Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Characteristics of Soil and Climatic Conditions
2.3. Evaluation of Pre-Emergence Damping-Off of Broad Bean Seedlings
2.4. Isolation and Identification of Fungi Colonizing Pre-Emergence-Infested Seedlings
2.5. Statistical Analysis
2.6. Descriptive Statistics
3. Results and Discussion
3.1. Pre-Emergence Damping-Off of Seedlings Depending on the Examined Factors
3.2. Qualitative Analysis of Fungi and Oomycota Colonizing the Seedlings That Died Due to Pre-Emergence Damping-Off
3.3. Biodiversity and Trophic Structure of Microbial Populations Isolated from Pre-Emergence-Infested Broad Bean Seedlings
3.4. Assessment of the Relationship between Fungal Communities Colonizing Infected Broad Bean Seedlings, Soil Chemical Properties and Disease Severity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswas, B.; Gresshoff, P.M. The role of symbiotic nitrogen fixation in sustainable production of biofuels. Int. J. Mol. Sci. 2014, 15, 7380–7397. [Google Scholar] [CrossRef]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.H.; Oldroyd, G.E.; Poole, P.S.; et al. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.C.; van den Brand, G.J.; Vanlauwe, B.; Giller, K.E. Sustainable intensification through rotations with grain legumes in Sub-Saharan Africa: A review. Agric. Ecosyst. Environ. 2018, 261, 172–185. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Wahbi, S.; Prin, Y.; Thioulouse, J.; Sanguin, H.; Baudoin, E.; Maghraoui, T.; Oufdou, K.; Le Roux, C.; Galiana, A.; Hafidi, M.; et al. Impact of wheat/faba bean mixed cropping or rotation systems on soil microbial functionalities. Front. Plant Sci. 2016, 7, 1364. [Google Scholar] [CrossRef]
- Giménez, M.A.; Drag, S.R.; De Greef, D.; Gonzalez, R.J.; Lobo, M.O.; Samman, N.C. Rheological, functional and nutritional properties of wheat/broad bean (Vicia faba) flour blends for pasta formulation. Food Chem. 2012, 134, 200–206. [Google Scholar] [CrossRef]
- Łabuda, H.; Buczkowska, H. Biologically active substances in the broad bean green seeds after storage in the pods. Acta Sci. Pol.-Hortorum Cultus 2014, 13, 83–93. [Google Scholar]
- Revilla, I. Impact of thermal processing on faba bean (Vicia faba) composition. In Processing and Impact on Active Components in Food; Academic Press: Cambridge, MA, USA, 2015; Volume 40, pp. 337–343. [Google Scholar] [CrossRef]
- Turco, I.; Ferretti, G.; Bacchetti, T. Review of the health benefits of Faba bean (Vicia faba L.) polyphenols. J. Food Nutr. Res. 2016, 55, 283–293. [Google Scholar]
- Chaieb, N.; González, J.L.; Lópezesas, M.; Bouslama, M.; Valiente, M. Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Res. Int. 2011, 44, 970–977. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xie, W.; Jin, X.; Tao, B.; Chen, Q.; Zhu, W. Impact of sprouting pretreatment on phytic acid and polyphenol level of faba bean (Vicia fabae L.) flour. Int. Food Res. J. 2013, 20, 1133–1137. [Google Scholar]
- Gasim, S.; Hamad, S.A.A.; Abdelmula, A.; Ahmed, I.A.M. Yield and quality attributes of faba bean inbred lines grown under marginal environmental conditions of Sudan. Food Sci. Nutr. 2015, 3, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Alemu, G.Y.; Tadele, Y.A. Management of faba bean gall disease through the use of host resistance and fungicide foliar spray in Northwestern Ethiopia. Adv. Crop Sci. Tech. 2017, 5, 254. [Google Scholar] [CrossRef]
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop J. 2017, 5, 115–126. [Google Scholar] [CrossRef]
- Abdel-Monaim, M.F. Improvement of biocontrol of damping-off and root rot/wilt of fabae bean by salicylic acid and hydrogen peroxide. Mycobiology 2013, 41, 47–55. [Google Scholar] [CrossRef]
- Abdel-Monaim, M.F. Integrated management of damping-off, root and/or stem rot diseases of chickpea and efficacy of the suggested formula. Not. Sci. Biol. 2011, 3, 80–88. [Google Scholar]
- Chang, K.F.; Hwang, S.F.; Gossen, B.D.; Strelkov, S.E.; Turnbull, G.D.; Bing, D.J. Effect of seeding practices, temperature and seed treatments on fusarium seedling blight of narrow-leaved lupin. Can. J. Plant Sci. 2011, 91, 859–872. [Google Scholar] [CrossRef]
- Chang, K.F.; Conner, R.L.; Hwang, S.F.; Ahmed, H.U.; McLaren, D.L.; Gossen, B.D.; Turnbull, G.D. Effects of seed treatments and inoculum density of Fusarium avenaceum and Rhizoctonia solani on seedling blight and root rot of faba bean. Can. J. Plant Sci. 2014, 94, 693–700. [Google Scholar] [CrossRef]
- Mitiku, M. Management of root rot diseases of cool season food legumes crops in Ethiopia. J. Plant Sci. 2017, 5, 104–109. [Google Scholar] [CrossRef]
- Hwang, S.F.; Ahmed, H.U.; Turnbull, G.D.; Gossen, B.D.; Strelkov, S. Effect of seeding date and depth, seed size and fungicide treatment on Fusarium and Pythium seedling blight of canola. Can. J. Plant Sci. 2015, 95, 293–301. [Google Scholar] [CrossRef]
- Reznikov, S.; Vellicce, G.R.; González, V.; de Lisi, V.; Castagnaro, A.P.; Ploper, L.D. Evaluation of chemical and biological seed treatments to control charcoal rot of soybean. J. Gen. Plant Pathol. 2016, 82, 273. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messéan, A.; Aubertot, J.-N. Integrated management of damping-off diseases. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Kim, Y.-J.; Yang, D.-C. Cylindrocarpon destructans/Ilyonectria radicicola-species complex: Causative agent of ginseng root-rot disease and rusty symptoms. J. Ginseng Res. 2018, 42, 9–15. [Google Scholar] [CrossRef] [PubMed]
- El-Samawaty, A.M.; Omar, M.; El-Naggar, M.; Yassin, O. Amer. Pathological assessment of seed borne fungi involved in cotton seedlings damping-off. J. Plant Sci. 2012, 7, 85–95. [Google Scholar] [CrossRef]
- Gleń, K.; Boligłowa, E.; Gospodarek, J. Fungi colonising broad bean seeds depending on protection method. Pol. J. Agron. 2013, 12, 9–16. (In Polish) [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Gospodarek, J. Mycological purity of broad bean (Vicia faba L.) seeds in the conditions of companion planting and differentiated protection. J. Res. Appl. Agric. Eng. 2016, 61, 126–131. [Google Scholar]
- You, M.P.; Rensing, K.; Renton, M.; Barbetti, M.J. Modeling effects of temperature, soil, moisture, nutrition and variety as determinants of severity of Pythium damping-off and root disease in subterranean clover. Front. Microbiol. 2017, 8, 2223. [Google Scholar] [CrossRef]
- Kato, M.; Minamida, K.; Tojo, M.; Kokur, T.; Hamaguchi, H.; Shimada, S. Association of Pythium and Phytophthora with pre-emergence seedling damping-off of soybean grown in a field converted from a paddy field in Japan. Plant Prod. Sci. 2013, 16, 95–104. [Google Scholar] [CrossRef][Green Version]
- Carmona, M.A.; Sautua, F.J.; Grijalba, P.E.; Cassina, M.; Pérez-Hernández, O. Effect of potassium and manganese phosphites in the control of Pythium damping-off in soybean: A feasible alternative to fungicide seed treatments. Pest Manag. Sci. 2018, 74, 66–374. [Google Scholar] [CrossRef]
- Alcala, A.V.C.; Paulitz, T.C.; Schroeder, K.L.; Porter, L.D.; Derie, M.L.; du Toit, L.J. Pythium species associated with damping-off of pea in certified organic fields in the Columbia Basin of Central Washington. Plant Dis. 2016, 100, 916–925. [Google Scholar] [CrossRef][Green Version]
- Valenciano, J.B.; Casquero, P.A.; Boto, J.A.; Marcelo, V. Evaluation of the occurrence of root rots on bean plants (Phaseolus vulgaris) using different sowing methods and with different techniques of pesticide application. N. Zeal. J. Crop. Hortic. Sci. 2006, 34, 291–298. [Google Scholar] [CrossRef]
- Luna, M.P.R.; Mueller, D.; Mengistu, A.; Singh, A.K.; Hartman, G.L.; Wise, K.A. Advancing our understanding of charcoal rot in soybeans. J. Integr. Pest Manag. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Judelson, H.S.; Ah-Fong, A.M.V. Exchanges at the Plant-Oomycete Interface That Influence Disease. Plant Physiol. 2019, 179, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.R. Effect of seed quality and combination fungicide–Trichoderma spp. seed treatments on pre- and postemergence damping-off in cotton. Phytopathology 2007, 97, 66–71. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The effect of the Falcon 460 EC fungicide on soil microbial communities, enzyme activities and plant growth. Ecotoxicology 2016, 25, 1575–1587. [Google Scholar] [CrossRef]
- Singh, Z.; Kaur, J.; Kaur, R.; Hundal, S.S. Toxic effects of organochlorine pesticides: A Review. Am. J. BioSci. 2016, 4, 11–18. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; Van der Heijden, M.G.A. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability crossref. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—a meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Lehmann, J.; Camenzind, T.; Rauh, C. Soil biodiversity effects from field to fork. Trends Plant Sci. 2018, 23, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, L.; Liu, H.; Li, Y.; Li, C.; Wu, G.; Yu, X.; Guo, L.; Cheng, D.; Muminov, M.A.; et al. Biodiversity management of organic orchard enhances both ecological and economic profitability. PeerJ 2016, 4, e2137. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gleń-Karolczyk, K.; Gondek, K. Fungistatic activity of composts with the addition of polymers obtained from thermoplastic corn starch and polyethylene—An innovative cleaner production alternative. Sci. Total Environ. 2018, 635, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Habtegebriel, B.; Boydom, A. Biocontrol of faba bean black root rot caused by Fusarium solani using seed dressing and soil application of Trichoderma harzianum. J. Biol. Control 2016, 30, 169–176. [Google Scholar] [CrossRef]
- El-Shennawy, R.Z. Biological control of root-rot and wilt disease of faba bean using some bioagents. J. Plant Prot. Pathol. 2011, 2, 195–202. [Google Scholar]
- Benhamou, N.; Le Floch, G.; Vallance, J.; Gerbore, J.; Grizard, D.; Rey, P. Pythium oligandrum: An example of opportunistic success. Microbiology 2012, 158, 2679–2694. [Google Scholar] [CrossRef]
- Khabbaz, S.E.; Abbasi, P.A. Isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber. Can. J. Microbiol. 2014, 60, 25–33. [Google Scholar] [CrossRef]
- Takenaka, S.; Sekiguchi, H.; Nakaho, K.; Tojo, M.; Masunaka, A.; Takahashi, H. Colonization of Pythium oligandrum in the tomato rhizosphere for biological control of bacterial wilt disease analyzed by real-time PCR and confocal laser-scanning microscopy. Phytopathology 2008, 98, 187–195. [Google Scholar] [CrossRef]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef]
- Takenaka, S.; Ishikawa, S. Biocontrol of sugar beet seedling and taproot diseases caused by Aphanomyces cochlioides by Pythium oligandrum treatments before transplanting. Jpn. Agric. Res. Q. 2013, 47, 75–83. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Lamb, A.; Walker, S.; Farmer, A. Resolving conflicts between agriculture and the natural environment. PLoS Biol. 2015, 13, e1002242. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.J.A.; van Veen, J.D.; van Elsas, J.D. Microbial diversity in soil:Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Świtoniak, M.; Kabała, C.; Charzyński, P. Proposal of English equivalents for the soil taxa names in the Polish Soils Classification. Soil Sci. Annu. 2016, 67, 103–116. [Google Scholar] [CrossRef][Green Version]
- Cwalina-Ambroziak, B. The efficiency of biological and chemical protection of potato plants against late blight (Phytophthora infestans /Mont./de Bary) and early blight (Alternaria spp.). Pol. J. Agron. 2012, 11, 3–9. [Google Scholar]
- De Carolis, E.; Posteraro, B.P.; Lass-Flörl, C.; Vella, A.; Florio, A.R.; Torelli, R.; Girmenia, C.; Colozza, C.; Tortorano, A.M.; Sanguinetti, M.; et al. Species identification of Aspergillus, Fusarium and Mucorales with direct surface analysis by matrix-assisted laser desorption ionization timeof-flightmass spectrometry. Clin. Microbiol. Infect. 2012, 18, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Barnet, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 1998; p. 218. [Google Scholar]
- Dugan, F. The Identification of Fungi: An Illustrated Introduction with Keys, Glossary, and Guide to Literature; The American Phytopathological Society: St. Paul, MN, USA, 2006; p. 176. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing Ltd.: Oxford, UK; London, UK, 2006; p. 388. [Google Scholar]
- Klaus, H.; Domsch, W.; Gams, W.; Anderson, T. Compendium of Soil Fungi, 2nd ed.Wiley Subscription Services, Inc.: St. Paul, MN, USA, 2008; p. 672. [Google Scholar]
- Gardi, C.; Jeffery, S. Soil Biodiversity; European Commission, Institute for Environmental and Sustainability: Luxemburg, 2009; Volume 10, pp. 2788–7831. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Urrea, K.; Rupe, J.C.; Rothrock, C.S. Effect of fungicide seed treatments, cultivars, and soils on soybean stand establishment. Plant Dis. 2013, 97, 807–812. [Google Scholar] [CrossRef][Green Version]
- Li, Y.P.; You, M.P.; Colmer, T.D.; Barbetti, M.J. Effect of timing and duration of soil saturation on soilborne Pythium diseases of common bean (Phaseolus vulgaris). Plant Dis. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Macedo, R.; Sales, L.P.; Yoshida, F.; Silva-Abud, L.L.; Lobo, M. Potential worldwide distribution of Fusarium dry root rot in common beans based on the optimal environment for disease occurrence. PLoS ONE 2017, 12, e0187770. [Google Scholar] [CrossRef] [PubMed]
- Rego, C.; Farropas, L.; Nascimento, T.; Cabral, A.; Oliveira, T. Black foot of grapevine: Sensitivity of Cylindrocarpon destructans to fungicides. Phytopathol. Mediterr. 2006, 45, 93–100. [Google Scholar]
- Kurzawińska, H. Fungi inhabiting the rhizosphere of persian cyclamen and their impact. Phytopathologia 2010, 57, 5–10. [Google Scholar]
- Pastucha, A.; Kołodziej, B. The effect of irrigation and foliar fertilization on the colonization of american ginseng (Panax quinquefolium L.) Diseased parts by different micro-organisms. Acta Agrobot. 2010, 63, 179–188. [Google Scholar] [CrossRef]
- Jamali, S.; Nasimi, Z. First report of black-foot disease, caused by Cylindrocarpon destructans, on ornamental marigold (Tagetes minuta) in Iran. J. Plant Prot. Res. 2014, 54, 139–143. [Google Scholar] [CrossRef]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef]
- Torres, S.V.; Vargas, M.M.; Godoy-Lutz, G.; Porch, T.P.; Beaver, J.S. Isolates of Rhizoctonia solani can produce both web blight and root rot symptoms in common bean (Phaseolus vulgaris L.). Plant Dis. 2016, 100, 1351–1357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, J.P.; Sperschneider, J.; Win, J.; Kidd, B.; Yoshida, K.; Hane, J.; Saunders, D.G.; Singh, K.B. Comparative secretome analysis of Rhizoctonia solani isolates with different host ranges reveals unique secretomes and cell death inducing effectors. Sci. Rep. 2017, 7, 10410. [Google Scholar] [CrossRef]
- Brožová, J. Exploitation of the mycoparasitic fungus Pythium oligandrum in plant protection. Plant Protect. Sci. 2002, 38, 29–35. [Google Scholar]
- Marroni, I.V.; Moura, A.B.; Ueno, B. Chemical and biological treatments of castor bean seeds: Effect on germination, emergence and associated microorganismus. Rev. Bras. Sementes 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Sun, M.-H.; Zhou, M.; Li, S.-D. Transformation of the endochitinase gene Chi67-1 in Clonostachys rosea 67-1 increases its biocontrol activity against Sclerotinia sclerotiorum. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Koutb, M.; Ali, E.H. Potential of Epicoccum purpurascens Strain 5615 AUMC as a biocontrol agent of Pythium irregulare root rot in three leguminous plants. Mycobiology 2010, 38, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Anil Kumar, R.; Rajkumar, H.G. Antagonistic effect of rhizospheric Aspergillus species against Fusarium oxysporum f. sp. lycopersici. Int. J. Chem. Anal. Sci. 2014, 5, 39–42. [Google Scholar]
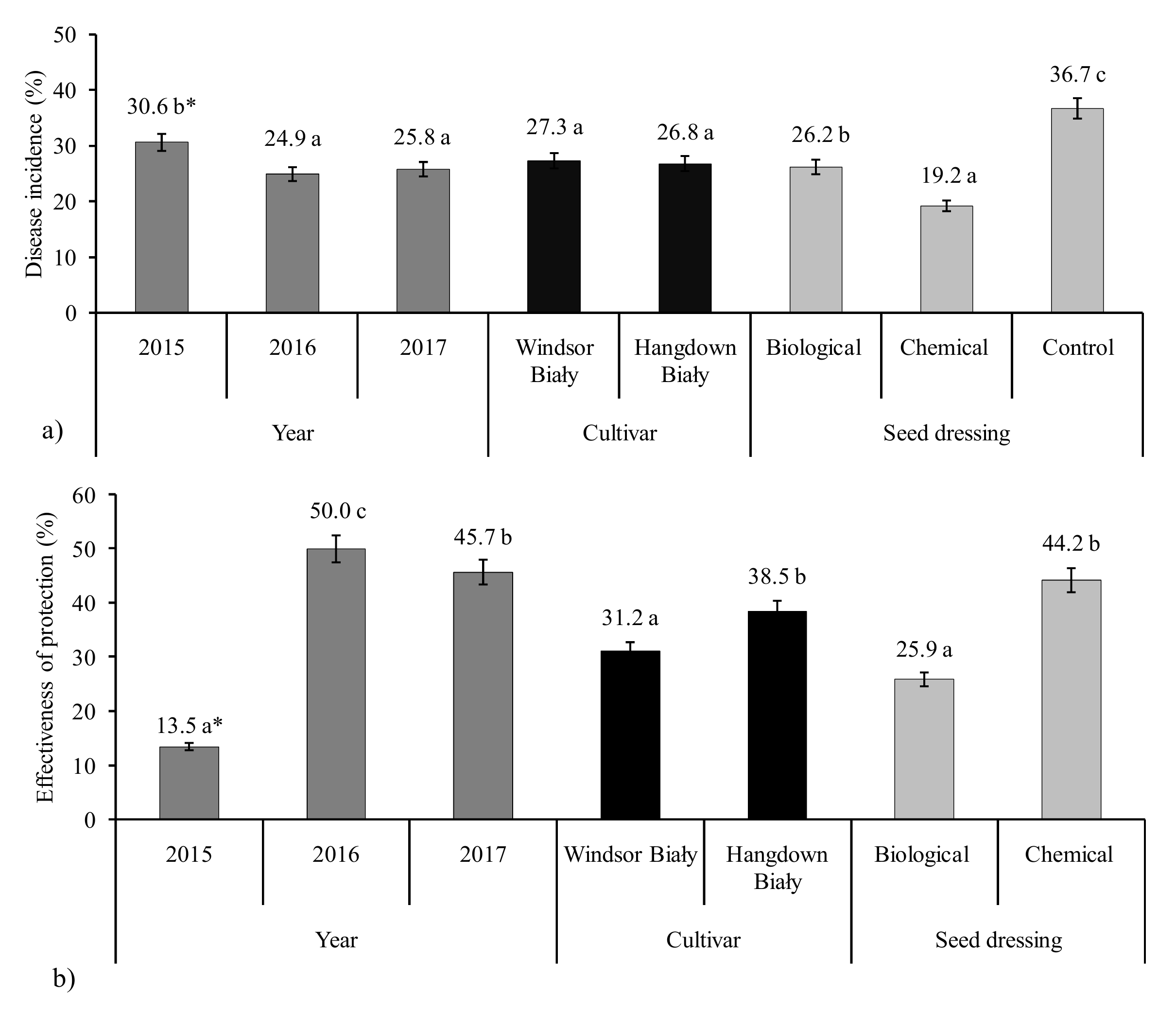
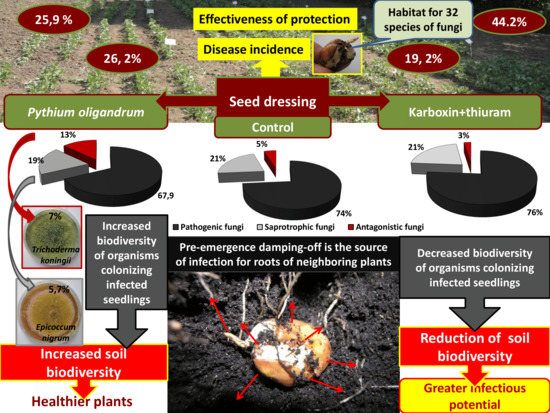
| Treatments | Cultivars | DI—Disease Incidence % | E-effectiveness of Protection in % | ||||
|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | ||
| Pythium oligandrum | Windsor Biały | 30.1 gh * | 21.3 c | 22.5 cd | 10.1 b | 45.4 f | 34.6 e |
| Hangdown Biały | 32.1 hi | 32.1 hi | 27.3 fg | 6.0 a | 33.9 e | 36.1 e | |
| Carboxin + thiuram | Windsor Biały | 28.4 g | 16.7 b | 23.4 cde | 15.2 c | 57.2 g | 32.5 e |
| Hangdown Biały | 25.3 ef | 14.9 b | 9.2 a | 25.8 d | 59.5 g | 78.4 h | |
| Control | Windsor Biały | 33.5 i | 37.9 k | 34.8 ijk | |||
| Hangdown Biały | 34.2 ij | 37.1 jk | l | ||||
| Species of Fungi | Cultivar | Year | Seed Dressing | Mean | Group of Attendance | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Windsor Biały | Hangdown Biały | 2015 | 2016 | 2017 | Control | Biological | Chemical | |||
| Pathogenic fungi | ||||||||||
| Ilyonectria destructans | 9.4 | 11.2 | 9.3 | 9.1 | 12.0 | 10.2 | 10.6 | 9.6 | 10.2 | Eudominants (10.2) |
| Globisporangium irregulare | 8.1 | 9.5 | 16.3 | 7.1 | 5.7 | 8.3 | 8.3 | 13.4 | 9.6 | Dominants (29.6) |
| Fusarium equiseti (2.54) * | 8.4 | 8.4 | 7.7 | 10.2 | 7.7 | 8.6 | 8.4 | 8.8 | 8.5 | |
| Rhizoctonia solani (2.80) | 6.2 | 5.2 | 6.8 | 6.9 | 3.7 | 2.5 | 6.3 | 10.7 | 6.0 | |
| Fusarium solani (2.47) | 6.0 | 4.5 | 6.1 | 5.9 | 5.0 | 7.3 | 4.7 | 4.4 | 5.5 | |
| Botrytis cinerea (2.92) | 4.5 | 5.9 | 4.0 | 4.1 | 6.5 | 5.6 | 4.8 | 3.9 | 5.0 | Subdominants (22.5) |
| Ascochyta fabae | 4.9 | 4.0 | 3.1 | 5.2 | 4.5 | 4.4 | 4.3 | 4.1 | 4.3 | |
| Fusarium proliferatum (2.72) | 3.9 | 3.8 | 4.5 | 4.1 | 3.2 | 4.2 | 3.2 | 4.4 | 3.9 | |
| Fusarium culmorum (2.51) | 4.7 | 2.6 | 2.4 | 3.7 | 4.9 | 3.3 | 3.5 | 4.7 | 3.7 | |
| Fusarium oxysporum (2.83) | 2.6 | 3.5 | 4.2 | 2.9 | 2.3 | 3.6 | 2.6 | 3.0 | 3.1 | |
| Fusarium avenaceum | 3.4 | 1.8 | 3.0 | 3.4 | 1.7 | 3.6 | 2.3 | 1.5 | 2.5 | |
| Alternaria alternata (2.96) | 1.7 | 1.7 | 1.2 | 1.6 | 2.2 | 3.1 | 0.1 | 1.4 | 1.6 | Recedents (5.6) |
| Didymella glomerata (2.39) | 1.7 | 1.6 | 2.6 | 1.6 | 1.0 | 1.9 | 1.9 | 1.3 | 1.7 | |
| Juxtiphoma eupyrena | 1.0 | 1.4 | 1.0 | 1.2 | 1.4 | 1.5 | 1.0 | 1.1 | 1.2 | |
| F. sporotrichioides | 1.0 | 1.3 | 1.7 | 0.6 | 1.0 | 0.4 | 2.4 | 0.6 | 1.1 | |
| Phoma herbarum (2.77) | 1.0 | 1.1 | 0.9 | 1.6 | 0.6 | 0.9 | 1.2 | 0.9 | 1.0 | Subrecedents (4.3) |
| Sclerotinia sclerotiorum | 1.2 | 0.7 | 1.0 | 0.6 | 1.2 | 1.4 | 0.6 | 0.7 | 0.9 | |
| Fusarium poae | 1.0 | 0.9 | 0.7 | 0.9 | 1.0 | 1.0 | 0.6 | 0.8 | 0.9 | |
| Ulocladium consortiale | 1.0 | 0.8 | - | 1.1 | 1.5 | 1.6 | 0.2 | 0.5 | 1.0 | |
| Verticillium albo-atrum | 0.6 | 0.5 | 0.3 | 0.5 | 0.7 | 0.3 | 1.0 | 0.2 | 0.5 | |
| Total | 72.4 | 70.7 | 77.2 | 72.5 | 67.8 | 73.6 | 67.9 | 76.2 | ||
| Nonpathogenic fungi | ||||||||||
| Saprotrophic fungi | ||||||||||
| Epicoccum nigrum (2.93) | 1.5 | 7.2 | 2.1 | 3.7 | 6.6 | 3.7 | 5.7 | 3.1 | 4.2 | Subdominants (16.1) |
| Mortierella alpina | 5.7 | 2.7 | 3.3 | 4.2 | 3.7 | 4.0 | 3.7 | 3.4 | 3.8 | |
| Cladosporium cladosporioides (2.61) | 3.8 | 4.2 | 2.6 | 4.7 | 3.6 | 4.6 | 3.9 | 1.7 | 3.6 | |
| Aspergillus niger (2.93) | 2.3 | 2.2 | 1.9 | 1.7 | 3.2 | 1.3 | 0.9 | 5.9 | 2.4 | |
| Rhizopus stolonifera (2.58) | 31 | 1.2 | 2.1 | 2.4 | 2.1 | 3.5 | 1.1 | 1.5 | 2.1 | |
| Penicillium expansum (2.49) | 1.3 | 2.0 | 1.7 | 1.1 | 2.0 | 2.1 | 0.4 | 2.4 | 1.6 | Recedents (4.1) |
| Mucor spp. | 1.4 | 1.4 | 1.4 | 1.4 | 1.5 | 0.7 | 2.2 | 1.5 | 1.4 | |
| Sarocladium strictum (2.74) | 1.5 | 0.5 | 0.9 | 1.4 | 0.9 | 1.3 | 0.1 | 1.8 | 1.1 | |
| Penicillium spp. | 0.5 | 0.4 | - | 0.9 | 0.4 | 0.3 | 0.9 | - | 0.4 | Subrecedents (0.43) |
| Acremonium rutilum | 0.1 | 0.0 | - | 0.1 | - | - | 0.1 | - | 0.03 | |
| Total saprotrophic fungi | 21.2 | 21.8 | 16.0 | 21.6 | 24.0 | 21.5 | 19.0 | 21.3 | ||
| Antagonistic fungi | ||||||||||
| Trichoderma koningii (2.69) | 3.2 | 2.8 | 3.7 | 2.9 | 2.8 | 1.5 | 7.0 | 0.7 | 3.1 | Subdominants (5.3) |
| Trichoderma harzianum | 1.9 | 2.8 | 1.7 | 1.5 | 3.4 | 2.4 | 2.8 | 1.3 | 2.2 | |
| Clonostachys rosea | 1.3 | 1.9 | 1.4 | 1.5 | 2.0 | 1.0 | 3.3 | 0.5 | 1.6 | Recedents (1.6) |
| Total antagonistic fungi | 6.4 | 7.5 | 6.8 | 5.9 | 8.2 | 4.9 | 13.1 | 2.5 | ||
| Number of isolates | 1341 | 1255 | 919 | 1285 | 1308 | 1702 | 1150 | 1041 | ||
| The species richness (S) | 33 | 32 | 30 | 33 | 32 | 32 | 32 | 31 | ||
| Simpson’s Reciprocal Index (1/D) | 18.43 | 16.95 | 14.82 | 19.43 | 18.82 | 19.25 | 18.93 | 14.90 | ||
| Shannon–Wiener index (H′) | 1.28 | 1.30 | 1.11 | 1.39 | 1.37 | 1.39 | 1.34 | 1.14 | ||
| Evenness (Shannon) (JH′) | 0.39 | 0.40 | 0.35 | 0.41 | 0.42 | 0.41 | 0.42 | 0.35 | ||
| Biological Seed Dressing | Chemical Seed Dressing | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |
| Pathogenic Fungi | |||||||||
| Ilyonectria destructans | 11.8 | 8.2 | 11.7 | 7.0 | 10.5 | 11.4 | 8.9 | 9.0 | 12.6 |
| Globisporangium irregulare | 12.4 | 6.0 | 6.4 | 27.4 | 8.0 | 4.9 | 11.8 | 7.5 | 5.5 |
| Fusarium equiseti | 8.4 | 8.2 | 8.5 | 5.7 | 13.0 | 7.6 | 8.4 | 10.2 | 7.2 |
| Rhizoctonia solani | 6.7 | 7.1 | 5.0 | 11.5 | 12.0 | 8.6 | 3.8 | 3.6 | |
| Fusarium solani | 4.5 | 4.9 | 4.6 | 3.2 | 4.5 | 5.4 | 9.3 | 7.5 | 5.2 |
| Botrytis cinerea | 4.5 | 3.4 | 6.4 | 1.9 | 4.0 | 5.9 | 5.1 | 4.8 | 6.9 |
| Ascochyta fabae | 3.4 | 6.3 | 3.2 | 2.5 | 5.5 | 4.3 | 3.4 | 4.2 | 5.7 |
| Fusarium proliferatum | 2.8 | 3.0 | 3.9 | 5.1 | 5.0 | 3.2 | 5.5 | 4.5 | 2.6 |
| Fusarium culmorum | 1.7 | 4.5 | 4.3 | 4.5 | 2.5 | 7.0 | 1.7 | 3.9 | 4.3 |
| Fusarium oxysporum | 3.4 | 2.2 | 2.1 | 4.5 | 3.0 | 1.6 | 4.6 | 3.3 | 2.9 |
| Fusarium avenaceum | 2.2 | 4.1 | 0.7 | 2.5 | 2.0 | 3.8 | 3.6 | 3.4 | |
| Alternaria alternata | 0.4 | 0.6 | 2.5 | 1.1 | 2.5 | 2.4 | 4.3 | ||
| Didymella glomerata | 3.9 | 1.5 | 0.4 | 1.3 | 2.0 | 0.5 | 2.5 | 1.5 | 1.7 |
| Juxtiphoma eupyrena | 1.1 | 0.7 | 1.1 | 0.6 | 1.0 | 1.6 | 1.3 | 1.8 | 1.4 |
| Fusarium sporotrichioides | 4.5 | 0.7 | 2.1 | 1.3 | 0.5 | 0.6 | 0.6 | ||
| Phoma herbarum | 1.1 | 2.2 | 0.4 | 0.6 | 2.0 | 0.8 | 0.9 | 1.1 | |
| Sclerotinia sclerotiorum | 0.7 | 1.1 | 0.6 | 1.0 | 0.5 | 2.1 | 0.3 | 1.7 | |
| Fusarium poae | 1.1 | 0.7 | 1.3 | 1.1 | 0.8 | 1.2 | 1.1 | ||
| Ulocladium consortiale | 0.7 | 0.5 | 1.1 | 1.8 | 2.9 | ||||
| Verticillium albo-atrum | 1.5 | 1.4 | 0.6 | 0.4 | 0.6 | ||||
| Including the type of Fusarium | 27.5 | 28.7 | 26.9 | 28.1 | 30.5 | 25.9 | 34.1 | 34.8 | 27.3 |
| Total | 76.6 | 67.0 | 64.4 | 82.9 | 79.5 | 65.9 | 76.7 | 72.4 | 71.7 |
| Saprotrophic Fungi | |||||||||
| Epicoccum nigrum | 3.9 | 4.9 | 8.2 | 1.3 | 1.5 | 6.5 | 1.3 | 4.2 | 5.5 |
| Mortierella alpina | 2.8 | 3.7 | 4.6 | 1.9 | 4.0 | 4.3 | 4.6 | 4.8 | 2.6 |
| Cladosporium cladosporioides | 2.2 | 5.6 | 3.9 | 2.5 | 2.7 | 4.6 | 5.4 | 3.7 | |
| Aspergillus niger | 1.5 | 1.1 | 5.1 | 4.0 | 8.6 | 1.3 | 0.6 | 2.0 | |
| Rhizopus stolonifer | 1.1 | 0.7 | 1.4 | 1.3 | 1.0 | 2.2 | 3.4 | 4.5 | 2.6 |
| Penicillium expansum | 0.4 | 0.7 | 2.5 | 2.5 | 2.2 | 2.5 | 0.9 | 2.9 | |
| Mucor spp. | 2.2 | 1.9 | 2.5 | 2.5 | 1.0 | 1.1 | 1.2 | 0.9 | |
| Sarocladium strictum | 0.4 | 1.3 | 2.0 | 2.2 | 1.3 | 1.8 | 0.9 | ||
| Penicillium spp. | 1.9 | 0.7 | 0.6 | 0.3 | |||||
| Acremonium rutilum | 0.4 | ||||||||
| Total | 12.2 | 21.4 | 23.1 | 15.9 | 18.5 | 29.8 | 19.1 | 24.0 | 21.5 |
| Antagonistic Fungi | |||||||||
| Trichoderma koningii | 9.0 | 6.0 | 6.0 | 0.6 | 1.0 | 0.5 | 1.7 | 1.5 | 1.4 |
| Trichoderma harzianum | 1.7 | 3.0 | 3.6 | 0.6 | 0.5 | 2.7 | 2.5 | 0.9 | 3.7 |
| Clonostachys rosea | 4.5 | 2.6 | 2.8 | 0.5 | 1.1 | 1.2 | 1.7 | ||
| Total | 15.2 | 11.6 | 12.5 | 1.2 | 2.0 | 4.3 | 4.2 | 3.6 | 6.8 |
| Number of isolates | 1175 | 1250 | 1025 | 1025 | 900 | 1200 | 1700 | 1380 | 2025 |
| Indicators of the Biodiversity of Fungal Communities | |||||||||
| The species richness (S) | 23 | 31 | 29 | 28 | 29 | 27 | 27 | 31 | 31 |
| Simpson’s Reciprocal Index (1/D) | 16.24 | 22.27 | 18.26 | 9.66 | 16.40 | 18.62 | 18.55 | 19.62 | 19.56 |
| Shannon-Wiener (H′) | 1.05 | 1.62 | 1.35 | 1.01 | 1.21 | 1.21 | 1.28 | 1.33 | 1.56 |
| Evenness (Shannon) (JH′) | 0.33 | 0.47 | 0.40 | 0.30 | 0.36 | 0.37 | 0.39 | 0.39 | 0.45 |
| Group of Attendance | >10% | -Eudominants | 5.1–10% | -Dominants | 2.1–5.0% | -Subdominants | |||
| 1.1–2.0% | -Recedents | <1.0% | -Subrecedents | ||||||
| Independent Variables (x) | Dependent Variables (y) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Incidence | Number of Isolates | Share | |||||||||||||
| Pathogens | Saprobiontic | Antagonists | |||||||||||||
| B* | CH | C | B | CH | C | B | CH | C | B | CH | C | B | CH | C | |
| pH H2O | 0.86 | 0.97 | −0.88 | −0.05 | 0.15 | 0.23 | 0.84 | 0.45 | 0.93 | −0.92 | −0.44 | −0.99 | 0.99 | −0.50 | −0.10 |
| Humus content | −0.99 | −0.93 | −0.99 | −0.50 | 0.42 | 0.33 | −0.99 | −0.86 | −0.98 | 0.98 | 0.86 | 0.64 | −0.84 | 0.89 | 0.63 |
| N | 0.46 | 0.15 | −0.42 | 1.00 | −0.99 | −0.98 | 0.48 | 0.87 | 0.31 | −0.33 | −0.87 | 0.34 | −0.05 | −0.83 | −0.98 |
| P | −0.45 | −0.15 | 0.42 | −1.00 | 0.99 | 0.98 | −0.48 | −0.86 | −0.31 | 0.33 | 0.87 | −0.34 | 0.05 | 0.83 | 0.98 |
| K | −0.35 | −0.03 | 0.32 | −0.99 | 0.99 | 0.99 | −0.38 | −0.80 | −0.20 | 0.22 | 0.81 | −0.44 | 0.17 | 0.76 | 0.96 |
| Ca | 0.99 | 0.97 | −0.99 | 0.37 | −0.28 | −0.19 | 0.99 | 0.78 | 0.99 | −0.99 | −0.77 | −0.75 | 0.91 | −0.82 | −0.51 |
| Mg | 0.75 | 0.92 | −0.77 | −0.25 | 0.34 | 0.42 | 0.73 | 0.27 | 0.84 | −0.83 | −0.25 | −0.99 | 0.98 | −0.33 | 0.09 |
| Cu | −0.57 | −0.28 | 0.54 | −0.99 | 0.97 | 0.95 | −0.59 | −0.92 | −0.44 | 0.45 | 0.93 | −0.21 | −0.08 | 0.90 | 0.99 |
| Zn | −0.56 | −0.26 | 0.52 | −0.99 | 0.98 | 0.95 | −0.58 | −0.91 | −0.42 | 0.43 | 0.92 | −0.23 | −0.06 | 0.89 | 0.99 |
| Mn | −0.88 | −0.69 | 0.87 | −0.82 | 0.76 | 0.70 | −0.89 | −0.99 | −0.80 | 0.81 | 0.99 | 0.26 | −0.53 | 0.99 | 0.89 |
| Fe | −0.91 | −0.99 | 0.92 | −0.04 | −0.05 | −0.14 | −0.89 | −0.53 | −0.96 | 0.93 | 0.52 | 0.93 | −0.99 | 0.67 | 0.30 |
| Participation: | |||||||||||||||
| Pathogens | 0.99 | 0.62 | −0.99 | Explanations: | rxy > 0.9 | Very strong dependence | |||||||||
| Saprobiontic | −0.99 | −0.61 | 0.70 | 0.7 < rxy < 0.9 | Quite strong dependence | ||||||||||
| Antagonists | 0.86 | −0.67 | 0.56 | 0.4 < rxy < 0.7 | Moderate dependence | ||||||||||
| The species richness (S) | −0.86 | −0.04 | 0.97 | 0.2 < rxy < 0.4 | Weak dependence | ||||||||||
| Simpson’s Reciprocal Index (1/D) | −0.54 | −0.96 | 0.95 | rxy < 0.2 | No dependency | ||||||||||
| Shannon-Wiener (H′) | −0.71 | −0.99 | 0.81 | ||||||||||||
| Evenness (Shannon) (JH′) | −0.68 | −0.98 | 0.70 | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gleń-Karolczyk, K.; Boligłowa, E.; Gospodarek, J.; Antonkiewicz, J.; Luty, L. Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings. Agronomy 2021, 11, 1889. https://doi.org/10.3390/agronomy11091889
Gleń-Karolczyk K, Boligłowa E, Gospodarek J, Antonkiewicz J, Luty L. Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings. Agronomy. 2021; 11(9):1889. https://doi.org/10.3390/agronomy11091889
Chicago/Turabian StyleGleń-Karolczyk, Katarzyna, Elżbieta Boligłowa, Janina Gospodarek, Jacek Antonkiewicz, and Lidia Luty. 2021. "Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings" Agronomy 11, no. 9: 1889. https://doi.org/10.3390/agronomy11091889
APA StyleGleń-Karolczyk, K., Boligłowa, E., Gospodarek, J., Antonkiewicz, J., & Luty, L. (2021). Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings. Agronomy, 11(9), 1889. https://doi.org/10.3390/agronomy11091889