Seed Priming with Sulfhydral Thiourea Enhances the Performance of Camelina sativa L. under Heat Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Husbandry
2.2. Growth Parameters
2.3. Gas Exchange Parameters
2.4. Water Relations
2.5. Yield and Related Attributes
2.6. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Physiological Parameters
3.2.1. Gas Exchange Attributes
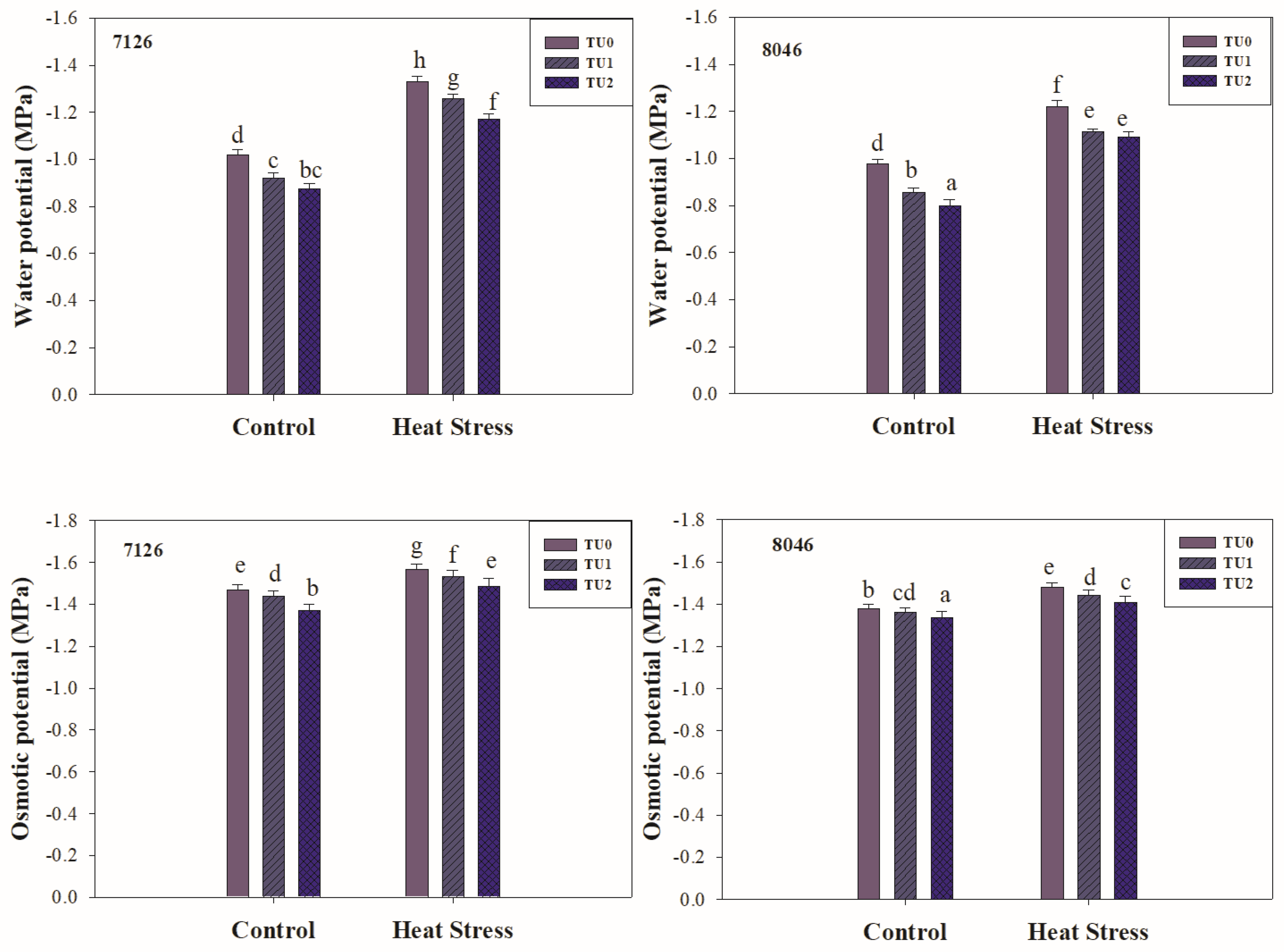
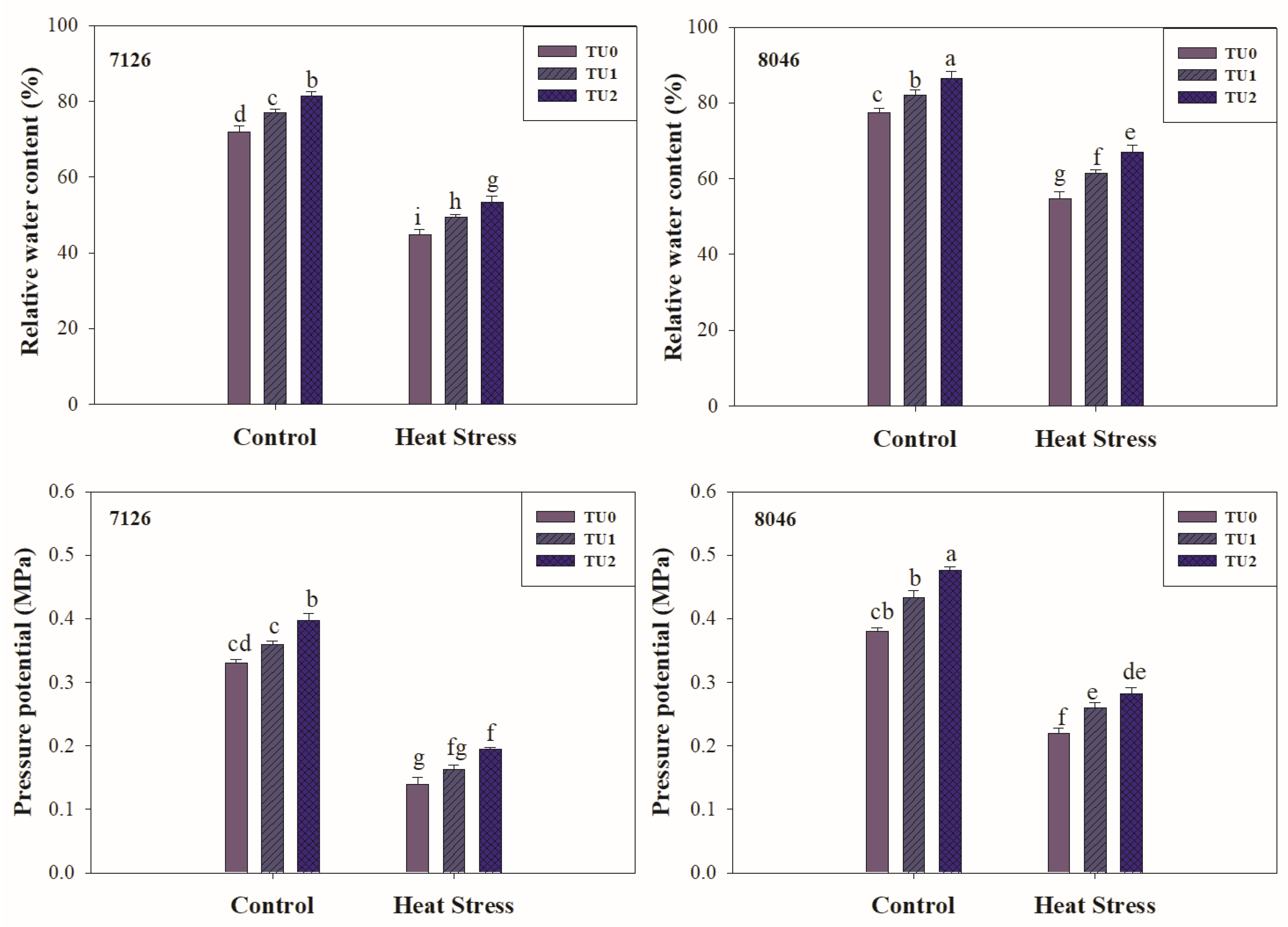
3.2.2. Water Relations
3.3. Yield and Related Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faisal, M.; Iqbal, M.A.; Aydemir, S.K.; Hamid, A.; Rahim, N.; El Sabagh, A.; Khaliq, A.; Siddiqui, M.H. Exogenously foliage applied micronutrients efficacious impact on achene yield of sunflower under temperate conditions. Pak. J. Bot. 2020, 52, 1215–1221. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- IPCC. IPCC Expert Meeting Report: Towards New Scenarios for Analysis of Emissions, Climate Change, Impacts, and Response Strategies; IPCC Secretariat: Geneva, Switzerland, 2007. [Google Scholar]
- NASA. What Is Climate Change? Available online: https://climatekids.nasa.gov/climate-change-meaning/ (accessed on 14 May 2019).
- Olesen, J.E.; Bindi, M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002, 16, 239–262. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Ahmad, R.; Shahbaz, M. Modulation in water relations, chlorophyll contents and antioxidants activity of maize by foliar phosphorus application under drought stress. Pak. J. Bot. 2017, 49, 11–19. [Google Scholar]
- Stern, N. Stern Review: The Economics of Climate Change; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F. Temperature response of photosynthesis and its interaction with light intensity insweet orange leaf discs under non-photorespiratory condition. Ciênc. Agrotecnol. 2006, 30, 670–678. [Google Scholar] [CrossRef]
- Rout, G.R.; Das, A.B. Molecular Stress Physiology of Plants; Springer: New Delhi, India, 2013; ISBN 978-81-322-0806-8. [Google Scholar]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—Fromgene to biotechnology. AoB Plants. 2017, 1, 9. [Google Scholar]
- Kumar, S.; Kaur, R.; Kaur, N.; Bhandhari, K.; Kaushal, N.; Gupta, K.; Bains, T.S.; Nayyar, H. Heat-stress induced inhibition ingrowth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related toreduction in oxidative stress. Acta Physiol. Plant. 2011, 33, 2091–2101. [Google Scholar] [CrossRef]
- Rai, A.C.; Singh, M.; Shah, K. Effect of water withdrawal on formation of free radical, proline accumulation and activities of antioxidant enzymes in ZAT12-transformed transgenic tomato plants. Plant Physiol. Biochem. 2012, 61, 108–114. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Silva, J.A.T.D.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antiox-idant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica. Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Tanveer, A.; Anwar-Ul-Haq, M. Foliar Applied Thiourea Improved Physiological Traits and Yield of Camelina and Canola Under Normal and Heat Stress Conditions. J. soil Sci. Plant Nutr. 2021, 21, 1666–1678. [Google Scholar] [CrossRef]
- Chen, S.; Stefanova, K.; Siddique, K.H.M.; Cowling, W.A. Transient daily heat stress during the early reproductive phase disrupts pod and seed development in Brassica napus L. Food Energy Secur. 2021, 10, 262. [Google Scholar] [CrossRef]
- Ihsan, M.Z.; Daur, I.; Alghabari, F.; Alzamanan, S.; Rizwan, S.; Ahmad, M.; Waqas, M.; Shafqat, W. Heat stress and plant development: Role of sulphur metabolites and management strategies. Acta Agric. Scand. Sect. B—Plant Soil Sci. 2019, 69, 332–342. [Google Scholar] [CrossRef]
- Dawood, M.G. Stimulating Plant Tolerance against Abiotic Stress Through Seed Priming. In Advances in Seed Priming; Springer International Publishing: Cham, Switzerland, 2018; pp. 147–183. [Google Scholar]
- Ahmad, Z.; Waraich, E.A.; Barutçular, C.; Alharby, H.; Bamagoos, A.; Kizilgeci, F.; Öztürk, F.; Hossain, A.; Bayoumi, Y.; El Sabagh, A. Enhancing drought tolerance in Camelina sativa L. and Canola napus L. through application of selenium. Pak. J. Bot. 2020, 52, 1927–1939. [Google Scholar] [CrossRef]
- Dutta, P. Seed Priming: New Vistas and Contemporary Perspectives. In Advances in Seed Priming; Springer: Singapore, 2018; pp. 3–22. [Google Scholar]
- Nathawat, N.S.; Nair, J.S.; Kumawat, S.M.; Yadava, N.S.; Singh, G.; Ramaswamy, N.K.; Sahu, M.P.; D’Souza, S.F. Effect of seed soaking with thiols on the antioxidant enzymes and photosystem activities in wheat subjected to water stress. Biol. Plant. 2007, 51, 93–97. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Nahar, K.; Mohsin, S.M.; Parvin, K.; Fujita, M. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal. Behav. 2018, 13, e1477905. [Google Scholar] [CrossRef] [PubMed]
- Skudra, I.; Ruza, A. Effect of Nitrogen and Sulphur Fertilization on Chlorophyll Content in Winter Wheat. Rural. Sustain. Res. 2017, 37, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Srivastava, A.K.; D’Souza, S.F.; Penna, S. Thiourea, a ROS scavenger, regulates source-to-sink relationship to enhance crop yield and oil content in Brassica juncea (L.). PLoS ONE 2013, 8, e73921. [Google Scholar]
- Patade, V.Y.; Nikalje, G.C.; Srivastava, S. Role of Thiourea in Mitigating Different Environmental Stresses in Plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Roychoudhury, A., Tripathi, D.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 467–482. [Google Scholar] [CrossRef]
- Akladious, S.A. Influence of thiourea application on some physiological and molecular criteria of sunflower (Helianthus annuus L.) plants under conditions of heat stress. Protoplasma 2013, 251, 625–638. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Zulfiqar, U.; Ullah, A.; Farooq, M. Thiourea application improves heat tolerance in camelina (Camelina sativa L. Crantz) by modulating gas exchange, antioxidant defense and osmoprotection. Ind. Crop. Prod. 2021, 170, 113826. [Google Scholar] [CrossRef]
- Hao, Z.; Singh, V.P. Drought characterization from a multivariate perspective: A review. J. Hydrol. 2015, 527, 668–678. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Martínez-Force, E.; Mandrioli, M.; Toschi, T.G.; Monti, A. Shifting sowing of camelina from spring to autumn enhances the oil quality for bio-based applications in response to temperature and seed carbon stock. Ind. Crop. Prod. 2019, 137, 66–73. [Google Scholar] [CrossRef]
- Righini, D.; Zanetti, F.; Monti, A. The bio-based economy can serve as the springboard for camelina and crambe to quit the limbo. OCL 2016, 23, D504. [Google Scholar] [CrossRef] [Green Version]
- Gesch, R.; Dose, H.; Forcella, F. Camelina growth and yield response to sowing depth and rate in the northern Corn Belt USA. Ind. Crop. Prod. 2017, 95, 416–421. [Google Scholar] [CrossRef]
- Murphy, E.J. Camelina (Camelina sativa). In Industrial Oil Crops; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 207–230. [Google Scholar]
- Zubr, J. Qualitative variation of Camelina sativa seed from different locations. Ind. Crop. Prod. 2003, 17, 161–169. [Google Scholar] [CrossRef]
- Dixon, G.R. Vegetable Brassicas and Related Crucifers (No. 14); CABI: Wallingford, UK, 2007. [Google Scholar]
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; van Loo, E.N.; Puttick, D.; Monti, A. Agronomic performance and seed quality attributes of Camelina (Camelina sativa L. crantz) in multi-environment trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H.; Deekey., D.A. Principles and Procedures of Statistics. In A Biometrical Approach; McGraw Hill Book. Int. Co.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Shah, A.N.; Wu, C.; Yousaf, M.; Nasim, W.; Alharby, H.; et al. Exogenously Applied Plant Growth Regulators Enhance the Morpho-Physiological Growth and Yield of Rice under High Temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asthir, B.; Thapar, R.; Farooq, M.; Bains, N.S. Exogenous application of thiourea improves the performance of late sown wheat by inducing terminal heat resistance. Int. J. Agric. Biol. 2013, 15, 1337–1342. [Google Scholar]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Chauhan, B.; Khan, F.; Ihsan, M.Z.; Ullah, A.; Wu, C.; Bajwa, A.; et al. Responses of Rapid Viscoanalyzer Profile and Other Rice Grain Qualities to Exogenously Applied Plant Growth Regulators under High Day and High Night Temperatures. PLoS ONE 2016, 11, e0159590. [Google Scholar] [CrossRef]
- Jiang, Y. Effect of heat stress on pollen development and seed set of field pea (Pisum sativum L.). Ph.D. Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2016. [Google Scholar]
- Farooq, M.; Bramley, H.; Palta, J.; Siddique, K. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Weymann, W.; Böttcher, U.; Sieling, K.; Kage, H. Effects of weather conditions during different growth phases on yield formation of winter oilseed rape. Field Crop. Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Afzal, I.; Basra, S.; Shahid, M.; Farooq, M.; Saleem, M. Priming enhances germination of spring maize (Zea mays L.) under cool conditions. Seed Sci. Technol. 2008, 36, 497–503. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Shafique, M.W.; Gull, S.; Naveed, W.A.; Javed, T.; Yousef, A.F.; Mauro, R.P. Alleviation of Heat Stress in Tomato by Exogenous Application of Sulfur. Horticulturae 2021, 7, 21. [Google Scholar] [CrossRef]
- Singh, S.; Rathore, P. Influence of phosphorus and thiourea on yield and economics of greengram [Vigna radiata var. aureus (L.) Wilczek]. Res. Crop. 2003, 4, 210–212. [Google Scholar]
- Ahmad, M.; Waraich, E.A.; Hussain, S.; Ayyub, C.M.; Ahmad, Z.; Zulfiqar, U. Improving Heat Stress Tolerance in Camelina sativa and Brassica napus Through Thiourea Seed Priming. J. Plant Growth Regul. 2021, 1–17. [Google Scholar] [CrossRef]
- Rezaei, M.; Bagherian, F. Influence of planting date and sulfur coating in seed coating solution on cotton (Gossypium hirsutum L.) seeds: Physiological traits. Iran. J. Plant Physiol. 2013, 4, 917–923. [Google Scholar]
- Brooks, A. Effects of Phosphorus Nutrition on Ribulose-1,5-Bisphosphate Carboxylase Activation, Photosynthetic Quantum Yield and Amounts of Some Calvin-Cycle Metabolites in Spinach Leaves. Funct. Plant Biol. 1986, 13, 221–237. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Tuna, A.L.; Polat, T.; Aydemir, S. Exogenous application of thiamin promotes growth and antioxidative defense system at initial phases of development in salt-stressed plants of two maize cultivars differing in salinity tolerance. Acta Physiol. Plant. 2014, 37, 1741. [Google Scholar] [CrossRef]
- Mobin, M.; Khan, M.N.; Abbas, Z.K.; Ansari, H.R.; Al-Mutairi, K. Significance of sulfur in heat stressed cluster bean (Cymopsis tetragonoloba L. Taub) genotypes: Responses of growth, sugar and antioxidative metabolism. Arch. Agron. Soil Sci. 2016, 63, 288–295. [Google Scholar] [CrossRef]
- Orman, S.; Kaplan, M. Effects of elemental sulphur and farmyard manure on pH and salinity of calcareous sandy loam soil and some nutrient elements in tomato plant. J. Agric. Sci. Technol. 2011, 5, 20–26. [Google Scholar]
- Sohag, A.A.M.; Arif, T.-U.; Brestic, M.; Afrin, S.; Sakil, A.; Hossain, T.; Hossain, M.A.; Hossain, A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Daud, M.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant. Physiol. Biochem. 2017, 119, 50–58. [Google Scholar] [CrossRef]
- Javeed, H.; Ali, M.; Skalicky, M.; Nawaz, F.; Qamar, R.; Rehman, A.; Faheem, M.; Mubeen, M.; Iqbal, M.; Rahman, M.; et al. Lipoic Acid Combined with Melatonin Mitigates Oxidative Stress and Promotes Root Formation and Growth in Salt-Stressed Canola Seedlings (Brassica napus L.). Molecules 2021, 26, 3147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.; Tariq, R.M.S.; Ayub, M.; Hossain, A.; Hassan, M.; Brestic, M.; Islam, M.S.; et al. Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Marchand, F.L.; Mertens, S.; Kockelbergh, F.; Beyens, L.; Nijs, I. Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Glob. Chang. Biol. 2005, 11, 2078–2089. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of heat stress tolerance in winter wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
| Varieties (V) | Heat Stress (T) | Thiourea (TU) Applications | Plant Height (cm) | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|---|---|
| 7126 | Control | TU0 | 52.7 ± 1.25 d | 9.19 ± 0.15 f | 4.04 ± 0.06 d | 1.41 ± 0.004 e | 0.56 ± 0.02 d | 0.08 ± 0.003 f |
| TU1 | 55.5 ± 0.56 c | 10.3 ± 0.51 e | 4.61 ± 0.21 c | 1.47 ± 0.02 c,d | 0.60 ± 0.01 c | 0.09 ± 0.002 d,e | ||
| TU2 | 58.5 ± 2.17 b | 11.9 ± 0.61 c | 5.35 ± 0.20 b | 1.50 ± 0.005 c | 0.72 ± 0.01 b | 0.10 ± 0.002 b | ||
| Heat stress | TU0 | 33.6 ± 1.00 g | 7.37 ± 0.17 g | 2.13 ± 0.10 h | 1.11 ± 0.01 i | 0.21 ± 0.002 g | 0.04 ± 0.001 i | |
| TU1 | 37.6 ± 1.21 f | 8.91 ± 1.12 f | 2.47 ± 0.05 g | 1.15 ± 0.01 h | 0.22 ± 0.01 g | 0.05 ± 0.001 h | ||
| TU2 | 45.2 ± 1.42 e | 11.6 ± 0.22 c,d | 2.92 ± 0.40 f | 1.21 ± 0.01 g | 0.29 ± 0.01 f | 0.09 ± 0.00 f | ||
| 8046 | Control | TU0 | 54.5 ± 0.15 c,d | 12.09 ± 1.17 c | 4.27 ± 0.02 d | 1.45 ± 0.002 d,e | 0.63 ± 0.01 c | 0.09 ± 0.002 e |
| TU1 | 60.6 ± 0.62 | 13.2 ± 1.57 b | 5.13 ± 0.14 b | 1.55 ± 0.02 b | 0.72 ± 0.02 b | 0.10 ± 0.002 c | ||
| TU2 | 64.5 ± 0.86 a | 17.2 ± 0.50 a | 6.01 ± 0.17 a | 1.60 ± 0.04 a | 0.82 ± 0.004 a | 0.13 ± 0.005 a | ||
| Heat stress | TU0 | 39.2 ± 0.66 f | 10.7 ± 0.05 d,e | 2.74 ± 0.03 f,g | 1.15 ± 0.01 h | 0.29 ± 0.01 f | 0.06 ± 0.00 h | |
| TU1 | 46.6 ± 1.69 e | 13.1 ± 0.53 b | 2.96 ± 0.04 f | 1.22 ± 0.01 g | 0.32 ± 0.01 f | 0.07 ± 0.00 g | ||
| TU2 | 55.0 ± 2.00 c | 16.3 ± 0.74 a | 3.56 ± 0.03 e | 1.29 ± 0.02 f | 0.47 ± 0.03 e | 0.09 ± 0.00 d |
| Varieties (V) | Heat Stress (T) | Thiourea (TU) Applications | Photosynthetic Rate (μmol H2O m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) | Intercellular CO2 Concentration (μmol m−2 s−1) |
|---|---|---|---|---|---|---|
| 7126 | Control | TU0 | 4.05 ± 0.02 f | 0.41 ± 0.00 h | 0.05 ± 0.00 e | 278.1 ± 1.43 e |
| TU1 | 4.75 ± 0.06 e | 0.47 ± 0.00 g | 0.05 ± 0.00 d | 267.8 ± 1.72 f | ||
| TU2 | 5.78 ± 0.06 c | 0.50 ± 0.02 f | 0.06 ± 0.00 c | 254.3 ± 2.15 g | ||
| Heat stress | TU0 | 2.05 ± 0.03 j | 0.43 ± 0.00 h | 0.04 ± 0.00 h | 344.3 ± 3.72 a | |
| TU1 | 2.51 ± 0.02 j | 0.48 ± 0.01 g | 0.04 ± 0.00 g | 331.0 ± 2.75 b | ||
| TU2 | 2.87 ± 0.00 h | 0.49 ± 0.00 e | 0.04 ± 0.00 f | 311.0 ± 2.75 c | ||
| 8046 | Control | TU0 | 5.42 ± 0.37 d | 0.56 ± 0.00 e | 0.06 ± 0.00 c | 248.4 ± 5.37 g |
| TU1 | 6.42 ± 0.37 b | 0.63 ± 0.01 c | 0.07 ± 0.00 b | 237.1 ± 3.19 h | ||
| TU2 | 7.45 ± 0.01 a | 0.67 ± 0.01 a,b | 0.07 ± 0.00 a | 224.2 ± 6.10 i | ||
| Heat stress | TU0 | 3.07 ± 0.06 h | 0.60 ± 0.00 d | 0.04 ± 0.00 g | 329.2 ± 3.51 b | |
| TU1 | 3.53 ± 0.11 g | 0.64 ± 0.03 b,c | 0.04 ± 0.00 f | 311.4 ± 2.76 c | ||
| TU2 | 4.76 ± 0.08 e | 0.68 ± 0.00 a | 0.05 ± 0.00 d | 296.4 ± 7.28 d |
| Varieties (V) | Heat Stress (T) | Thiourea (TU) Applications | No. of Silicle Plant−1 | No. of Seeds Silicle−1 | 1000-Seed Weight (g) | Seed Yield Pot−1 (g) |
|---|---|---|---|---|---|---|
| 7126 | Control | TU0 | 33.3 ± 1.52 e | 9.73 ± 0.64 e | 0.76 ± 0.04 ef | 2.45 ± 0.06 d |
| TU1 | 40.6 ± 0.57 c | 11.7 ± 0.68 c | 0.87 ± 0.05 cd | 2.92 ± 0.17 b,c | ||
| TU2 | 45.6 ± 1.15 b | 13.6 ± 0.57 b | 1.00 ± 0.03 b | 3.81 ± 0.24 a | ||
| Heat stress | TU0 | 23.0 ± 1.00 g | 7.42 ± 0.46 h | 0.48 ± 0.01 h | 0.57 ± 0.02 h | |
| TU1 | 27.0 ± 1.00 f | 8.30 ± 0.26 f,g | 0.53 ± 0.01 g | 0.62 ± 0.01 h | ||
| TU2 | 32.6 ± 0.58 e | 9.66 ± 0.58 e | 0.73 ± 0.01 f | 1.31 ± 0.24 f | ||
| 8046 | Control | TU0 | 38.0 ± 1.03 d | 10.5 ± 0.25 d | 0.81 ± 0.01 d,e | 2.66 ± 0.04 c,d |
| TU1 | 44.0 ± 1.00 b | 13.3 ± 0.57 b | 0.89 ± 0.02 c | 2.96 ± 0.15 b | ||
| TU2 | 50.6 ± 1.52 a | 15.3 ± 0.35 a | 1.08 ± 0.05 a | 3.89 ± 0.28 a | ||
| Heat stress | TU0 | 28.1 ± 1.25 f | 7.97 ± 0.16 g,h | 0.53 ± 0.01 h | 0.72 ± 0.03 g,h | |
| TU1 | 33.0 ± 2.64 e | 9.00 ± 0.05 e,f | 0.60 ± 0.02 g | 0.96 ± 0.09 g | ||
| TU2 | 36.3 ± 0.57 d | 12.3 ± 0.57 c | 0.84 ± 0.02 cd | 1.61 ± 0.18 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waraich, E.A.; Ahmad, M.; Soufan, W.; Manzoor, M.T.; Ahmad, Z.; Habib-Ur-Rahman, M.; Sabagh, A.E. Seed Priming with Sulfhydral Thiourea Enhances the Performance of Camelina sativa L. under Heat Stress Conditions. Agronomy 2021, 11, 1875. https://doi.org/10.3390/agronomy11091875
Waraich EA, Ahmad M, Soufan W, Manzoor MT, Ahmad Z, Habib-Ur-Rahman M, Sabagh AE. Seed Priming with Sulfhydral Thiourea Enhances the Performance of Camelina sativa L. under Heat Stress Conditions. Agronomy. 2021; 11(9):1875. https://doi.org/10.3390/agronomy11091875
Chicago/Turabian StyleWaraich, Ejaz Ahmad, Muhammad Ahmad, Walid Soufan, Muhammad Taimoor Manzoor, Zahoor Ahmad, Muhammad Habib-Ur-Rahman, and Ayman EL Sabagh. 2021. "Seed Priming with Sulfhydral Thiourea Enhances the Performance of Camelina sativa L. under Heat Stress Conditions" Agronomy 11, no. 9: 1875. https://doi.org/10.3390/agronomy11091875
APA StyleWaraich, E. A., Ahmad, M., Soufan, W., Manzoor, M. T., Ahmad, Z., Habib-Ur-Rahman, M., & Sabagh, A. E. (2021). Seed Priming with Sulfhydral Thiourea Enhances the Performance of Camelina sativa L. under Heat Stress Conditions. Agronomy, 11(9), 1875. https://doi.org/10.3390/agronomy11091875








