Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Separation Columns
2.3. Reagents
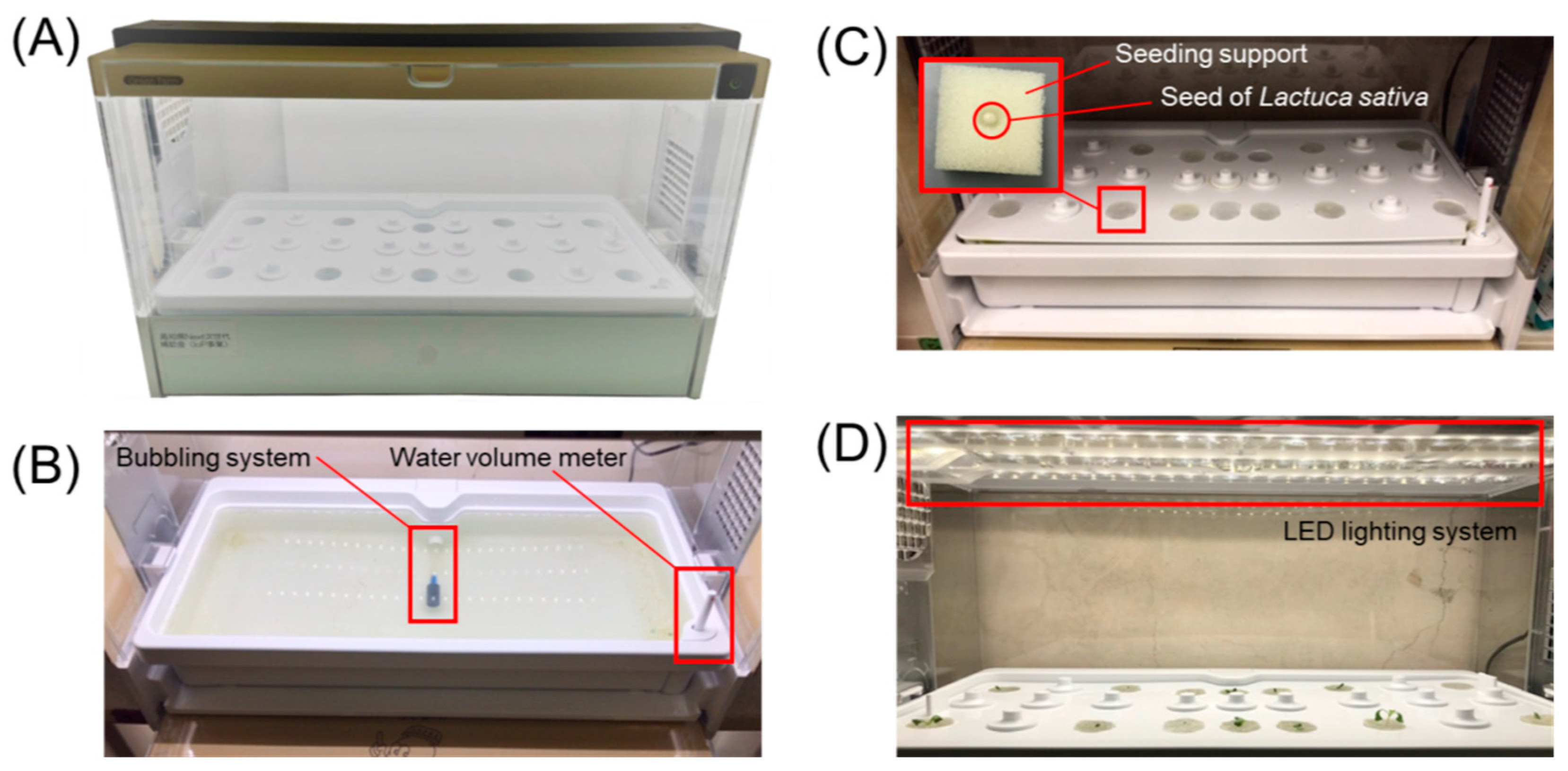
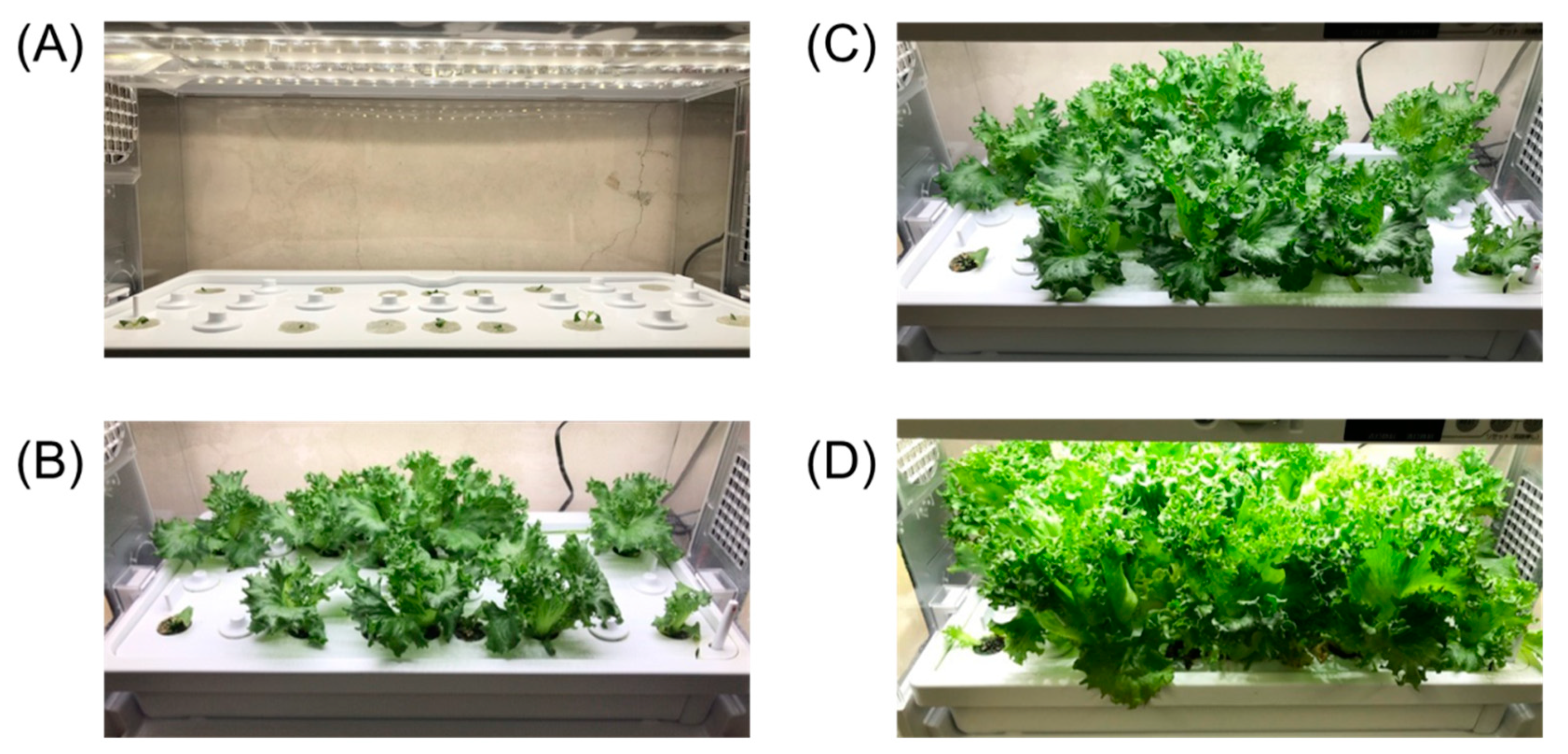
2.4. Hydroponic System
3. Results and Discussion
3.1. Selection of Separation Columns
3.2. Eluent Optimization
3.3. Analytical Performance
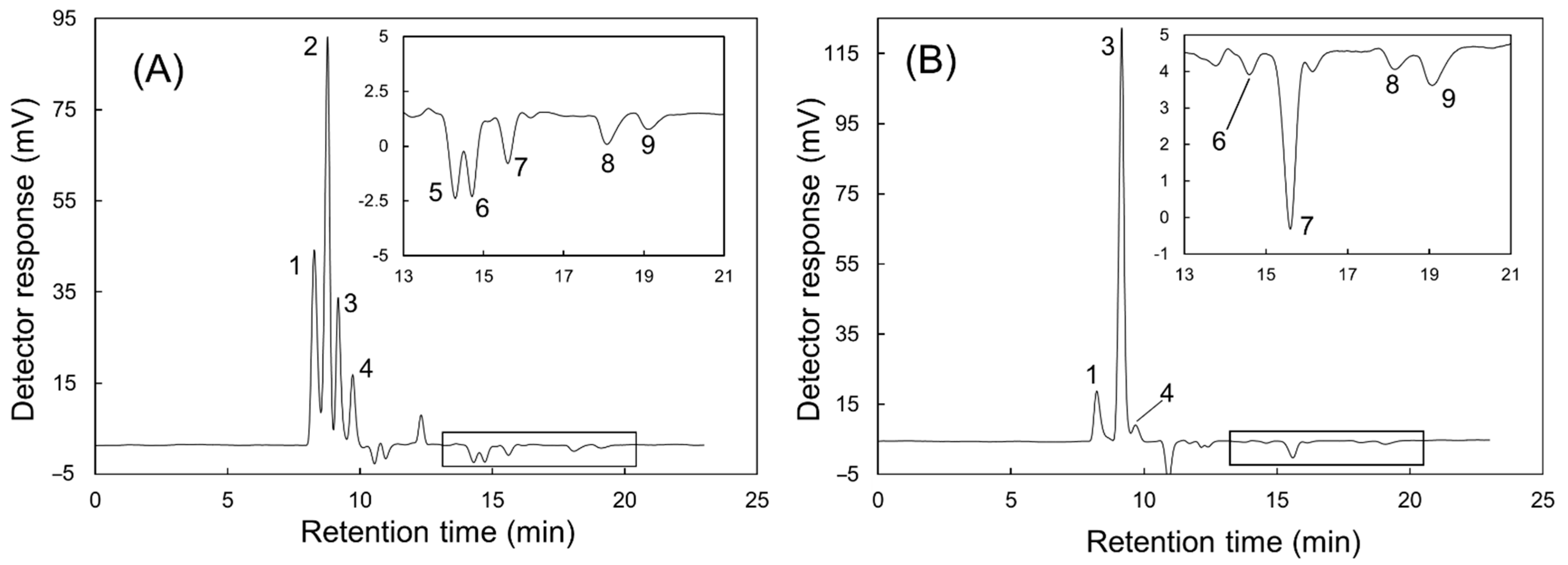
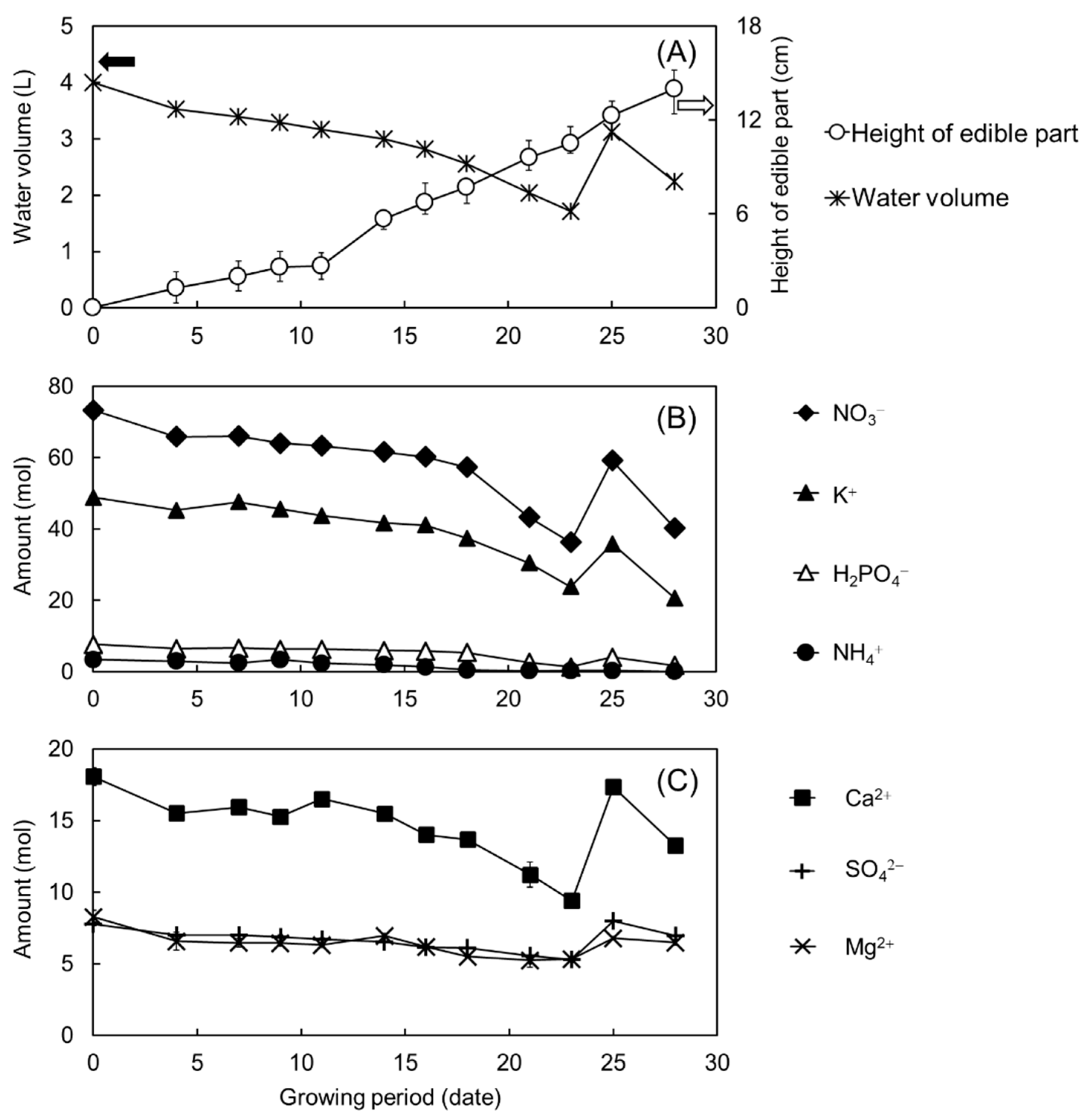
3.4. Monitoring the Fertilizer Solution Used in Hydroponic Culture
3.5. Validations of Analyte Ion Concentrations
4. Conclusions
- It helps to understand the behavior of ionic nutrients in fertilizer solution during hydroponic cultivation.
- It is potentially useful for the fertilization of ionic nutrients based on the understanding of all major ionic nutrients’ concentration compared with the conventional monitoring system (pH and EC glass electrode).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregory, P.J.; Nortcliff, S. Soil Conditions and Plant Growth; Wiley-Blackwell Publishing Ltd.: West Sussex, UK, 2013; pp. 1–21. [Google Scholar]
- Stoltzenberg, D. Fritz Haber: Chemist, Nobel Laureate, German, Jew; Chemical Heritage Press: Philadelphia, PA, USA, 2004; pp. 101–113. [Google Scholar]
- Aftalion, F. A History of the International Chemical Industry, 2nd ed.; Chemical Heritage Press: Philadelphia, PA, USA, 2001; pp. 88–89. [Google Scholar]
- Barrett, G.; Alexander, P.; Robinson, J.; Bragg, N. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Good Agricultural Practices for Greenhouse Vegetable Crops, Principles for Mediterranean Climate Areas; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2013; pp. 303–354. [Google Scholar]
- Bamsey, M.; Graham, T.; Thompson, C.; Berinstain, A.; Scott, A.; Dixon, M. Ion-Specific Nutrient Management in Closed Systems: The Necessity for Ion-Selective Sensors in Terrestrial and Space-Based Agriculture and Water Management Systems. Sensors 2012, 12, 13349–13392. [Google Scholar] [CrossRef] [PubMed]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.A.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Katsoulas, N.; Savvas, D.; Kitta, E.; Bartzanas, T.; Kittas, C. Extension and evaluation of a model for automatic drainage solution management in tomato crops grown in semi-closed hydroponic systems. Comput. Electron. Agric. 2015, 113, 61–71. [Google Scholar] [CrossRef]
- Nakatani, N.; Kozaki, D.; Mori, M.; Tanaka, K. Recent Progress and Applications of Ion-exclusion/Ion-exchange Chromatography for Simultaneous Determination of Inorganic Anions and Cations. Anal. Sci. 2012, 28, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Mori, M. Milestone Studies on Ion-exclusion Chromatography of Ionic and Nonionic Substances Utilizing Multifunctional Separation Mechanism of Ion-exchange Resins. Anal. Sci. 2021, 37, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ohta, K.; Haddad, P.R.; Fritz, J.S.; Miyanaga, A.; Hu, W.; Hasebe, K.; Lee, K.-P.; Sarzanini, C. High-performance ion-exclusion/cation-exchange chromatography of anions and cations in acid rain waters no a weakly acidic cation-exchange resin. J. Chromatogr. A 2001, 920, 239–245. [Google Scholar] [CrossRef]
- Kozaki, D.; Ozaki, T.; Nakatani, N.; Mori, M.; Tanaka, K. Utilization of Ion-Exclusion Chromatography for Water Quality Monitoring in a Suburban River in Jakarta, Indonesia. Water 2014, 6, 1945–1960. [Google Scholar] [CrossRef] [Green Version]
- Kozaki, D.; Harun, N.I.B.; Chong, C.H.; Esraruddin, M.H.B.; Yunus, N.A.B.; Derahman, A.S.B.; Pu, K.S.; Alias, N.S.B.; Annamalai, K.A.; Nagappan, S.; et al. Identification of Polluted Sites in Four Major Rivers in Kuantan, Malaysia based on Water Chemistry Estimates of Aquatic Microbial Activity. Sustainability 2019, 11, 3813. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, N.; Kozaki, D.; Mori, M.; Hasebe, K.; Nakagoshi, N.; Tanaka, K. Ion-exclusion/cation-exchange Chromatography with Dual Detection of the Conductivity and Spectrophotometry for the Simultaneous Determination of Common Inorganic Anionic Species and Cations in River and Wastewater. Anal. Sci. 2011, 27, 499. [Google Scholar] [CrossRef] [Green Version]
- Kozaki, D.; Tanihata, S.; Yamamoto, A.; Nakatani, N.; Mori, M.; Tanaka, K. Single injection ion-exclusion/cation-exchange chromatography for simultaneous determination of organic/inorganic anions, inorganic cations, and ethanol in beer samples. Food Chem. 2018, 274, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Japan Greenhouse Horticulture Association (JGHA). Survey of Protected Horticulture. Available online: https://www.maff.go.jp/j/seisan/ryutu/engei/sisetsu/pdf/daikibo_1.pdf (accessed on 1 September 2021).
- Guilherme, L.B.; Francisca, D.A.G.; Natalya, K.; Alan, P.; Lucas, R.; Emily, W.; Gregory, M.W.; Rolf, U.H. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [Green Version]
- USDA. Vegetables 2019 Summary. National Agricultural Statistics Service. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf (accessed on 1 September 2021).
- Tanaka, K.; Haddad, P.R. Encyclopedia of Separation Science, Ion Exclusion Chromatography; Wilson, I.D., Ed.; Academic Press, Inc.: New York, NY, USA, 2000; pp. 3193–3201. [Google Scholar]
- Fritz, J.S.; Gjerde, D.T. Ion Chromatography, 4th ed.; Completely Revised and Enlarged Edition; Fritz, J.S., Gjerde, D.T., Eds.; WILEY-VCH: Weinheim, Germany, 2009; pp. 207–235. [Google Scholar]
- Kwon, S.-M.; Lee, K.-P.; Tanaka, K.; Ohta, K. Simultaneous determination of anions and cations by ion-exclusion chromatography-cation-exchange chromatography with tartaric acid/18-crown-6 as eluent. J. Chromatogr. A 1999, 850, 79–84. [Google Scholar] [CrossRef]
- Izatt, R.M.; Terry, R.E.; Haymore, B.L.; Hansen, L.D.; Dalley, N.K.; Avondet, A.G.; Christensen, J.J. Calorimetric titration study of the interaction of several uni- and bivalent cations with 15-crown-5, 18-crown-6, and two isomers of dicyclohexo-18-crown-6 in aqueous solution at 25.degree.C and .mu. = 0.1. J. Am. Chem. Soc. 1976, 98, 7620–7626. [Google Scholar] [CrossRef]
- Izatt, R.; Bradshaw, J.S.; Nielsen, S.A.; Lamb, J.D.; Christensen, J.J.; Sen, D. Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 1985, 85, 271–339. [Google Scholar] [CrossRef]
- USEPA. Dissolved Sodium, Ammonium, Potassium, Magnesium, and Calcium in Wet Deposition by Chemically Suppressed Ion Chromatography; Method 300.7; USEPA: Cincinnati, OH, USA, 1986. [Google Scholar]
- JIS K 0102: 2019. Testing Methods for Industrial Wastewater. Available online: https://www.kikakurui.com/k0/K0102-2019-01.html (accessed on 29 July 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozaki, D.; Sago, Y.; Fujiwara, T.; Mori, M.; Kubono, C.; Koga, T.; Mitsui, Y.; Tachibana, T. Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture. Agronomy 2021, 11, 1847. https://doi.org/10.3390/agronomy11091847
Kozaki D, Sago Y, Fujiwara T, Mori M, Kubono C, Koga T, Mitsui Y, Tachibana T. Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture. Agronomy. 2021; 11(9):1847. https://doi.org/10.3390/agronomy11091847
Chicago/Turabian StyleKozaki, Daisuke, Yuki Sago, Taku Fujiwara, Masanobu Mori, Chihiro Kubono, Tougo Koga, Yuta Mitsui, and Tomotaka Tachibana. 2021. "Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture" Agronomy 11, no. 9: 1847. https://doi.org/10.3390/agronomy11091847
APA StyleKozaki, D., Sago, Y., Fujiwara, T., Mori, M., Kubono, C., Koga, T., Mitsui, Y., & Tachibana, T. (2021). Ion-Exclusion/Cation-Exchange Chromatography Using Dual-Ion-Exchange Groups for Simultaneous Determination of Inorganic Ionic Nutrients in Fertilizer Solution Samples for the Management of Hydroponic Culture. Agronomy, 11(9), 1847. https://doi.org/10.3390/agronomy11091847







