A Review of Factors to Consider for Permanent Cordon Establishment and Maintenance
Abstract
1. Introduction
2. Physiology of the Grapevine
2.1. Water and Nutrient Transport
2.2. Xylem Morphology
2.3. Phloem Morphology
2.4. Effects of Water Stress
2.5. Impact of Cordon Health on Reproduction and Vine Balance
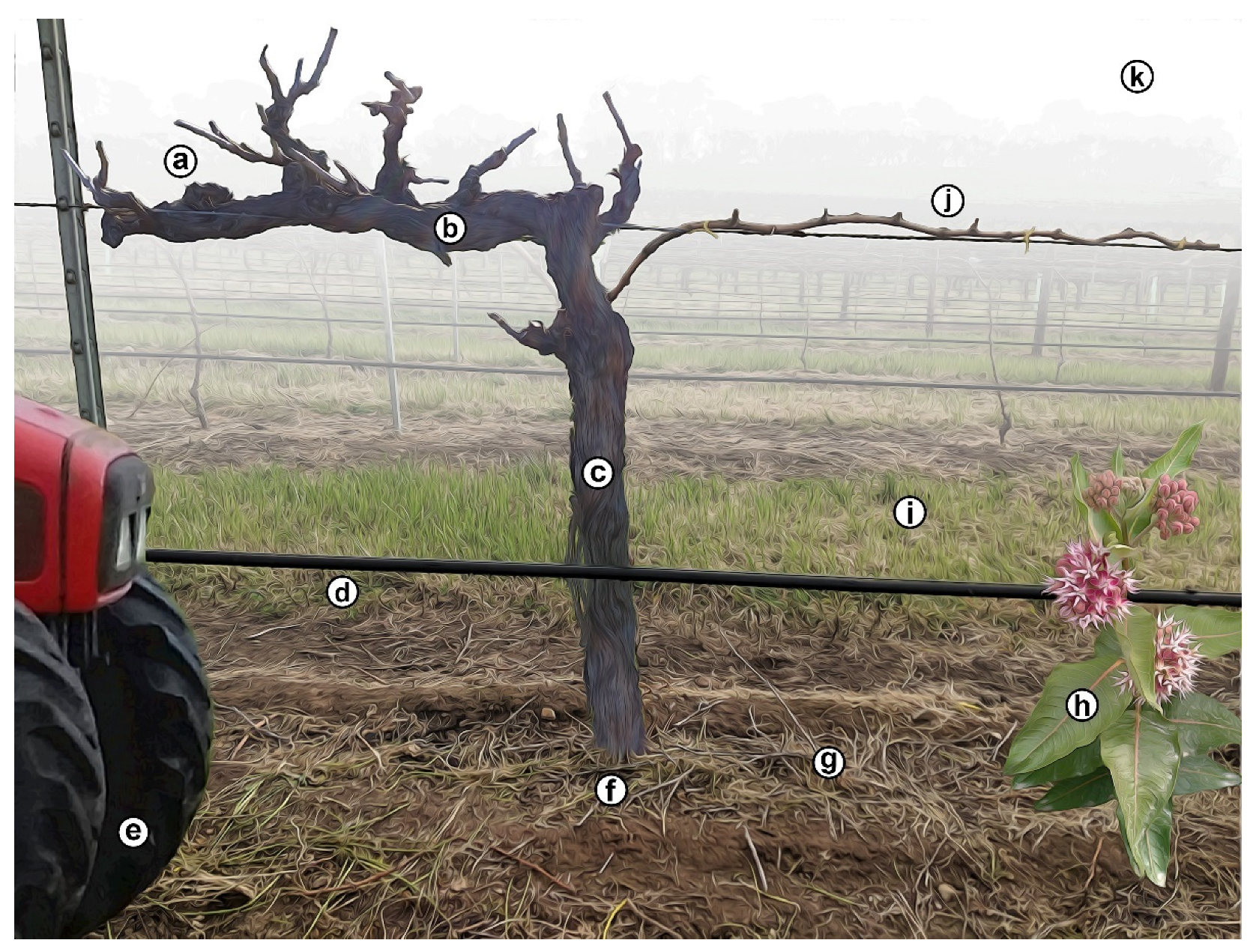
3. Cordon Establishment and Maintenance
3.1. Selection of Training System
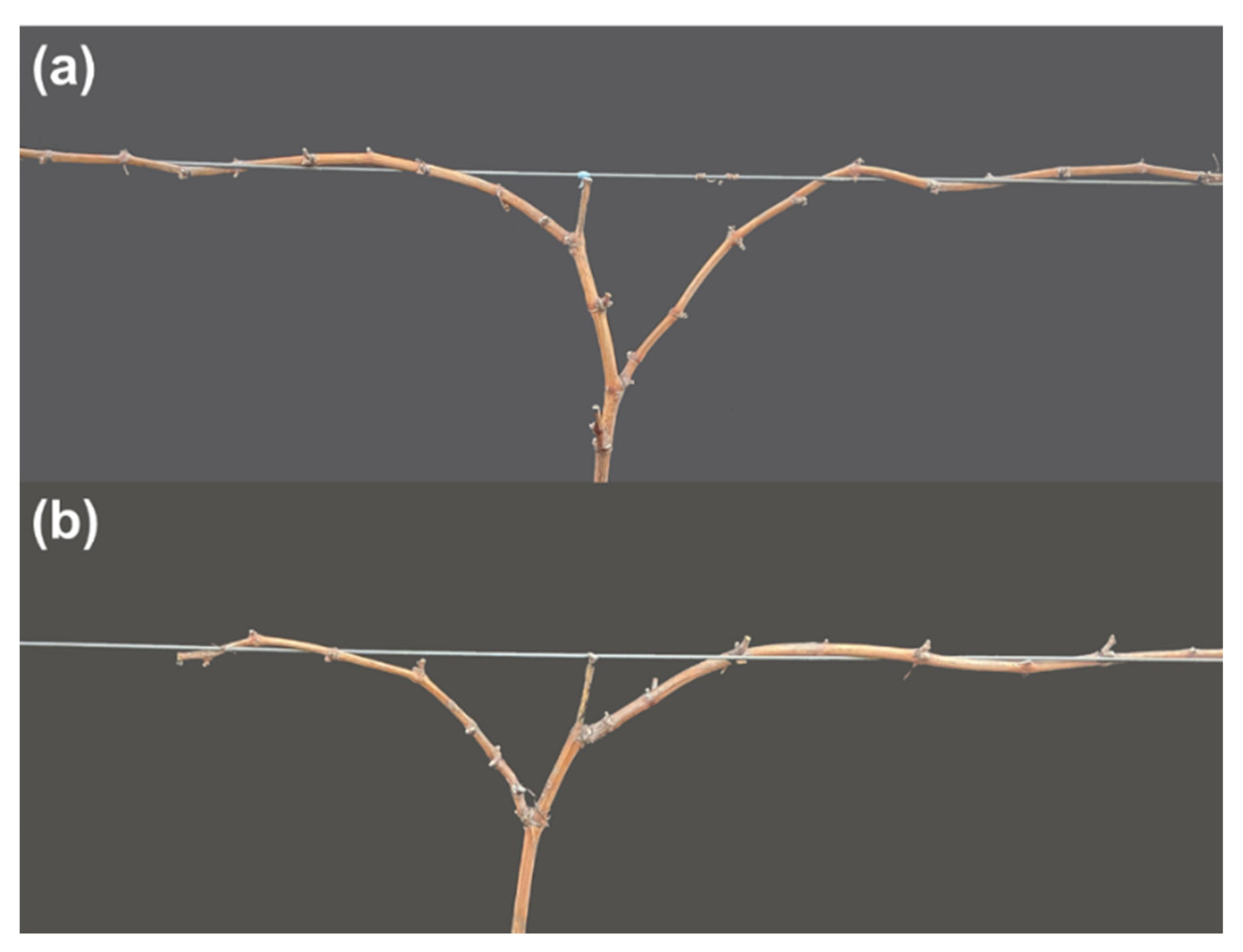
3.2. Wrapping Canes Tightly around the Cordon Wire
3.3. Establishment of Cordon Arms
3.4. Maintenance of Cordon Arms
4. Vascular Diseases of Grapevine
4.1. Mechanism of Infection and Implicated Pathogens
4.2. Relationship between Stress and Disease Symptom Expression
4.3. Relationship between Strangulation and Trunk Disease
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grigg, D.; Methven, D.; de Bei, R.; Rodríguez López, C.M.; Dry, P.; Collins, C. Effect of vine age on vine performance of Shiraz in the Barossa Valley, Australia. Aust. J. Grape Wine Res. 2017, 24, 75–87. [Google Scholar] [CrossRef]
- Vršič, S.; Ivančič, A.; Šušek, A.; Valdhuber, B.; Šiško, J.; Zagradišnik, M. The World’s oldest living grapevine specimen and its genetic relationships. Vitis 2011, 50, 167–171. [Google Scholar]
- Heymann, H.; Noble, A. Descriptive analysis of commercial Cabernet Sauvignon wines from California. Am. J. Enol. Vitic. 1987, 38, 41–44. [Google Scholar]
- Reynolds, A.G.; Pearson, E.G.; De Savigny, C.; Coventry, J.; Strommer, J. Interactions of vine age and reflective mulch upon berry, must, and wine composition of five Vitis vinifera cultivars. Int. J. Fruit Sci. 2008, 7, 85–119. [Google Scholar] [CrossRef]
- Bou Nader, K.; Stoll, M.; Rauhut, D.; Patz, C.D.; Jung, R.; Loehnertz, O.; Schultz, H.R.; Hilbert, G.; Renaud, C.; Roby, J.P.; et al. Impact of grapevine age on water status and productivity of Vitis vinifera L. cv. Riesling. Eur. J. Agron. 2019, 104, 1–12. [Google Scholar] [CrossRef]
- Rahman, L.; Creecy, H.; Orchard, B. Impact of citrus nematode (Tylenchulus semipenetrans) densities in soil on yield of grapevines (Vitis vinifera ‘Shiraz’) in south-eastern New South Wales. Vitis 2008, 47, 175–180. [Google Scholar]
- Siebert, J. Eutypa: The economic toll on vineyards. Wines Vines 2001, 4, 50–56. [Google Scholar]
- Sipiora, M.J.; Cuellar, S. Economic impact of Eutypa dieback. Wine Bus. Mon. 2014, 21, 46–49. [Google Scholar]
- Sosnowski, M.R.; McCarthy, G. Economic impact of grapevine trunk disease management in Sauvignon blanc vineyards of New Zealand. Wine Vitic. J. 2017, 32, 42. [Google Scholar]
- Atallah, S.S.; Gomez, M.I.; Fuchs, M.F.; Martinson, T.E. Economic impact of grapevine leafroll disease on Vitis vinifera cv. Cabernet franc in Finger Lakes vineyards of New York. Am. J. Enol. Vitic. 2011, 63, 73–79. [Google Scholar] [CrossRef]
- Credi, R.; Babini, A.R. Effects of virus and virus-like infections on growth, yield, and fruit quality of Albana and Trebbiano Romagnolo grapevines. Am. J. Enol. Vitic. 1997, 48, 7–12. [Google Scholar]
- Ripamonti, M.; Pacifico, D.; Roggia, C.; Palmano, S.; Rossi, M.; Bodino, N.; Marzachì, C.; Bosco, D.; Galetto, L. Recovery from grapevine flavescence dorée in areas of high infection pressure. Agronomy 2020, 10, 1479. [Google Scholar] [CrossRef]
- White, R.E. Understanding Vineyard Soils, 2nd ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Duchêne, E.; Schneider, C. Grapevine and climatic changes: A glance at the situation in Alsace. Agron. Sustain. Dev. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Keller, M. Managing grapevines to optimise fruit development in a challenging environment: A climate change primer for viticulturists. Aust. J. Grape Wine Res. 2010, 16, 56–69. [Google Scholar] [CrossRef]
- Schultz, H.R. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Caravia, L.; Collins, C.; Shepherd, J.; Tyerman, S. Wrapping arms for cordon establishment: Is it a stressful practice for grapevines? Wine Vitic. J. 2015, 30, 48–50. [Google Scholar]
- Sperry, J.S. Evolution of water transport and xylem structure. Int. J. Plant. Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef]
- Lucas, W.J.; Groover, A.; Lichtenberger, R.; Furuta, K.; Yadav, S.R.; Helariutta, Y.; He, X.Q.; Fukuda, H.; Kang, J.; Brady, S.M.; et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013, 55, 294–388. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.H.; Joly, J. On the ascent of sap. Philos. Trans. R. Soc. B 1895, 186, 563–576. [Google Scholar]
- Pickard, W.F. The ascent of sap in plants. Prog. Biophys. Mol. Biol. 1981, 37, 181–229. [Google Scholar] [CrossRef]
- Brown, H.R. The theory of the rise of sap in trees: Some historical and conceptual remarks. Phys. Perspect. 2013, 15, 320–358. [Google Scholar] [CrossRef]
- Venturas, M.D.; Sperry, J.S.; Hacke, U.G. Plant xylem hydraulics: What we understand, current research, and future challenges. J. Integr. Plant. Biol. 2017, 59, 356–389. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Sperry, J.S. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant. Physiol. 1988, 88, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Choné, X.; van Leeuwen, C.; Dubourdieu, D.; Gaudillère, J. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant. Anatomy: Meristems, Cells, and Tissues of the Plant. Body: Their Structure, Function, and Development, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lovisolo, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Munitz, S.; Netzer, Y.; Shtein, I.; Schwartz, A. Water availability dynamics have long-term effects on mature stem structure in Vitis vinifera. Am. J. Bot. 2018, 105, 1443–1452. [Google Scholar] [CrossRef]
- Ewers, F.W.; Fisher, J.B. Variation in vessel length and diameter in stems of six tropical and subtropical lianas. Am. J. Bot. 1989, 76, 1452–1459. [Google Scholar] [CrossRef]
- Jacobsen, A.L.; Pratt, R.B.; Tobin, M.F.; Hacke, U.G.; Ewers, F.W. A global analysis of xylem vessel length in woody plants. Am. J. Bot. 2012, 99, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.L.; Rodriguez-Zaccaro, F.D.; Lee, T.F.; Valdovinos, J.; Toschi, H.S.; Martinez, J.A.; Pratt, R.B. Grapevine xylem development, architecture, and function. In Functional and Ecological Xylem Anatomy; Hacke, U., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–162. [Google Scholar]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae). Am. J. Bot. 2006, 93, 1567–1576. [Google Scholar] [CrossRef]
- Schultz, H.R.; Matthews, M.A. Xylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): Evidence that low light uncouples water transport capacity from leaf area. Planta 1993, 190, 393–406. [Google Scholar] [CrossRef]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Biol. 1989, 40, 19–38. [Google Scholar] [CrossRef]
- Sun, Q.; Rost, T.L.; Matthews, M.A. Wound-induced vascular occlusions in Vitis vinifera (Vitaceae): Tyloses in summer and gels in winter. Am. J. Bot. 2008, 95, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Bonsen, K.J.M.; Kučera, L.J. Vessel occlusions in plants: Morphological, functional and evolutionary aspects. IAWA Bull. 1990, 11, 393–399. [Google Scholar] [CrossRef]
- Esau, K. Anatomy of Seed Plants, 2nd ed.; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Pérez-Donoso, A.G.; Lenhof, J.J.; Pinney, K.; Labavitch, J.M. Vessel embolism and tyloses in early stages of Pierce’s disease. Aust. J. Grape Wine Res. 2016, 22, 81–86. [Google Scholar] [CrossRef]
- Dute, R.R.; Duncan, K.M.; Duke, B. Tyloses in abscission scars of loblolly pine. IAWA J. 1999, 20, 67–74. [Google Scholar] [CrossRef]
- Saitoh, T.; Ohtani, J.; Fukazawa, K. The occurrence and morphology of tyloses and gums in the vessels of Japanese hardwoods. IAWA J. 1993, 14, 359–371. [Google Scholar] [CrossRef]
- Schulz, A. Phloem. Structure related to function. In Progress in Botany; Springer: Berlin/Heidelberg, Germany, 1998; pp. 429–475. [Google Scholar]
- Ruiz-Medrano, R.; Xoconostle-Cázares, B.; Lucas, W.J. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant. Biol. 2001, 4, 202–209. [Google Scholar] [CrossRef]
- Esau, K. Phloem structure in the grapevine, and its seasonal changes. Hilgardia 1948, 18, 217–296. [Google Scholar] [CrossRef][Green Version]
- Gonzalez Antivilo, F.; Paz, R.C.; Tognetti, J.; Keller, M.; Cavagnaro, M.; Barrio, E.E.; Roig Juñent, F. Winter injury to grapevine secondary phloem and cambium impairs budbreak, cambium activity, and yield formation. J. Plant. Growth Regul. 2019, 39, 1095–1106. [Google Scholar] [CrossRef]
- Esau, K. Anatomy and cytology of Vitis phloem. Hilgardia 1965, 37, 17–72. [Google Scholar] [CrossRef]
- Bates, T.R.; Dunst, R.M.; Joy, P. Seasonal dry matter, starch, and nutrient distribution in ‘Concord’ grapevine roots. HortScience 2002, 37, 313–316. [Google Scholar] [CrossRef]
- Zapata, C.; Deleens, E.; Chaillou, S.; Magne, C. Partitioning and mobilization of starch and N reserves in grapevine (Vitis vinifera L.). J. Plant Physiol. 2004, 161, 1031–1040. [Google Scholar] [CrossRef]
- Holzapfel, B.P.; Smith, J.P. Developmental stage and climatic factors impact more on carbohydrate reserve dynamics of Shiraz than cultural practice. Am. J. Enol. Vitic. 2012, 63, 333–342. [Google Scholar] [CrossRef]
- Aloni, R.; Raviv, A.; Peterson, C.A. The role of auxin in the removal of dormancy callose and resumption of phloem activity in Vitis vinifera. Can. J. Bot. 1991, 69, 1825–1832. [Google Scholar] [CrossRef]
- Stoll, M. Effects of Partial Rootzone Drying on Grapevine Physiology and Fruit Quality. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2000. [Google Scholar]
- Dry, P.R.; Loveys, B.R.; Botting, D.; Düring, H. Effects of partial root-zone drying on grapevine vigour, yield, composition of fruit and use of water. In Proceedings of the IX Australian Wine Industry Technical Conference, Adelaide, Australia, 16–19 July 1995; Winetitles: Adelaide, Australia, 1996; pp. 126–131. [Google Scholar]
- Schultz, H.R.; Matthews, M.A. Resistance to water transport in shoots of Vitis vinifera L.: Relation to growth at low water potential. Plant Physiol. 1988, 88, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, R.; Raschi, A.; Béres, C.; Fenyvesi, A.; Ridder, H.W. Comparison of sap flow, cavitation and water status of Quercus petraea and Quercus cerris trees with special reference to computer tomography. Plant Cell Environ. 1996, 19, 928–938. [Google Scholar] [CrossRef]
- Dayer, S.; Prieto, J.A.; Galat, E.; Perez Peña, J. Carbohydrate reserve status of Malbec grapevines after several years of regulated deficit irrigation and crop load regulation. Aust. J. Grape Wine Res. 2013, 19, 422–430. [Google Scholar] [CrossRef]
- Keller, M.; Romero, P.; Gohil, H.; Smithyman, R.P.; Riley, W.R.; Casassa, L.F.; Harbertson, J.F. Deficit irrigation alters grapevine growth, physiology, and fruit microclimate. Am. J. Enol. Vitic. 2016, 67, 426–435. [Google Scholar] [CrossRef]
- Rossouw, G.C.; Smith, J.P.; Barril, C.; Deloire, A.; Holzapfel, B.P. Implications of the presence of maturing fruit on carbohydrate and nitrogen distribution in grapevines under postveraison water constraints. J. Am. Soc. Hortic. Sci. 2017, 142, 71–84. [Google Scholar] [CrossRef]
- Marciniak, M.; Reynolds, A.G.; Brown, R. Influence of water status on sensory profiles of Ontario Riesling wines. Food Res. Int. 2013, 54, 881–891. [Google Scholar] [CrossRef]
- McCarthy, M.G. The effect of transient water deficit on berry development of cv. Shiraz (Vitis vinifera L.). Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Naor, A.; Bravdo, B.; Hepner, Y. Effect of post-veraison irrigation level on Sauvignon blanc yield, juice quality and water relations. S. Afr. J. Enol. Vitic. 1993, 14, 19–25. [Google Scholar] [CrossRef]
- Romero, P.; Muñoz, R.G.; Fernández-Fernández, J.I.; del Amor, F.M.; Martínez-Cutillas, A.; García-García, J. Improvement of yield and grape and wine composition in field-grown Monastrell grapevines by partial root zone irrigation, in comparison with regulated deficit irrigation. Agrar. Water Manag. 2015, 149, 55–73. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. Interactions of crop level and late season water stress on growth and physiology of field-growth Concord grapevines. Am. J. Enol. Vitic. 1994, 45, 252–258. [Google Scholar]
- Lopez, G.; Girona, J.; Marsal, J. Response of winter root starch concentration to severe water stress and fruit load and its subsequent effects on early peach fruit development. Tree Physiol. 2007, 27, 1619–1626. [Google Scholar] [CrossRef][Green Version]
- Smith, J.P.; Holzapfel, B.P. Cumulative responses of Semillon grapevines to late season perturbation of carbohydrate reserve status. Am. J. Enol. Vitic. 2009, 60, 461–470. [Google Scholar]
- Dry, P.R. Canopy management for fruitfulness. Aust. J. Grape Wine Res. 2000, 6, 109–115. [Google Scholar] [CrossRef]
- Bennett, J.; Jarvis, P.; Creasy, G.L.; Trought, M.C.T. Influence of defoliation on overwintering carbohydrate reserves, return bloom, and yield of mature Chardonnay grapevines. Am. J. Enol. Vitic. 2005, 56, 386–393. [Google Scholar]
- Cox, C.M.; Favero, A.C.; Dry, P.R.; McCarthy, M.G.; Collins, C. Rootstock effects on primary bud necrosis, bud fertility, and carbohydrate storage in Shiraz. Am. J. Enol. Vitic. 2012, 63, 277–283. [Google Scholar] [CrossRef]
- Nuzzo, V.; Matthews, M.A. Response of fruit growth and ripening to crop level in dry-farmed Cabernet Sauvignon on four rootstocks. Am. J. Enol. Vitic. 2006, 57, 314–324. [Google Scholar]
- Holzapfel, B.P.; Smith, J.P.; Field, S.K.; Hardie, W.J. Dynamics of carbohydrate reserves in cultivated grapevines. Hortic. Rev. 2010, 37, 143. [Google Scholar]
- Guilpart, N.; Metay, A.; Gary, C. Grapevine bud fertility and number of berries per bunch are determined by water and nitrogen stress around flowering in the previous year. Eur. J. Agron. 2014, 54, 9–20. [Google Scholar] [CrossRef]
- Collins, C.; Rawnsley, B. Factors influencing primary bud necrosis (PBN) in Australian vineyards. In Proceedings of the VII International Symposium on Grapevine Physiology and Biotechnology, Davis, CA, USA, 21–25 June 2004; Volume 689, pp. 81–86. [Google Scholar]
- Vasudevan, L.; Wolf, T.K.; Welbaum, G.G.; Wisniewski, M.E. Reductions in bud carbohydrates are associated with grapevine bud necrosis. Vitis 1998, 37, 189–190. [Google Scholar]
- Collins, C.; Rawnsley, B. National survey reveals primary bud necrosis is widespread in Australian vineyards. Aust. N. Z. Grapegrow. Winemak. 2004, 485, 46–49. [Google Scholar]
- Bernizzoni, F.; Gatti, M.; Civardi, S.; Poni, S. Long-term performance of Barbera grown under different training systems and within-row vine spacings. Am. J. Enol. Vitic. 2009, 60, 339–348. [Google Scholar]
- Carbonneau, A.; Garrier, G.; Deloire, A. Quelques éléments historiques de l’évolution des architectures de vigne (première partie). Le Progrès Agric. Et Vitic. 2001, 118, 155–161. [Google Scholar]
- Eltom, M.; Winefield, C.S.; Trought, M.C.T. Effect of pruning system, cane size and season on inflorescence primordia initiation and inflorescence architecture of Vitis vinifera L. Sauvignon blanc. Aust. J. Grape Wine Res. 2014, 20, 459–464. [Google Scholar] [CrossRef]
- Trought, M.C.T. Fruitset—Possible implications on wine quality. In Proceedings of the ASVO Transforming Flowers to Fruit Seminar, Mildura, Australia, 25 July 2005; pp. 32–36. [Google Scholar]
- Jackson, D. Pruning and Training; Rev. ed.; Lincoln University Press: Lincoln, NZ, USA, 2001. [Google Scholar]
- May, P.; Brien, C.J.; Clingeleffer, P.R. Pruning Sultana vines by the arched cane system. Aust. J. Exp. Agric. Anim. Husb. 1978, 18, 301–308. [Google Scholar] [CrossRef]
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture; University of California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Tassie, E.; Freeman, B.M. Pruning. In Viticulture Volume 2, Practices; Coombe, B.G., Dry, P.R., Eds.; Winetitles: Adelaide, Australia, 1992; pp. 66–84. [Google Scholar]
- Intrieri, C.; Poni, S. Integrated evolution of trellis training systems and machines to improve grape quality and vintage quality of mechanized Italian vineyards. Am. J. Enol. Vitic. 1995, 46, 116–127. [Google Scholar]
- Gasparinetti, P.; Biasi, W.; Teot, G.; Maschio, T.; Peratoner, C.; Bertamini, M. Materiali d’impianto in viticoltura: Un occhio ai particolari. Inf. Agrar. 1999, 55, 33–42. [Google Scholar]
- Caravia, L. Heat Wave Mitigation Strategies for Wine Grape Production and Measures of the Impact of Heat on Berry Ripening and Wine Composition. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2016. [Google Scholar]
- Tombesi, S.; Farinelli, D. Trunk constriction effects on vegetative vigour and yield efficiency in olive tree (Olea europaea L.). J. Agric. Sci. Technol. 2016, 18, 1667–1680. [Google Scholar]
- Tombesi, S.; Farinelli, D.; Palliotti, A.; Poni, S.; DeJong, T.M. Xylem manipulation techniques affecting tree vigour in peach and olive trees. In Proceedings of the XI International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Bologna, Italy, 28 August–2 September 2016; Volume 1228, pp. 91–96. [Google Scholar]
- Dann, I.R.; Wildes, R.A.; Chalmers, D.J. Effects of limb girdling on growth and development of competing fruit and vegetative tissues of peach trees. Aust. J. Plant Physiol. 1984, 11, 49–58. [Google Scholar] [CrossRef]
- Rufato, L.; Machado, B.D.; Luz, A.R.; Marcon Filho, J.L.; Hipólito, J.S.; Kretzschmar, A.A. Effect of trunk girdling on growth and crop yield of ‘Packham’s Triumph’ pear. In Proceedings of the XII International Pear Symposium, Leuven, Belgium, 14–18 July 2014; Volume 1094, pp. 265–268. [Google Scholar]
- Sousa, R.; Calouro, F.; Oliveira, C. Influence of trunk girdling on growth and fruit production of ‘Rocha’/BA29. In Proceedings of the X International Pear Symposium, Peniche, Portugal, 22–26 May 2007; Volume 800, pp. 319–324. [Google Scholar]
- Day, K.R.; DeJong, T.M. Improving fruit size: Thinning and girdling nectarines, peaches, and plums. Compact Fruit Tree 1999, 32, 49–51. [Google Scholar]
- Jensen, F.; Swanson, F.; Peacock, W.; Leavitt, G. The effect of width of cane and trunk girdles on berry weight and soluble solids in table ‘Thompson Seedless’ vineyards. Am. J. Enol. Vitic. 1975, 26, 90–91. [Google Scholar]
- Li, K.T.; Chang, J.C.; Wang, L.L.; Liu, Y.T.; Lee, C.L. Girdling improved berry coloration in summer but suppressed return growth in the following spring in “Kyoho” grapevines cultivated in the subtropical double cropping system. Vitis 2015, 54, 59–63. [Google Scholar]
- Eltom, M.; Trought, M.C.T.; Winefield, C.S. The effects of cane girdling before budbreak on shoot growth, leaf area and carbohydrate content of Vitis vinifera L. Sauvignon blanc grapevines. Funct. Plant Biol. 2013, 40, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, R. Prevenire l’invecchiamento del cordone della vite. Inf. Agrar. 2008, 4, 89–91. [Google Scholar]
- Dry, P.R.; Loveys, B.R. Factors influencing grapevine vigour and the potential for control with partial rootzone drying. Aust. J. Grape Wine Res. 1998, 4, 140–148. [Google Scholar] [CrossRef]
- Castaldi, R. Vite: Mantenere funzionale il cordone permanente. Inf. Agrar. 2016, 2, 81–84. [Google Scholar]
- Bernizzoni, F.; Civardi, S.; Van Zeller, M.; Gatti, M.; Poni, S. Shoot thinning effects on seasonal whole-canopy photosynthesis and vine performance in Vitis vinifera L. cv. Barbera. Aust. J. Grape Wine Res. 2011, 17, 351–357. [Google Scholar] [CrossRef]
- Simonit, M.; Deledda, F.; Giudici, M.; Manfreda, L.; Ostan, M.; Turata, R.; Zanutta, A. Vite: Potatura «virtuosa» applicazione e vantaggi. Inf. Agrar. 2012, 37, 36–46. [Google Scholar]
- Simonit, M.; Sirch, P. «Potatura soffi ce» contro il deperimento dei vigneti. Inf. Agrar. 2009, 36, 43–46. [Google Scholar]
- Carter, M.V. Eutypa armeniacae Hansf. & Carter, sp. nov., an airborne vascular pathogen of Prunus armeniaca L. in southern Australia. Aust. J. Bot. 1957, 5, 21–35. [Google Scholar]
- Lecomte, P.; Diarra, B.; Boisseau, M.; Weingartner, S.; Rey, P. Prévenir l’esca chez Vitis vinifera en proscrivant les modes de conduite ou les systèmes de taille mutilants. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Henderson, B.; Sosnowski, M.R.; McCarthy, M.G.; Scott, E.S. Incidence and severity of Eutypa dieback in grapevines are related to total surface area of pruning wounds. Aust. J. Grape Wine Res. 2020, 27, 87–93. [Google Scholar] [CrossRef]
- Faúndez-López, P.; Delorenzo-Arancibia, J.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y. Pruning cuts affect wood necrosis but not the percentage of budburst or shoot development on spur pruned vines for different grapevine varieties. Vitis 2021, 60, 137–141. [Google Scholar]
- Cholet, C.; Bruez, É.; Lecomte, P.; Barsacq, A.; Martignon, T.; Giudici, M.; Simonit, M.; Dubourdieu, D.; Gény, L. Plant resilience and physiological modifications induced by curettage of Esca-diseased grapevines. OENO One 2021, 55, 153–169. [Google Scholar] [CrossRef]
- Travadon, R.; Lecomte, P.; Diarra, B.; Lawrence, D.P.; Renault, D.; Ojeda, H.; Rey, P.; Baumgartner, K. Grapevine pruning systems and cultivars influence the diversity of wood-colonizing fungi. Fungal Ecol. 2016, 24, 82–93. [Google Scholar] [CrossRef]
- Simonit, M.; Deledda, F.; Giudici, M.; Manfreda, L.; Ostan, M.; Turata, R.; Zanutta, A. Potatura vite: Il taglio è indipendente dalla forma di allevamento. Inf. Agrar. 2013, 36, 32–35. [Google Scholar]
- Simonit, M.; Deledda, F.; Giudici, M.; Manfreda, L.; Ostan, M.; Turata, R.; Zanutta, A. Pergola: Le conseguenze dei tagli di potatura. Inf. Agrar. 2013, 36, 36–39. [Google Scholar]
- Castaldi, R. Vite: Tendenza all’abbandono del cordone permanente. Inf. Agrar. 2011, 28, 58–60. [Google Scholar]
- van Niekerk, J.M.; Bester, W.; Halleen, F.; Crous, P.W.; Fourie, P.H. The distribution and symptomatology of grapevine trunk disease pathogens are influenced by climate. Phytopathol. Mediterr. 2011, 50, 98–111. [Google Scholar]
- Moller, W.J.; Kasimatis, A.N. Dieback of grapevines caused by Eutypa armeniacae. Plant. Dis. Rep. 1978, 62, 254–258. [Google Scholar]
- Trouillas, F.P.; Úrbez-Torres, J.R.; Gubler, W.D. Diversity of diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 2010, 102, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.M.; Huang, R.; Steel, C.C.; Savocchia, S. Identification, distribution and current taxonomy of Botryosphaeriaceae species associated with grapevine decline in New South Wales and South Australia. Aust. J. Grape Wine Res. 2010, 16, 258–271. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [CrossRef]
- van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 2004, 96, 781–798. [Google Scholar] [CrossRef]
- van Niekerk, J.M.; Groenewald, J.; Farr, D.; Fourie, P.; Halleer, F.; Crous, P. Reassessment of Phomopsis species on grapevines. Australas. Plant Path. 2005, 34, 27–39. [Google Scholar] [CrossRef]
- Fischer, M. Biodiversity and geographic distribution of Basidiomycetes causing Esca-associated white rot in grapevine: A worldwide perspective. Phytopathol. Mediterr. 2006, 45, 30–42. [Google Scholar]
- Crous, P.W.; Gams, W.; Wingfield, M.J.; van Wyk, P.S. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 1996, 88, 786–796. [Google Scholar] [CrossRef]
- Travadon, R.; Lawrence, D.P.; Rooney-Latham, S.; Gubler, W.D.; Wilcox, W.F.; Rolshausen, P.E.; Baumgartner, K. Cadophora species associated with wood-decay of grapevine in North America. Fungal Biol. 2015, 119, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Larignon, P.; Dubos, B. Fungi associated with Esca disease in grapevine. Eur. J. Plant Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Trouillas, F.; Gubler, W.D. Identification of Eutypa lata by PCR-RFLP. Plant. Dis. 2004, 88, 925–929. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Voegel, T.M.; Gubler, W.D. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant. Dis. 2006, 90, 1490–1503. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Úrbez-Torres, J.R.; Rooney-Latham, S.; Eskalen, A.; Smith, R.J.; Gubler, W.D. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 2010, 61, 113–119. [Google Scholar]
- Chatelet, D.S.; Matthews, M.A.; Rost, T.L. Xylem structure and connectivity in grapevine (Vitis vinifera) shoots provides a passive mechanism for the spread of bacteria in grape plants. Ann. Bot. 2006, 98, 483–494. [Google Scholar] [CrossRef]
- Sosnowski, M.R.; Ayres, M.; Wicks, T.; McCarthy, M. In search of resistance to grapevine trunk diseases. Wine Vitic. J. 2013, 28, 55. [Google Scholar]
- Sosnowski, M.R.; Shtienberg, D.; Creaser, M.L.; Wicks, T.J.; Lardner, R.; Scott, E.S. The influence of climate on foliar symptoms of Eutypa dieback in grapevines. Phytopathology 2007, 97, 1284–1289. [Google Scholar] [CrossRef]
- Hopkins, D.L. Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Annu. Rev. Phytopathol. 1989, 27, 271–290. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, Y.; Walker, M.A.; Labavitch, J.M. Vascular occlusions in grapevines with Pierce’s disease make disease symptom development worse. Plant Physiol. 2013, 161, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Thorne, E.T.; Stevenson, J.F.; Rost, T.L.; Labavitch, J.M.; Matthews, M.A. Pierce’s disease symptoms: Comparison with symptoms of water deficit and the impact of water deficits. Am. J. Enol. Vitic. 2006, 57, 1–11. [Google Scholar]
- Edwards, J.; Salib, S.; Thomson, F.; Pascoe, I.G. The impact of Phaeomoniella chlamydospora infection on the grapevine’s physiological response to water stress—Part 1: Zinfandel. Phytopathol. Mediterr. 2007, 46, 26–37. [Google Scholar]
- Edwards, J.; Salib, S.; Thomson, F.; Pascoe, I.G. The impact of Phaeomoniella chlamydospora infection on the grapevine’s physiological response to water stress—Part 2: Cabernet Sauvignon and Chardonnay. Phytopathol. Mediterr. 2007, 46, 38–49. [Google Scholar]
- Ferreira, J.H.S.; van Wyk, P.S.; Calitz, F.J. Slow dieback of grapevine in South Africa: Stress-related predisposition of young vines for infection by Phaeoacremonium chlamydosporum. S. Afr. J. Enol. Vitic. 1999, 20, 43–46. [Google Scholar]
- Fischer, M.; Kassemeyer, H.H. Water regime and its possible impact on expression of Esca symptoms in Vitis vinifera: Growth characters and symptoms in the greenhouse after artificial infection with Phaeomoniella chlamydospora. Vitis 2012, 51, 129–135. [Google Scholar]
- Sosnowski, M.R.; Luque, J.; Loschiavo, A.P.; Martos, S.; Garcia-Figueres, F.; Wicks, T.J.; Scott, E.S. Studies on the effect of water and temperature stress on grapevines inoculated with Eutypa lata. Phytopathol. Mediterr. 2011, 50, 127–138. [Google Scholar]
- Sosnowski, M.R.; Ayres, M.; Scott, E. The influence of water deficit stress on the grapevine trunk disease pathogens Eutypa lata and Diplodia seriata. Plant. Dis. 2021. [Google Scholar] [CrossRef]
- Oswald, B. The Effect of Water Stress on Colonisation of Grapevines by Eutypa lata. Bachelor’s Thesis, University of Adelaide, Adelaide, Australia, 2017. [Google Scholar]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant Sci. 2017, 8, 1442. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.H.; Devay, J.E.; Meredith, C.P. Roles of water stress and phytotoxins in the development of Pierce’s disease of the grapevine. Physiol. Mol. Plant Pathol. 1988, 32, 1–15. [Google Scholar] [CrossRef]
- Choi, H.K.; Iandolino, A.; da Silva, F.G.; Cook, D.R. Water deficit modulates the response of Vitis vinifera to the Pierce’s disease pathogen Xylella fastidiosa. Mol. Plant-Microbe Interact. 2013, 26, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Gambetta, G.A.; Wada, H.; Shackel, K.A.; Matthews, M.A. The effects of Pierce’s disease on leaf and petiole hydraulic conductance in Vitis vinifera cv. Chardonnay. Physiol. Plant 2009, 136, 384–394. [Google Scholar] [CrossRef]
- Carter, M.V. The Status of Eutypa Lata as a Pathogen; Published on behalf of C.A.B. International; Mycological Institute by C.A.B. International: Wallingford, UK, 1991. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, P.; De Bei, R.; Sosnowski, M.; Collins, C. A Review of Factors to Consider for Permanent Cordon Establishment and Maintenance. Agronomy 2021, 11, 1811. https://doi.org/10.3390/agronomy11091811
O’Brien P, De Bei R, Sosnowski M, Collins C. A Review of Factors to Consider for Permanent Cordon Establishment and Maintenance. Agronomy. 2021; 11(9):1811. https://doi.org/10.3390/agronomy11091811
Chicago/Turabian StyleO’Brien, Patrick, Roberta De Bei, Mark Sosnowski, and Cassandra Collins. 2021. "A Review of Factors to Consider for Permanent Cordon Establishment and Maintenance" Agronomy 11, no. 9: 1811. https://doi.org/10.3390/agronomy11091811
APA StyleO’Brien, P., De Bei, R., Sosnowski, M., & Collins, C. (2021). A Review of Factors to Consider for Permanent Cordon Establishment and Maintenance. Agronomy, 11(9), 1811. https://doi.org/10.3390/agronomy11091811





