderchet, M.; Eullaffroy, P. Fungicides and Herbicide Removal in Scenedesmus Cell Suspensions. Chemosphere 2010, 79, 117–123. [Google Scholar] [CrossRef]
- Encarnação, T.; Santos, D.; Ferreira, S.; Valente, A.J.M.; Pereira, J.C.; Campos, M.G.; Burrows, H.D.; Pais, A.A.C.C. Removal of Imidacloprid from Water by Microalgae Nannochloropsis sp. and Its Determination by a Validated RP-HPLC Method. Bull. Environ. Contam. Toxicol. 2021, 107, 131–139. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Fatima, N.; Pruthi, V.; Verma, M.; Chauhan, P.; Vlaskin, M.; Grigorenko, A. Detoxification Mechanism of Organophosphorus Pesticide via Carboxylestrase Pathway that Triggers de Novo TAG Biosynthesis in Oleaginous Microalgae. Aquat. Toxicol. 2019, 209, 49–55. [Google Scholar] [CrossRef]
- Ni, Y.; Lai, J.; Wan, J.; Chen, L. Photosynthetic Responses and Accumulation of Mesotrione in Two Freshwater Algae. Environ. Sci. Process. Impacts. 2014, 16, 2288–2294. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro algae Gracilaria verrucosa as a biosorbent: A study of sorption mechanisms. Algal Res. 2012, 1, 194–204. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs. 2018, 16, 65. [Google Scholar] [CrossRef] [Green Version]
- Kabra, A.N.; Ji, M.K.; Choi, J.; Kim, J.R.; Govindwar, S.P.; Jeon, B.H. Toxicity of Atrazine and Its Bioaccumulation and Biodegradation in a Green Microalga, Chlamydomonas mexicana. Environ. Sci. Pollut. Res. 2014, 21, 12270–12278. [Google Scholar] [CrossRef]
- Swackhamer, D.L.; Skoglund, R.S. Bioaccumulation of PCBs by Algae: Kinetics Versus Equilibrium. Environ. Toxicol. Chem. 1993, 12, 831–838. [Google Scholar] [CrossRef]
- Pérez-Legaspi, I.A.; Ortega-Clemente, L.A.; Moha-León, J.D.; Ríos-Leal, E.; Gutiérrez, S.C.-R.; Rubio-Franchini, I. Effect of the Pesticide Lindane on the Biomass of the Microalgae Nannochloris oculata. J. Environ. Sci. Health Part B 2015, 51, 103–106. [Google Scholar] [CrossRef]
- Qiu, Y.W.; Zeng, E.Y.; Qiu, H.; Yu, K.; Cai, S. Bioconcentration of Polybrominated Diphenyl Ethers and Organochlorine Pesticides in Algae Is an Important Contaminant Route to Higher Trophic Levels. Sci. Total Environ. 2017, 579, 1885–1893. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Field, L.M. Gene Amplification and Insecticide Resistance. Annu. Rev. Entomol. 1991, 36, 1–21. [Google Scholar] [CrossRef]
- Ortiz-Hernández, M.L.; Sánchez-Salinas, E.; Dantán-González, E.; Castrejón-Godínez, M.L. Pesticide Biodegradation: Mechanisms, Genetics and Strategies to Enhance the Process. In Biodegradation—Life of Science; Chamy, R., Rosenkranz, F., Eds.; InTech: London, UK, 2013; pp. 251–287. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Huang, L. Stereoselective Bioaccumulation, Transformation, and Toxicity of Triadimefon in Scenedesmus obliquus. Chirality 2017, 29, 61–69. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W. A Tale of Two Databases: The Use of Web of Science and Scopus in Academic Papers. Scientometrics 2020, 123, 321–335. [Google Scholar] [CrossRef] [Green Version]
- Linnenluecke, M.K.; Marrone, M.; Singh, A.K. Conducting Systematic Literature Reviews and Bibliometric Analyses. Aust. J. Manag. 2020, 45, 175–194. [Google Scholar] [CrossRef]
- AlRyalat, S.A.S.; Malkawi, L.W.; Momani, S.M. Comparing Bibliometric Analysis using PubMed, Scopus, and Web of Science Databases. J. Vis. Exp. 2019, 152, e58494. [Google Scholar] [CrossRef]
- Hood, W.W.; Wilson, C.S. The Literature of Bibliometrics, Scientometrics, and Informetrics. Scientometrics 2001, 52, 291–314. [Google Scholar] [CrossRef]
- Moral-Muñoz, J.A.; Herrera-Viedma, E.; Santisteban-Espejo, A.; Cobo, M.J. Software Tools for Conducting Bibliometric Analysis in Science: An up-to-Date Review. Prof. Inf. 2020, 29, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Pedraza, S.; Clerici, N.; Gaviria, J.D.Z.; Sanchez, A. Global Research on Riparian Zones in the Xxi Century: A Bibliometric Analysis. Water 2021, 13, 1836. [Google Scholar] [CrossRef]
- De Bakker, F.G.A.; Groenewegen, P.; Hond, F.D. A Bibliometric Analysis of 30 Years of Research and Theory on Corporate Social Responsibility and Corporate Social Performance. Bus. Soc. 2005, 44, 283–317. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Erazo, C.P.; Álvarez-García, J.; Río-Rama, M.d.l.C.d.; Durán-Sánchez, A. Scientific Mapping on the Impact of Climate Change on Cultural and Natural Heritage: A Systematic Scientometric Analysis. Land 2021, 10, 76. [Google Scholar] [CrossRef]
- Gorraiz, J.; Schloegl, C. A Bibliometric Analysis of Pharmacology and Pharmacy Journals: Scopus versus Web of Science. J. Inf. Sci. 2008, 34, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Md Khudzari, J.; Kurian, J.; Tartakovsky, B.; Raghavan, G.S.V. Bibliometric Analysis of Global Research Trends on Microbial Fuel Cells Using Scopus Database. Biochem. Eng. J. 2018, 136, 51–60. [Google Scholar] [CrossRef]
- Harzing, A.-W.; Alakangas, S. Google Scholar, Scopus and the Web of Science: A Longitudinal and Cross-Disciplinary Comparison. Scientometrics 2015, 106, 787–804. [Google Scholar] [CrossRef]
- Parlina, A.; Ramli, K.; Murfi, H. Theme Mapping and Bibliometrics Analysis of One Decade of Big Data Research in the Scopus Database. Information 2020, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Asiri, F.Y.; Kruger, E.; Tennant, M. Global Dental Publications in PubMed Databases between 2009 and 2019—A Bibliometric Analysis. Molecules 2020, 25, 4747. [Google Scholar] [CrossRef]
- Scopus. Available online: https://www.scopus.com/search/form.uri?display=basic#basic (accessed on 10 August 2021).
- Kulkarni, A.V.; Aziz, B.; Shams, I.; Busse, J.W. Comparisons of Citations in Web of Science, Scopus, and Google Scholar for Articles Published in General Medical Journals. JAMA J. Am. Med. Assoc. 2009, 302, 1092–1096. [Google Scholar] [CrossRef]
- Kiduk, Y.; Meho, L.I. Citation Analysis: A Comparison of Google Scholar, Scopus, and Web of Science. Proc. Am. Soc. Inf. Sci. Technol. 2006, 43, 1–15. [Google Scholar] [CrossRef] [Green Version]
- van Eck, N.J.; Waltman, L. VOSviewer Manual: Manual for VOSviewer Version 1.6.7. Univeristeit Leiden. 2018, 51, 1–50. [Google Scholar]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Datawrapper: Create Charts, Maps, and Tables. Available online: https://www.datawrapper.de/ (accessed on 18 August 2021).
- Abdel-Razek, M.A.; Abozeid, A.M.; Eltholth, M.M.; Abouelenien, F.A.; El-Midany, S.A.; Moustafa, N.Y.; Mohamed, R.A. Bioremediation of a Pesticide and Selected Heavy Metals in Wastewater from Various Sources Using a Consortium of Microalgae and Cyanobacteria. Slov. Vet. Res. 2019, 56, 61–73. [Google Scholar] [CrossRef]
- Contreras-Blancas, E.; Ruiz-Ordaz, N.; Galíndez-Mayer, J.; Torres-Gómez, R.E.; Arias Ruiz, A.; Juárez-Ramírez, C. Permeable Reactive Surface-Biobarriers. Testing and Evaluation of an Ecotechnology for the Removal of Agrotoxic Compounds Carried by Agricultural Runoffs. J. Environ. Health Sci. Eng. 2020, 18, 559–571. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Gutiérrez, R.; Uggetti, E.; Matamoros, V.; García, J.; Ferrer, I. Use of Full-Scale Hybrid Horizontal Tubular Photobioreactors to Process Agricultural Runoff. Biosyst. Eng. 2018, 166, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Nicodemus, T.J.; DiRusso, C.C.; Wilson, M.; Black, P.N. Reactive Oxygen Species (ROS) Mediated Degradation of Organophosphate Pesticides by the Green Microalgae Coccomyxa subellipsoidea. Bioresour. Technol. Rep. 2020, 11, 100461. [Google Scholar] [CrossRef]
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of Microalgal and Cyanobacterial Biomass on a Way to Safe, Cleaner and a Sustainable Environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Chaudhary, R.; Tong, Y.W.; Dikshit, A.K. Kinetic Study of Nutrients Removal from Municipal Wastewater by Chlorella Vulgaris in Photobioreactor Supplied with CO2-Enriched Air. Environ. Technol. 2020, 41, 617–626. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, Biodegradation, and Metabolic Fate of Organophosphorus Pesticide Trichlorfon on the Freshwater Algae Chlamydomonas reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and Micro Algae in Pollution Control and Biofuel Production—A Review. ChemBioEng Rev. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Lukk, T.; Karpichev, Y.; Thakur, V.K.; Kumar, V.; Allaoui, A.; Awasthi, A.K.; Gupta, V.K. Developments in Enzyme and Microalgae Based Biotechniques to Remediate Micropollutants from Aqueous Systems—A Review. Crit. Rev. Environ. Sci. Technol. 2020, 1–46. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Monllor-Alcaraz, L.S.; Postigo, C.; Uggetti, E.; López de Alda, M.; Díez-Montero, R.; García, J. Microalgae-Based Bioremediation of Water Contaminated by Pesticides in Peri-Urban Agricultural Areas. Environ. Pollut. 2020, 265, 114579. [Google Scholar] [CrossRef]
- Molina, D.; de Carvalho, J.C.; Júnior, A.I.M.; Faulds, C.; Bertrand, E.; Soccol, C.R. Biological Contamination and Its Chemical Control in Microalgal Mass Cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9345–9358. [Google Scholar] [CrossRef]
- Chin, Y.-Y.; Chu, W.-L.; Kok, Y.-Y.; Phang, S.-M.; Wong, C.-Y.; Tan, B.-K.; Mustafa, E.M. Sensitivity of Selected Tropical Microalgae Isolated from a Farmland and a Eutrophic Lake to Atrazine and Endosulfan. J. Appl. Phycol. 2019, 31, 2981–2998. [Google Scholar] [CrossRef]
- Graça, C.A.L.; Maniero, M.G.; De Andrade, L.M.; Roberto Guimarães, J.; Teixeira, A.C.S.C. Evaluation of Amicarbazone Toxicity Removal through Degradation Processes Based on Hydroxyl and Sulfate Radicals. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 1126–1143. [Google Scholar] [CrossRef]
- Saccà, M.L.; Ferrero, V.E.V.; Loos, R.; Di Lenola, M.; Tavazzi, S.; Grenni, P.; Ademollo, N.; Patrolecco, L.; Huggett, J.; Caracciolo, A.B.; et al. Chemical Mixtures and Fluorescence in Situ Hybridization Analysis of Natural Microbial Community in the Tiber River. Sci. Total Environ. 2019, 673, 7–19. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhang, Q.-Q.; Wu, Y.-H.; Dao, G.-H.; Zhang, T.-Y.; Tao, Y.; Hu, H.-Y. The Light-Dependent Lethal Effects of 1,2-Benzisothiazol-3(2H)-One and Its Biodegradation by Freshwater Microalgae. Sci. Total Environ. 2019, 672, 563–571. [Google Scholar] [CrossRef]
- Hernández-Moreno, D.; Blázquez, M.; Andreu-Sánchez, O.; Bermejo-Nogales, A.; Fernández-Cruz, M.L. Acute Hazard of Biocides for the Aquatic Environmental Compartment from a Life-Cycle Perspective. Sci. Total Environ. 2019, 658, 416–423. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Phytoremediation for the Elimination of Metals, Pesticides, PAHs, and Other Pollutants from Wastewater and Soil. In Phytobiont and Ecosystem Restitution; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H. Effects of Fungal-Assisted Algal Harvesting through Biopellet Formation on Pesticides in Water. Biodegradation 2018, 29, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Fouilland, E.; Galès, A.; Beaugelin, I.; Lanouguère, E.; Pringault, O.; Leboulanger, C. Influence of Bacteria on the Response of Microalgae to Contaminant Mixtures. Chemosphere 2018, 211, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.K.; Singh, R.; Singh, D.P. Phycotechnological Approaches toward Wastewater Management; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Kannikka, B.; Sesha, C.P.; Subhasha, N. Bioremediation by Microalgae. In The Role of Photosynthetic Microbes in Agriculture and Industry; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 151–171. [Google Scholar]
- Singh, S.; Kumar, A.; Kumar, S.P.J.; Imran, M.; Kumar, M.; Singh, A.N.; Tripathi, M.K. Bioremediation of Pesticides Residues: A Psychological Approach. In The Role of Photosynthetic Microbes in Agriculture and Industry; Keshawanand, T., Abraham, G., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 209–218. [Google Scholar] [CrossRef]
- Taştan, B.E.; Tekinay, T.; Çelik, H.S.; Özdemir, C.; Cakir, D.N. Toxicity Assessment of Pesticide Triclosan by Aquatic Organisms and Degradation Studies. Regul. Toxicol. Pharmacol. 2017, 91, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Tabinda, A.B.; Iqbal, S.; Yasar, A.; Abbas, M.; Khan, A.M.; Mahfooz, Y.; Baqar, M. Phytoremediation of Organochlorine and Pyrethroid Pesticides by Aquatic Macrophytes and Algae in Freshwater Systems. Int. J. Phytoremediat. 2017, 19, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.A.U.; Lavoie, M.; Song, H.; Jin, Y.; Fu, Z.; Qian, H. Interaction of Chiral Herbicides with Soil Microorganisms, Algae and Vascular Plants. Sci. Total Environ. 2017, 580, 1287–1299. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Mahmoud, Y.A.G. Technological Approach of Bioremediation Using Microbial Tools: Bacteria, Fungi, and Algae. In Handbook of Research on Inventive Bioremediation Techniques; Bhakta, J.N., Ed.; IGI Global: Hershey, PA, USA, 2017; pp. 134–154. [Google Scholar] [CrossRef]
- Kumari, M.; Ghosh, P.; Thakur, I.S. Landfill Leachate Treatment Using Bacto-Algal Co-Culture: An Integrated Approach Using Chemical Analyses and Toxicological Assessment. Ecotoxicol. Environ. Saf. 2016, 128, 44–51. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y. Batch vs. Continuous-Feeding Operational Mode for the Removal of Pesticides from Agricultural Run-off by Microalgae Systems: A Laboratory Scale Study. J. Hazard. Mater. 2016, 309, 126–132. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H.; Ardal, E.; Asp, H. Effect of Microalgal Treatments on Pesticides in Water. Environ. Technol. 2016, 37, 893–898. [Google Scholar] [CrossRef]
- Furey, P.C.; Deininger, A.; Liess, A. Substratum-Associated Microbiota. Water Environ. Res. 2016, 88, 1637–1671. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of Microalgae-Based Wastewater Treatment Systems to Remove Emerging Organic Contaminants: A Pilot-Scale Study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, L.; Wong, Y.S.; Tam, N.F.Y. Toxicity and Removal of Organic Pollutants by Microalgae: A Review. In Microalgae: Biotechnology, Microbiology and Energy; Johansen, M.N., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 101–140. [Google Scholar]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Mixotrophic Cyanobacteria and Microalgae as Distinctive Biological Agents for Organic Pollutant Degradation. Environ. Int. 2013, 51, 59–72. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and Degradation of Pesticide Fluroxypyr Are Associated with Toxic Tolerance in Green Alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef]
- Kumar Singh, N.; Dhar, D.W. Microalgal Remediation of Sewage Effluent. Proc. Indian Natl. Sci. Acad. 2010, 76, 209–221. [Google Scholar]
- Chelinho, S.; Moreira-Santos, M.; Lima, D.; Silva, C.; Viana, P.; André, S.; Lopes, I.; Ribeiro, R.; Fialho, A.M.; Viegas, C.A.; et al. Cleanup of Atrazine-Contaminated Soils: Ecotoxicological Study on the Efficacy of a Bioremediation Tool with Pseudomonas sp. ADP. J. Soils Sediments 2010, 10, 568–578. [Google Scholar] [CrossRef]
- Benemann, J.R.; WoertzIan, I.; Lundquist, T. Autotrophic Microalgae Biomass Production: From Niche Markets to Commodities. Ind. Biotechnol. 2018, 14, 3–10. [Google Scholar] [CrossRef]
- Habibah, R.; Iswanto, B.; Rinanti, A. The Significance of Tropical Microalgae Chlorella sorokiniana as a Remediate of Polluted Water caused by Chlorpyrifos. Int. J. Sci. Technol. Res. 2020, 9, 4460–4463. [Google Scholar]
- Pacheco, D.; Rocha, A.C.; Pereira, L.; Verdelhos, T. Microalgae Water Bioremediation: Trends and Hot Topics. Appl. Sci. 2020, 10, 1886. [Google Scholar] [CrossRef] [Green Version]
- Ugya, A.Y.; Ajibade, F.O.; Hua, X. The Efficiency of Microalgae Biofilm in the Phycoremediation of Water from River Kaduna. J. Environ. Manag. 2021, 295, 113109. [Google Scholar] [CrossRef]
- Raymond Sunday, E. Phycoremediation: An Eco-Solution to Environmental Protection and Sustainable Remediation. J. Chem. Environ. Biol. Eng. 2018, 2, 5. [Google Scholar] [CrossRef]

| Active Ingredient | Type of Pesticide | Target Organism | Mode Action | Agricultural Application | Availability Status | References |
|---|---|---|---|---|---|---|
| Herbicides | ||||||
| 2,4-D (2,4-Dichlorophenoxyacetic acid) | Chlorophenoxy herbicide (systemic herbicide) | Broadleaf weeds and evasive weeds in the aquatic environment | Acts as a growth regulator. Induce uncontrolled cell division in vascular tissue due to abnormal increases in cell wall plasticity, biosynthesis of proteins, and production of ethylene | Field corn, soybean, spring wheat, hazelnuts, sugarcane, cereals | Current | [33,34] |
| Glyphosate | Organophosphate herbicide and desiccant (systemic herbicide) | Annual and perennial weeds, broadleaf weeds and grasses | Disrupts the shikimic acid pathway via inhibition of 5-enolpyruvylshikimate-3-phosphate synthase. | Fruits, vegetables, grains, legumes, herbs, and spices | Current | [35] |
| Dicamba (3,6-dichloro-2-methoxy-benzoic acid) | Benzoic acid herbicide (systemic herbicide) | Annual and perennial broadleaf weeds and woody plants (e.g., bedstraw, buttercup, pigweed) | Acts as a growth regulator. Induces abnormal and uncontrollable growth at high dicamba concentrations. | Corn, cereals, dicamba tolerant (DT) soybeans and cotton | Current | [36,37] |
| Atrazine | Triazine herbicide (systemic herbicide) | Broadleaf weeds and grasses (e.g., morning glory, crabgrass, barnyard grass) | Competitive inhibition for plastoquinone binding leads to inhibition of photosynthesis. | Corn, sorghum, sugarcane | Current | [38] |
| Paraquat/paraquat dichloride | Bipyridinium herbicide and desiccant (contact herbicide) | Broadleaf weeds (e.g., cocksfoot) and grasses (e.g., ryegrass, wild oats, fescue Yorkshire fog grass) | Inhibits photosynthesis and disrupt cell membranes, which allows water to escape leads to rapid desiccation. | Beans and peas, cotton, maize, rubber, tomato, citrus orchards, vineyards | Banned in the European Union nations, China, and Thailand | [39] |
| Monosodium methanearsonate (MSMA) | Organic arsenical herbicide (contact herbicide) | Broadleaf weed, grasses and sedges | Mode of action is not known. The rapid desiccation of plant suggests cell membrane destruction. | Cotton | Severely restricted. Not permitted for other agricultural crops | [33,40] |
| Insecticides | ||||||
| Chlorpyrifos | Organophosphate insecticide, acaricide and miticide | Foliage and soil-borne insect pests (e.g., scale, armyworm, flea beetles, fire ants) | Disrupts the nervous system by inhibiting acetylcholinesterase. | Food crops (e.g., cereals, cotton, fruits, tomatoes, nuts, vegetables) and livestock | Current | [33,40] |
| Malathion | Organophosphate insecticide and acaricide | Insect pests (e.g., aphids, leafhoppers, Japanese beetles) | Disrupts the nervous system by inhibiting acetylcholinesterase. | Food, feed, and ornamental crops | Current | [41] |
| Tetrachlorvinphos | Organophosphate insecticide and acaricide | Insect pests (e.g., fleas, ticks, flies, lice, insect larvae) | Disrupts the nervous system by inhibiting acetylcholinesterase. | Poultry (applied dermal), horses, cattle, goats, and swine (oral feed-through) | Not permitted for food crop use in U.S. and banned for all uses in the European Union | [42] |
| Methomyl | Carbamate insecticide | Foliage and soil-borne insect pests (e.g., lepidoptera, coleoptera, diptera) | Disrupts the nervous system by reversibly inhibiting acetylcholinesterase. | Field vegetables, orchard crops, cotton, sugar beet | Certain uses in U.S. are cancelled (barley, oat and rye) or reduced (wheat, corn and lettuce) | [33,40] |
| Carbofuran | Carbamate insecticide, nematicide and miticide | Soil insect and nematode (e.g., corn rootworms, spider mites, nematodes,) | Disrupts the nervous system by reversibly inhibiting acetylcholinesterase. | Potatoes, rice, citrus fruits, vegetables, cotton, alfalfa | Banned in many countries | [40] |
| Cypermethrin | Synthetic pyrethroid insecticide | Insect pests (e.g., pod midge, yellow cereal fly, blossom beetles) | Disrupts the nervous system, which prolongs the opening of the sodium channel leading to hyperexcitation. | Cereals, peas and beans, oilseed rape, potatoes | Current | [43] |
| Imidacloprid | neonicotinoid insecticide | Sucking and soil insects (e.g., plant hoppers, aphids, termites, craneflies, crickets) | Interferes neurotransmission by postsynaptic antagonism of nicotinic acetylcholine receptors. | Rice, cereals, maize, potatoes, sugar beet | Current | [44] |
| Fungicide | ||||||
| Mancozeb | Dithiocarbamate fungicide (contact fungicide) | Fungal pathogens (potato blight, leaf spot, scab) | Prohibits chelating properties, which interferes with enzymes containing sulfhydryl group in fungi. | Potatoes, fruit, cotton, corn, ornamental shrubs | Current | [40,45] |
| Pesticides | Species of Algae | Mode of Action | Concentration of Pesticides Tested | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| α-endosulfan | Chlorococcum sp. | Biodegradation | 1000 μg/μL | 65–75 | [67] |
| Scenedesmus sp. | |||||
| Atrazine | Chlorella vulgaris | Biosorption | 10 μg/L | 96.29 | [6] |
| Chlamydomonas mexicana | Biodegradation | 10 μg/L | 36 | [68] | |
| Bifenthrin | Parachlorella kessleri | Biodegradation | 5 mg/L | 85 | [48] |
| Chlorpyrifos | Chlorella sorokiniana | Biosorption | (100, 300, 500) ppm | (99.18, 99.85, 97.86) | [50] |
| Diazinon | Chlorella vulgaris | Biodegradation | 20 mg/L | 94 | [69] |
| Dimethomorph | Scenedesmus obliquus | Biodegradation | 600 μg/L | 24 | [70] |
| Scenedesmus quadricauda | 15 | ||||
| Fluroxypyr | Chlamydomonas reinhardtii | Biodegradation | 0.5 mg/L | 57 | [58] |
| Imidacloprid | Nannochloropsis sp. | Biodegradation | 9.59 μg/L | 50 | [71] |
| Isoproturon | Chlamydomonas reinhardtii | Bioaccumulation, biodegradation | 50 μg/L | 15.1 | [65] |
| Lindane | Nannochloris oculata | Bioaccumulation | 0.1 mg/L | 73 | [72] |
| Malathion | Chlorella sorokiniana | Biodegradation | 25, 100 ppm | (90, 70) | [73] |
| Mesotrione | Scenedesmus quadricauda | Biodegradation | 5 mg/L | 15.2 | [74] |
| Metalaxyl | Chlorella vulgaris | Biodegradation | 4 ppm | 100 | [75] |
| Prometryne | Chlamydomonas reinhartii | Biodegradation | 7.5 μg/L | 32 | [76] |
| Trichlorfon | Chlamydomonas reinhartii | Biodegradation | 200 mg/L | 96.2 | [59] |
| Element | Scopus [86,95,96] | PubMed [86,94,97] |
|---|---|---|
| Coverage | ~82 million items | ~32 million items |
| Discipline | Multidisciplinary | Medicine or biological sciences |
| Bibliographic | 1.7 billion | Not available |
| Provider | Elsevier | National Institute of Science (NIH) |
| Accessibility |
| Free |
| Features |
|
|
| No. | Year | Document Type | Title | Author Keywords | Reference |
|---|---|---|---|---|---|
| 1 | 2020 | Article | Permeable reactive surface-biobarriers. Testing and evaluation of an ecotechnology for the removal of agrotoxic compounds carried by agricultural runoffs | Ecotechnology, nonpoint source pollution; Micractinium sp.; microbial community; sustainable remediation | [106] |
| 2 | 2020 | Article | Microalgae-based bioremediation of water contaminated by pesticides in peri-urban agricultural areas | Agriculture; contaminants of emerging concern; ecotoxicity; low-cost treatment; photobioreactor | [107] |
| 3 | 2020 | Article | Potential for bifenthrin removal using microalgae from a natural source | Bifenthrin; bioremediation; microalgae; pesticide; phycoremediation; residual waters | [69] |
| 4 | 2020 | Article | Reactive Oxygen Species (ROS)-mediated degradation of organophosphate pesticides by the green microalgae Coccomyxa subellipsoidea | Algae; bioremediation; organophosphate pesticide; reactive oxygen species; water quality | [108] |
| 5 | 2020 | Review | Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner, and a sustainable environment | Biofuel; cyanobacteria; microalgae; secondary metabolites; value-added products; wastewater | [109] |
| 6 | 2020 | Article | Bioremediation of water containing pesticides by microalgae: mechanisms, methods, and prospects for future research | Biodiesel; green remediation; microalgae; pesticide; water pollution | [11] |
| 7 | 2020 | Article | Kinetic study of nutrients removal from municipal wastewater by Chlorella vulgaris in photobioreactor supplied with CO2-enriched air | Chlorella vulgaris; kinetic study; municipal wastewater; nutrient bioremediation; photobioreactor | [110] |
| 8 | 2020 | Article | Toxicity, Biodegradation, and Metabolic Fate of Organophosphorus Pesticide Trichlorfon on the Freshwater Algae Chlamydomonas reinhardtii | biodegradation; metabolic fate; microalgae; toxicity; trichlorfon | [111] |
| 9 | 2020 | Review | Macro and Micro Algae in Pollution Control and Biofuel Production—A Review | Algae; biofuel; biosorption; CO2 fixation; wastewater | [112] |
| 10 | 2020 | Article | Developments in enzyme and microalgae based biotechniques to remediate micropollutants from aqueous systems—a review | Bioremediation; laccase; microalgae; peroxidase; wastewater treatment | [113] |
| 11 | 2020 | Article | The significance of tropical microalgae Chlorella sorokiniana as a remediate of polluted water caused by chlorpyrifos | Bioremediation; Chlorella sorokiniana; chlorpyrifos; contact time; pesticide | [114] |
| 12 | 2019 | Review | Biological contamination and its chemical control in microalgal mass cultures | Algal parasites; contamination; control; mass cultures; microalgae | [115] |
| 13 | 2019 | Review | Microalgal bioremediation of emerging contaminants—Opportunities and challenges | Aquatic pollution; bioadsorption; Biodegradation; emerging contaminants; microalgal treatment systems | [9] |
| 14 | 2019 | Article | Sensitivity of selected tropical microalgae isolated from a farmland and a eutrophic lake to atrazine and endosulfan | Atrazine; chlorella; endosulfan; microalgae; oxidative stress; Scenedesmus | [116] |
| 15 | 2019 | Article | Evaluation of amicarbazone toxicity removal through degradation processes based on hydroxyl and sulfate radicals | amicarbazone degradation products; pesticide; sulfate and hydroxyl radicals-mediated degradation; toxicity assays | [117] |
| 16 | 2019 | Article | Chemical mixtures and fluorescence in situ hybridization analysis of natural microbial community in the Tiber river | Anthropogenic pollution and stressors; bioindicators; freshwater; microbial populations; water quality | [118] |
| 17 | 2019 | Article | The light-dependent lethal effects of 1,2-benzisothiazol-3(2H)-one and its biodegradation by freshwater microalgae | 1,2-Benzisothiazol-3(2H)-one; biodegradation; environmental risk estimation; light-dependent lethal effects; microalgae | [119] |
| 18 | 2019 | Review | Acute hazard of biocides for the aquatic environmental compartment from a life-cycle perspective | Aquatic compartment; biocide; metabolite; toxicity category | [120] |
| 19 | 2019 | Article | Bioremediation of a pesticide and selected heavy metals in wastewater from various sources using a consortium of microalgae and cyanobacteria | Bioremediation; heavy metals; microalgae; microorganisms; pesticides; wastewater | [105] |
| 20 | 2018 | Book Chapter | Phytoremediation for the elimination of metals, pesticides, PAHs, and other pollutants from wastewater and soil | - | [121] |
| 21 | 2018 | Article | Effects of fungal-assisted algal harvesting through biopellet formation on pesticides in water | Aspergillus niger; bioremediation; chlorella vulgaris; emerging pollutants; water quality | [122] |
| 22 | 2018 | Article | Influence of bacteria on the response of microalgae to contaminant mixtures | Metallic and pesticide contaminants; Microbial interactions; sediments | [123] |
| 23 | 2018 | Book Chapter | Phycotechnological approaches toward wastewater management | Microalgae; phycoremediation; pollutant; wastewater | [124] |
| 24 | 2018 | Book Chapter | Bioremediation by microalgae | Biosorption; contamination; environment; industrialization; microalgae | [125] |
| 25 | 2018 | Book Chapter | Bioremediation of pesticides residues: A psychological approach | Bioremediation; cyanobacteria; pesticides residue | [126] |
| 26 | 2017 | Article | Toxicity assessment of pesticide triclosan by aquatic organisms and degradation studies | 2,4-Dichlorophenol; biodegradation; cyanobacteria; microalgae; toxicity; triclosan | [127] |
| 27 | 2017 | Article | Phytoremediation of organochlorine and pyrethroid pesticides by aquatic macrophytes and algae in freshwater systems | organochlorine; phytoremediation; pyrethroids | [128] |
| 28 | 2017 | Review | Interaction of chiral herbicides with soil microorganisms, algae, and vascular plants | Biodegradation; chiral herbicide; enantiomer; enantioselective toxicology; herbicide toxicity; plant | [129] |
| 29 | 2017 | Book Chapter | Technological approach of bioremediation using microbial tools: bacteria, fungi, and algae | - | [130] |
| 30 | 2016 | Review | Growth regime and environmental remediation of microalgae | Bioremediation; microalgae; nutrient removal; wastewater | [10] |
| 31 | 2016 | Article | Landfill leachate treatment using bacto-algal coculture: an integrated approach using chemical analyses and toxicological assessment | Coculture; detoxification; landfill leachate; lysimeter; treatment | [131] |
| 32 | 2016 | Article | Batch vs. continuous-feeding operational mode for the removal of pesticides from agricultural run-off by microalgae systems: a laboratory scale study | Agricultural run-off; biodegradation; microalgae; pesticides; removal | [132] |
| 33 | 2016 | Article | Effect of microalgal treatments on pesticides in water | Bioremediation; biosorption; Chlorella vulgaris; organic pollutants; water treatment | [133] |
| 34 | 2016 | Article | Effect of the pesticide lindane on the biomass of the microalgae Nannochloris oculata | chronic toxicity; microalgae; organochloride pesticide; phycoremediation; removal | [81] |
| 35 | 2016 | Review | Substratum-associated microbiota | Algae; bacteria; biofilm; cyanobacteria; diatoms; microbes; periphyton | [134] |
| 36 | 2015 | Article | Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study | Biodegradation; emerging organic contaminants; high-rate algal pond; microalgae; photodegradation; volatilization | [135] |
| 37 | 2013 | Book Chapter | Toxicity and removal of organic pollutants by microalgae: A review | - | [136] |
| 38 | 2013 | Review | Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation | Biodegradation; biological agents; cyanobacteria; microalgae; mixotrophy; organic pollutants | [137] |
| 39 | 2011 | Article | Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii | Bioaccumulation; biodegradation; Chlamydomonas reinhardtii; fluroxypyr; oxidation | [138] |
| 40 | 2010 | Conference Paper | Microalgal remediation of sewage effluent | Heavy metals; microalgae; nitrogen; phosphorous; sewage; wastewater | [139] |
| 41 | 2010 | Article | Cleanup of atrazine-contaminated soils: Ecotoxicological study on the efficacy of a bioremediation tool with Pseudomonas sp. ADP | Atrazine; bioremediation; ecotoxicology; pesticides; soil contamination | [140] |
| 42 | 2010 | Article | Fungicides and herbicide removal in Scenedesmus cell suspensions | Bioremediation; chlorophyll fluorescence; Scenedesmus; toxicity; uptake | [73] |
| Cluster | Author Keyword | Links | TLS | Occurrences |
|---|---|---|---|---|
| 1 | Bioremediation | 35 | 43 | 11 |
| 2 | Pesticide | 14 | 16 | 4 |
| 3 | Chlorella vulgaris | 11 | 12 | 3 |
| 4 | Water quality | 11 | 12 | 3 |
| 5 | Microalgae | 57 | 78 | 18 |
| 6 | Cyanobacteria | 20 | 23 | 5 |
| 7 | Biosorption | 12 | 12 | 3 |
| 8 | Wastewater | 15 | 20 | 5 |
| 9 | Heavy metals | 9 | 10 | 2 |
| 10 | Biodegradation | 33 | 40 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verasoundarapandian, G.; Lim, Z.S.; Radziff, S.B.M.; Taufik, S.H.; Puasa, N.A.; Shaharuddin, N.A.; Merican, F.; Wong, C.-Y.; Lalung, J.; Ahmad, S.A. Remediation of Pesticides by Microalgae as Feasible Approach in Agriculture: Bibliometric Strategies. Agronomy 2022, 12, 117. https://doi.org/10.3390/agronomy12010117
Verasoundarapandian G, Lim ZS, Radziff SBM, Taufik SH, Puasa NA, Shaharuddin NA, Merican F, Wong C-Y, Lalung J, Ahmad SA. Remediation of Pesticides by Microalgae as Feasible Approach in Agriculture: Bibliometric Strategies. Agronomy. 2022; 12(1):117. https://doi.org/10.3390/agronomy12010117
Chicago/Turabian StyleVerasoundarapandian, Gayathiri, Zheng Syuen Lim, Syahirah Batrisyia Mohamed Radziff, Siti Hajar Taufik, Nurul Aini Puasa, Noor Azmi Shaharuddin, Faradina Merican, Chiew-Yen Wong, Japareng Lalung, and Siti Aqlima Ahmad. 2022. "Remediation of Pesticides by Microalgae as Feasible Approach in Agriculture: Bibliometric Strategies" Agronomy 12, no. 1: 117. https://doi.org/10.3390/agronomy12010117
APA StyleVerasoundarapandian, G., Lim, Z. S., Radziff, S. B. M., Taufik, S. H., Puasa, N. A., Shaharuddin, N. A., Merican, F., Wong, C.-Y., Lalung, J., & Ahmad, S. A. (2022). Remediation of Pesticides by Microalgae as Feasible Approach in Agriculture: Bibliometric Strategies. Agronomy, 12(1), 117. https://doi.org/10.3390/agronomy12010117









