Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities
Abstract
:1. Introduction
2. Materials and Methods
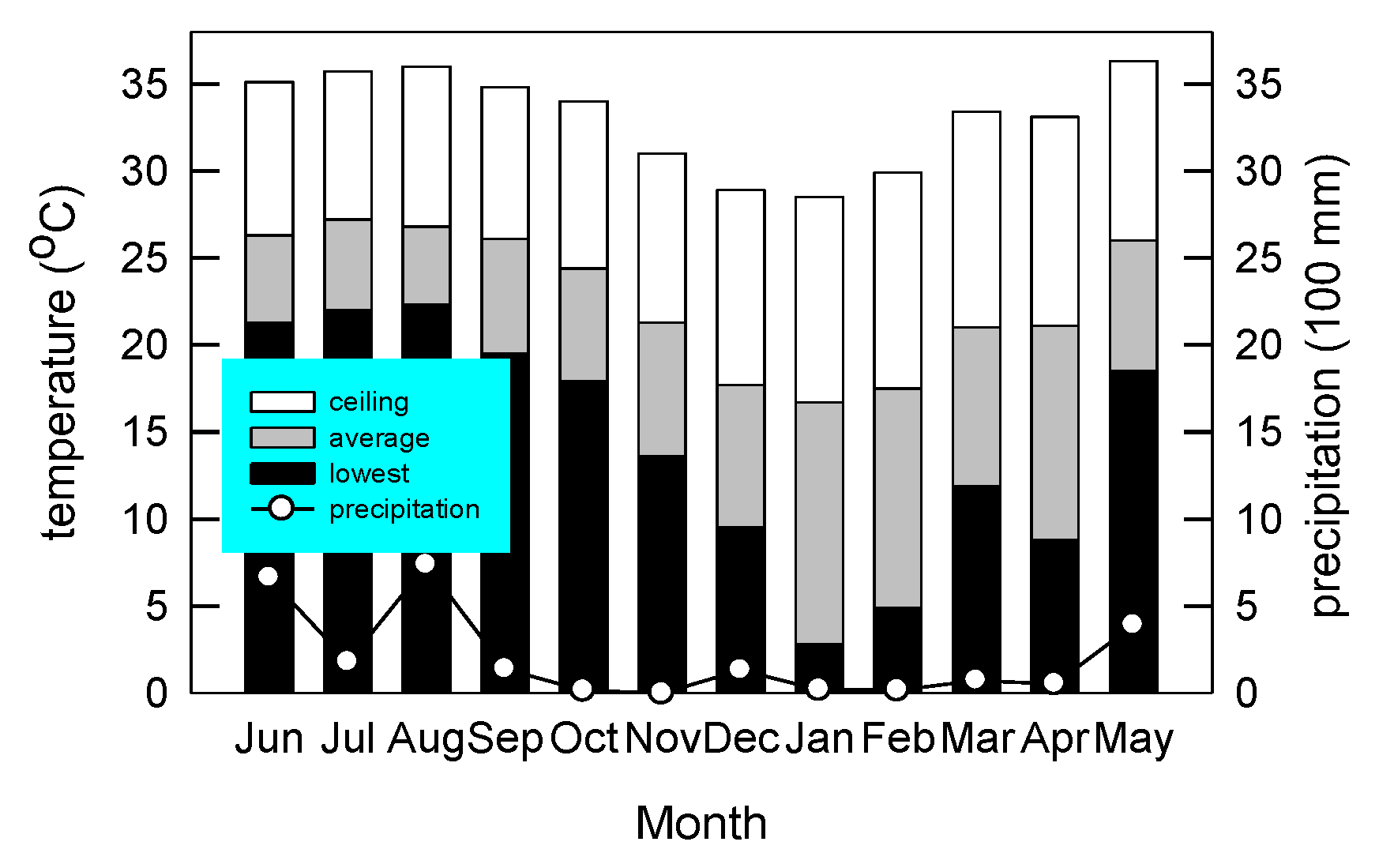
2.1. Site Description, Experimental Field Management, and Experimental Design
2.2. Determination of Photosynthetic Capacityand Chlorophyll Fluorescence (ChlF) Parameters with a Fixed Light Source
2.3. Statistical Analysis
3. Results
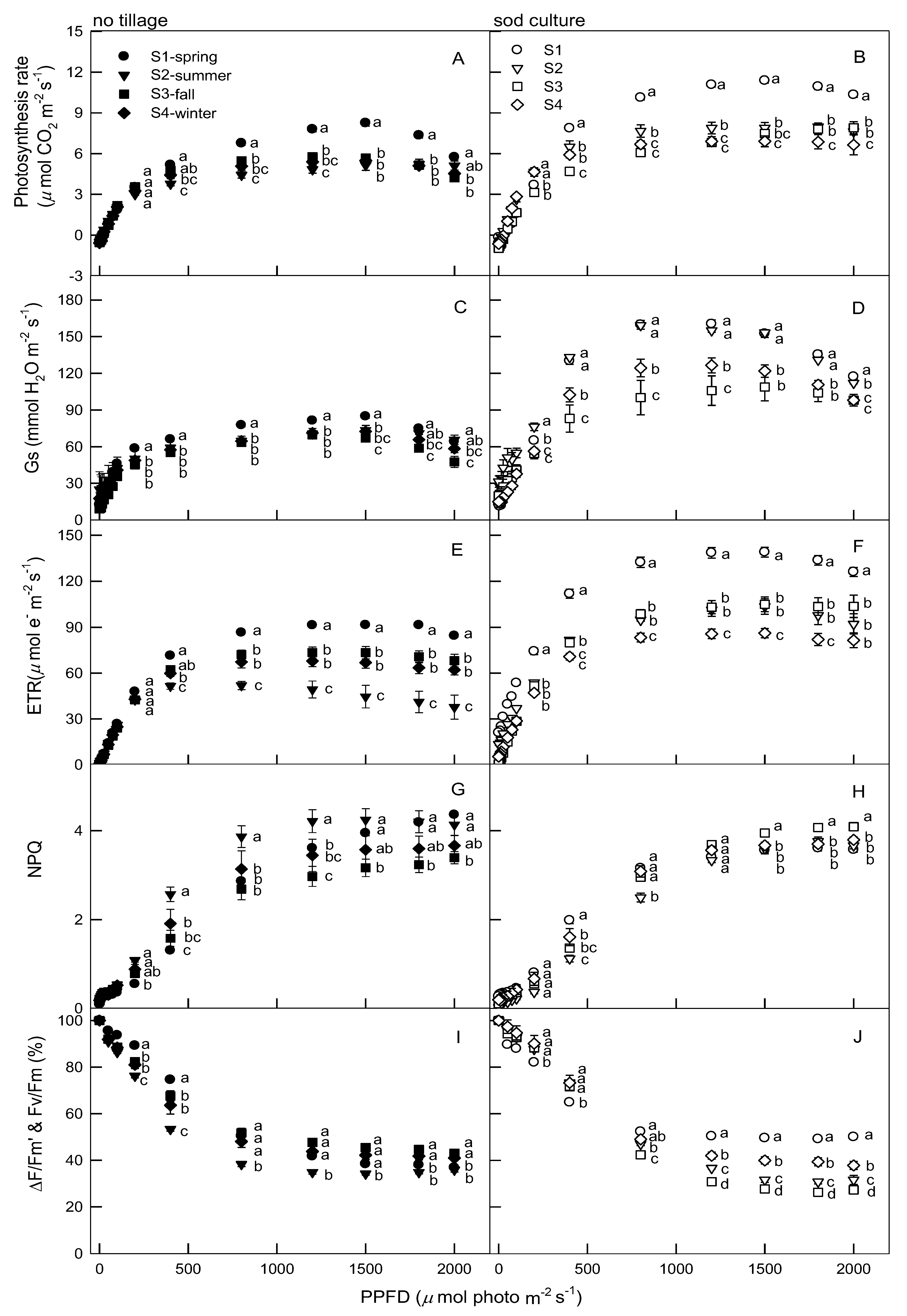
3.1. Trends in Citrus Photosynthetic Parameters under Varied Light Intensities, Tillages, and Seasons
3.2. Changes in Citrus Photosynthetic Parameters under Varied Tillages and Seasons
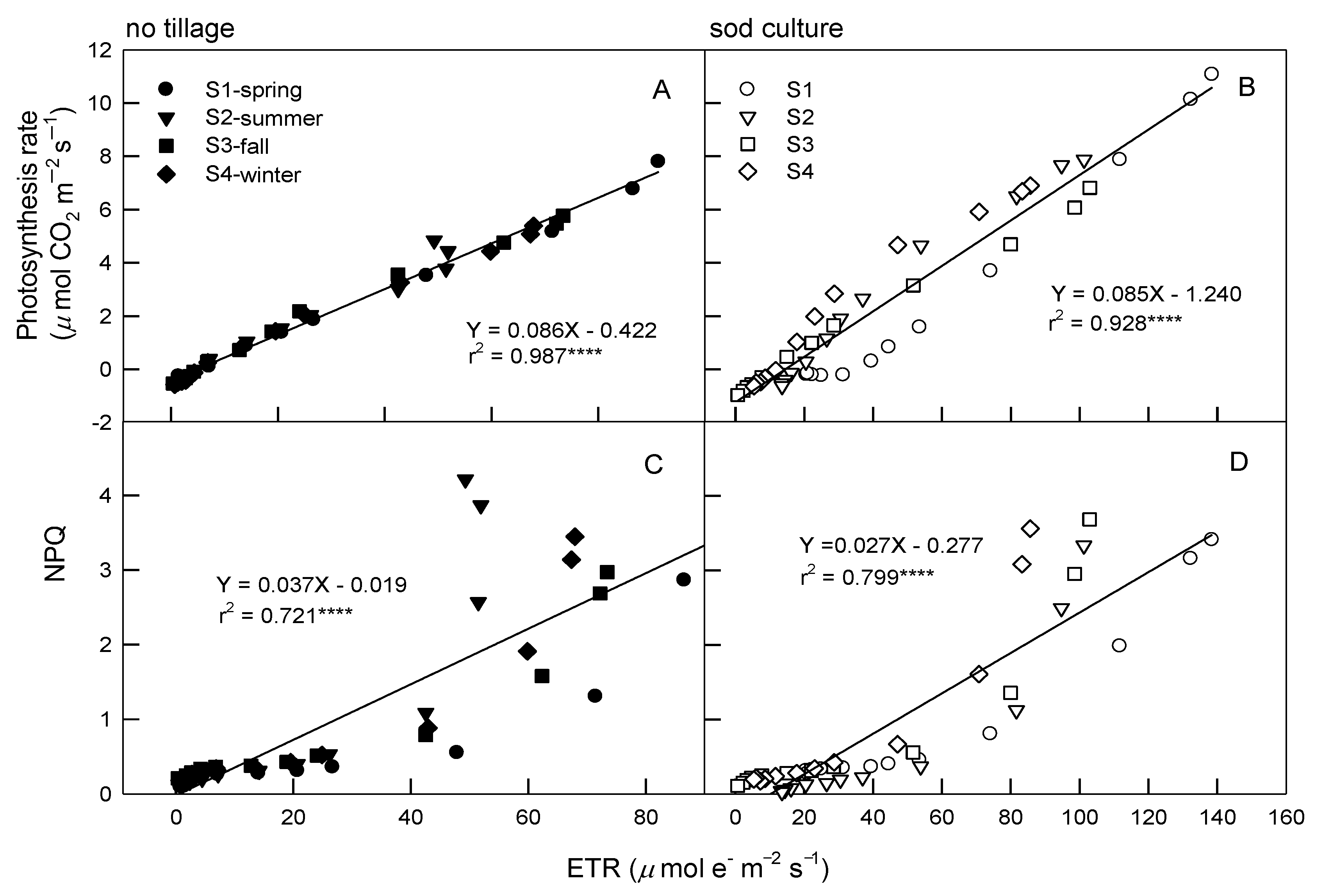
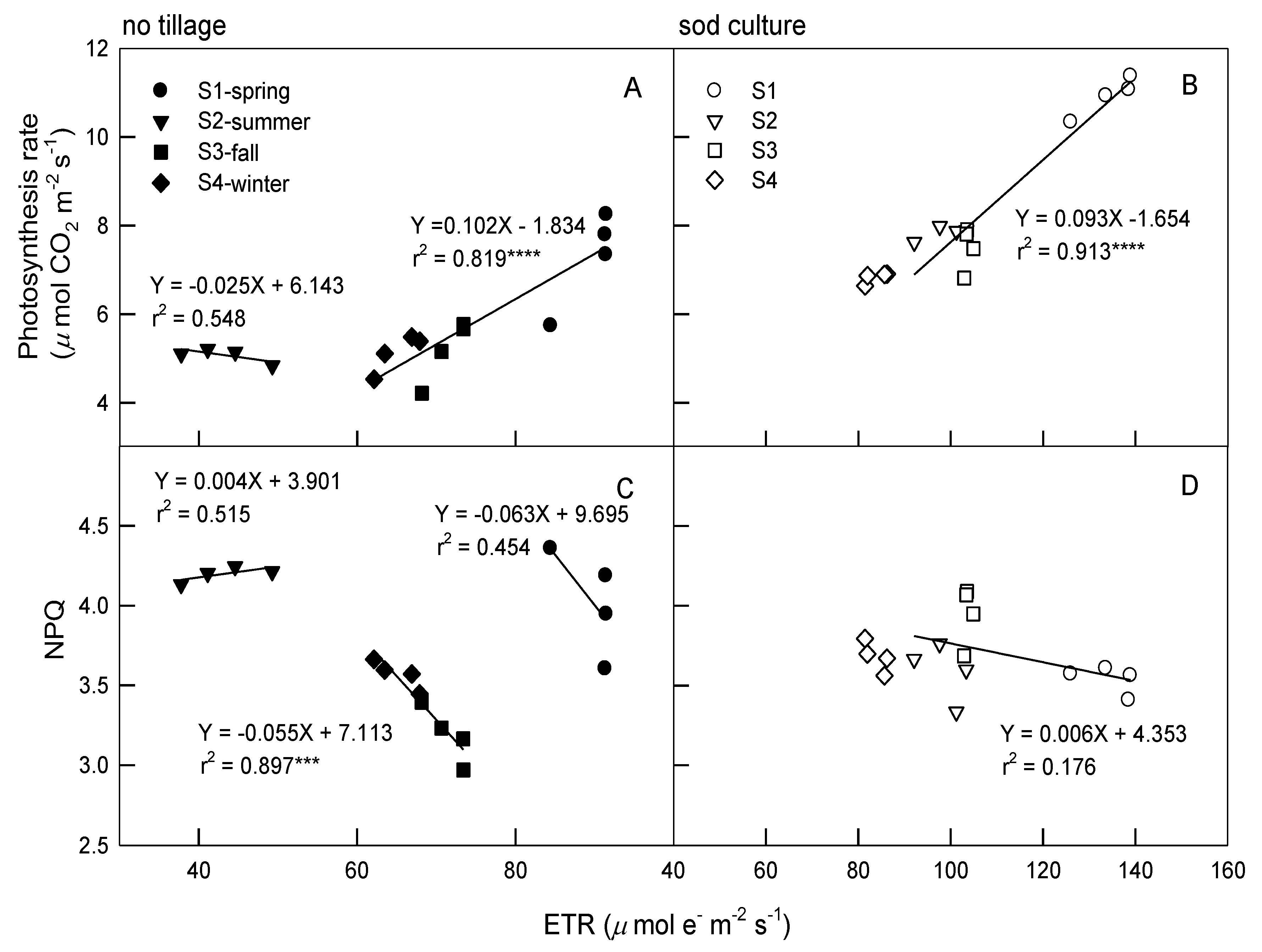
3.3. Relationships among Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ΔF/Fm’ | Actual quantum efficiency of PSII |
| CO2 | Carbon dioxide |
| ChlF | Chlorophyll fluorescence |
| Rd | Dark respiration rate of CO2 |
| ETR | Electron transport rate |
| LHC | Light-harvesting complexes |
| LCP | Light compensation point |
| Qy | Light quantum yield of CO2 |
| Amax | Maximum net assimilation of CO2 |
| Fv/Fm | Maximal quantum yield of PSII photochemistry |
| NT | No-tillage |
| Pn | Net photosynthesis rate |
| NPQ | Nonphotochemical quenching |
| PS | Photosystems |
| PPFD | Photosynthetic photon flux densities |
| Gs | Stomatal conductance |
| SC | Sod culture |
| WUE | Water use efficiency |
References
- Council of Agriculture, Executive Yuan. Citrus Reticulate Blanco × C. Sinensis Osbeck Production Survey in Taiwan. 2019. Available online: https://kmweb.coa.gov.tw/theme_data.php?theme=production_map&id=50 (accessed on 1 July 2021).
- Arai, M.; Miura, T.; Tsuzura, H.; Minamiya, Y.; Kaneko, N. Two-year responses of earthworm abundance, soil aggregates, and soil carbon to no-tillage and fertilization. Geoderma 2018, 332, 135–141. [Google Scholar] [CrossRef]
- Cerdà, A.; Rodrigo-Comino, J.; Yakupoğlu, T.; Dindaroğlu, T.; Terol, E.; Mora-Navarro, G.; Arabameri, A.; Radziemska, M.; Novara, A.; Kavian, A.; et al. Tillage Versus No-Tillage. Soil Properties and Hydrology in an Organic Persimmon Farm in Eastern Iberian Peninsula. Water 2020, 12, 1539. [Google Scholar] [CrossRef]
- Peixoto, D.S.; Silva, L.D.C.M.D.; de Melo, L.B.B.; Azevedo, R.P.; Araújo, B.C.L.; de Carvalho, T.S.; Moreira, S.G.; Curi, N.; Silva, B.M. Occasional tillage in no-tillage systems: A global meta-analysis. Sci. Total Environ. 2020, 745, 140887. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Wu, Q.-S. Effects of soil tillage and planting grass on arbuscular mycorrhizal fungal propagules and soil properties in citrus orchards in southeast China. Soil Tillage Res. 2016, 155, 54–61. [Google Scholar] [CrossRef]
- Zou, Y.-N.; Chen, X.; Srivastava, A.; Wang, P.; Xiang, L.; Wu, Q.-S. Changes in rhizosphere properties of trifoliate orange in response to mycorrhization and sod culture. Appl. Soil Ecol. 2016, 107, 307–312. [Google Scholar] [CrossRef]
- Bai, R.; Wang, Y.; Ma, Z.; Jia, Y. Research Advance on Sod Culture in Peach Orchard. J. Agric. Sci. Technol. 2017, 18, 270–284. Available online: https://www.proquest.com/scholarly-journals/research-advance-on-sod-culture-peach-orchard/docview/2202108940/se-2?accountid=8090 (accessed on 1 July 2021).
- Lin, Y.H.; Chiu, J.Y. Studies on the sod culture and the management of soil moisture for the improvement of wax apple quality. Afr. J. Biotechnol. 2019, 18, 719–725. [Google Scholar] [CrossRef]
- Deans, R.M.; Brodribb, T.J.; Busch, F.A.; Farquhar, G.D. Plant water-use strategy mediates stomatal effects on the light induction of photosynthesis. New Phytol. 2018, 222, 382–395. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.-M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2017, 231, 73–81. [Google Scholar] [CrossRef]
- Nakaji, T.; Oguma, H.; Fujinuma, Y. Seasonal changes in the relationship between photochemical reflectance index and photosynthetic light use efficiency of Japanese larch needles. Int. J. Remote Sens. 2006, 27, 493–509. [Google Scholar] [CrossRef]
- Wehr, R.; Munger, J.W.; McManus, J.B.; Nelson, D.D.; Zahniser, M.S.; Davidson, E.A.; Wofsy, S.C.; Saleska, S.R. Seasonality of temperate forest photosynthesis and daytime respiration. Nature 2016, 534, 680–683. [Google Scholar] [CrossRef]
- Sulman, B.N.; Roman, D.T.; Yi, K.; Wang, L.; Phillips, R.P.; Novick, K.A. High atmospheric demand for water can limit forest carbon uptake and transpiration as severely as dry soil. Geophys. Res. Lett. 2016, 43, 9686–9695. [Google Scholar] [CrossRef]
- Chen, C.-I.; Wang, Y.-N.; Lin, H.-H.; Wang, C.-W.; Yu, J.-C.; Chen, Y.-C. Seasonal Photosynthesis and Carbon Assimilation of Dynamics in a Zelkova serrata (Thunb.) Makino Plantation. Forests 2021, 12, 467. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Givnish, T.J. Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: Dynamic photosynthetic responses. Oecologia 2008, 155, 455–467. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant. Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murchie, E.H.; Niyogi, K.K. Manipulation of Photoprotection to Improve Plant Photosynthesis. Plant. Physiol. 2010, 155, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.-H.; Shih, F.-C.; Huang, M.-Y.; Weng, J.-H. Physiological Characteristics of Photosynthesis in Yellow-Green, Green and Dark-Green Chinese Kale (Brassica oleracea L. var. alboglabra Musil.) under Varying Light Intensities. Plants 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Forestry Bureau. Interviews of the Farmers with Green Conservation Label in Central Taiwan and Promotion to Develop Environment-Friendly Agriculture. 2019. Available online: https://conservation.forest.gov.tw/0002114 (accessed on 1 July 2021).
- Wong, S.L.; Huang, M.Y.; Chen, C.W.; Weng, J.H. Light induction of nonphotochemical quenching, CO2 fixation, and photoinhibition in woody and fern species adapted to different light regimes. Photosynthetica 2014, 52, 272–280. [Google Scholar] [CrossRef]
- Weng, J.H.; Chien, L.F.; Jiang, C.Y.; Shih, F.C.; Chen, H.Y. A comparison between yellow-green and green cultivars of four vegetable species in pigments, ascorbate, photosynthesis, energy dissipation, and photoinhibition. Photosynthetica 2011, 49, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Thornley, J.H.M. Instantaneous Canopy Photosynthesis: Analytical Expressions for Sun and Shade Leaves Based on Exponential Light Decay Down the Canopy and an Acclimated Non-rectangular Hyperbola for Leaf Photosynthesis. Ann. Bot. 2002, 89, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.P.; Ruban, A.V. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced △pH. J. Biolog. Chem. 2011, 286, 19973–19981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá, J.C.D.M.; Séguy, L.; Tivet, F.; Lal, R.; Bouzinac, S.; Borszowskei, P.R.; Briedis, C.; Dos Santos, J.B.; Hartman, D.D.C.; Bertoloni, C.G.; et al. Carbon Depletion by Plowing and its Restoration by No-Till Cropping Systems in Oxisols of Subtropical and Tropical Agro-Ecoregions in Brazil. Land Degrad. Dev. 2013, 26, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Telles, T.S.; Reydon, B.P.; Maia, A.G. Effects of no-tillage on agricultural land values in Brazil. Land Use Policy 2018, 76, 124–129. [Google Scholar] [CrossRef]
- Lachapelle, P.-P.; Shipley, B. Interspecific prediction of photosynthetic light response curves using specific leaf mass and leaf nitrogen content: Effects of differences in soil fertility and growth irradiance. Ann. Bot. 2012, 109, 1149–1157. [Google Scholar] [CrossRef] [Green Version]
- Lang, Y.; Wang, M.; Zhang, G.C.; Zhao, Q.K. Experimental and simulated light responses of photosynthesis in leaves of three tree species under different soil water conditions. Photosynthetica 2013, 51, 370–378. [Google Scholar] [CrossRef]
- Chen, C.-I.; Wang, Y.-N.; Yu, J.-C. Diurnal and Seasonal CO2 Assimilation by Four Plantation Species in Taiwan. For. Sci. 2018, 65, 68–76. [Google Scholar] [CrossRef]
- O’Leary, B.M.; Lee, C.P.; Atkin, O.K.; Cheng, R.; Brown, T.B.; Millar, A.H. Variation in Leaf Respiration Rates at Night Correlates with Carbohydrate and Amino Acid Supply. Plant. Physiol. 2017, 174, 2261–2273. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.G.; Dukes, J.S. Plant respiration and photosynthesis in global-scale models: Incorporating acclimation to temperature and CO2. Glob. Chang. Biol. 2012, 19, 45–63. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.-G.; Yoon, T.-M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Eyland, D.; van Wesemael, J.; Lawson, T.; Carpentier, S. The impact of slow stomatal kinetics on photosynthesis and water use efficiency under fluctuating light. Plant. Physiol. 2021, 186, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, N.; Arena, C.; De Santo, A.V. Temperature response of photosynthesis, excitation energy dissipation and alternative electron sinks to carbon assimilation in Beta vulgaris L. Environ. Exp. Bot. 2006, 55, 248–257. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Esteban, R.; Fernandez-Marin, B.; Hernandez, A.; Jiménez, E.T.; León, A.; García-Mauriño, S.; Silva, C.D.; Dolmus, J.R.; Dolmus, C.M.; Molina, M.J.; et al. Salt crystal deposition as a reversible mechanism to enhance photoprotection in black mangrove. Trees 2012, 27, 229–237. [Google Scholar] [CrossRef]
- Molina-Bravo, R.; Arellano, C.; Sosinski, B.R.; Fernandez, G.E. A protocol to assess heat tolerance in a segregating population of raspberry using chlorophyll fluorescence. Sci. Hortic. 2011, 130, 524–530. [Google Scholar] [CrossRef]
- La Porta, N.; Bertamini, M.; Nedunchezhian, N.; Raddi, P.; Muthuchelian, K. Photoinhibition of photosynthesis in needles of two cypress (Cupressus sempervirens) clones. Tree Physiol. 2005, 25, 1033–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schansker, G.; Toth, S.Z.; Strasser, R.J. Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: The qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Zulfugarov, I.S.; Ham, O.-K.; Mishra, S.R.; Kim, J.-Y.; Nath, K.; Koo, H.-Y.; Kim, H.-S.; Moon, Y.-H.; An, G.; Lee, C.-H. Dependence of reaction center-type energy-dependent quenching on photosystem II antenna size. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 773–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-W.; Lin, K.-H.; Lee, M.-C.; Peng, Y.-L.; Chou, T.-Y.; Chang, Y.-S. Using Chlorophyll Fluorescence and Vegetation Indices to Predict the Timing of Nitrogen Demand in Pentas lanceolata. Korean J. Hortic. Sci. Technol. 2015, 33, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Li, J.; Fan, Y.; Xu, S.; Zhan, Z. High Temperature Effects on Photo-Synthesis, PSII Functionality and Antioxidant Activity of Two Festuca arundinacea Cultivars with Different Heat Susceptibility. Bot. Stud. 2006, 47, 61–69. Available online: https://www.semanticscholar.org/paper/High-Temperature-Effects-on-Photosynthesis%2C-PSII-of-Cui-Li/3786d1211bf37d20f0ee0365098f733592f59c58 (accessed on 1 July 2021).
- Laza-Martínez, A.; Fernández-Marín, B.; García-Plazaola, J.I. Rapid colour changes in Euglena sanguinea (Euglenophyceae) caused by internal lipid globule migration. Eur. J. Phycol. 2018, 54, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Hirotsu, N.; Makino, A.; Yokota, S.; Mae, T. The Photosynthetic Properties of Rice Leaves Treated with Low Temperature and High Irradiance. Plant. Cell Physiol. 2005, 46, 1377–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| F-Value and Significance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variance | Pn | Gs | Slope | ETR | NPQ | ΔF/Fm’ (%) | Fv/Fm | Rd | Qy | LCP | Amax |
| Tillage (T) | 157.19 | 380.17 | 55.47 | 103.77 | 1.23 | 9.3 | 22.21 | 1.78 | 20.18 | 7.96 | 83.76 |
| **** | **** | **** | **** | NS | ** | **** | NS | **** | ** | **** | |
| Season (S) | 17.11 | 19.36 | 5.33 | 23.14 | 1.48 | 29.11 | 7.2 | 19.71 | 63.55 | 6.02 | 38.69 |
| **** | **** | ** | **** | NS | **** | *** | **** | **** | ** | **** | |
| T × S | 4.82 | 0.97 | 3.74 | 3.87 | 11.57 | 53.81 | 3.79 | 1.91 | 43.85 | 4.87 | 2.9 |
| ** | NS | * | * | **** | **** | * | NS | **** | ** | NS | |
| Photosynthetic Parameters | No Tillage (NT) | Sod Culture (SC) | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Summer | Fall | Winter | Spring | Summer | Fall | Winter | |
| Rd (µmol CO2 m−2 s−1) | 0.44 bc | 0.49 bc | 0.85 a | 0.71 a | 0.38 c | 0.64 b | 0.95 a | 0.78 a |
| Qy (CO2/PPFD) | 0.02 c | 0.03 b | 0.03 b | 0.03 b | 0.02 c | 0.03 b | 0.03 b | 0.06 a |
| LCP (µmol PPFD m−2 s−1) | 20.18 b | 16.84 b | 20.00 b | 21.14 b | 23.65 b | 19.13 b | 36.38 a | 21.21 b |
| Amax (µmol CO2 m−2 s−1) | 8.15 b | 5.21 d | 5.82 c | 5.61 d | 11.38 a | 8.10 b | 7.96 bc | 6.84 c |
| Fv/Fm | 0.76 b | 0.79 a | 0.74 c | 0.75 c | 0.79 a | 0.79 a | 0.79 a | 0.76 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-I.; Lin, K.-H.; Huang, M.-Y.; Yang, C.-K.; Lin, Y.-H.; Hsueh, M.-L.; Lee, L.-H.; Wang, C.-W. Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities. Agronomy 2021, 11, 1805. https://doi.org/10.3390/agronomy11091805
Chen C-I, Lin K-H, Huang M-Y, Yang C-K, Lin Y-H, Hsueh M-L, Lee L-H, Wang C-W. Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities. Agronomy. 2021; 11(9):1805. https://doi.org/10.3390/agronomy11091805
Chicago/Turabian StyleChen, Chung-I., Kuan-Hung Lin, Meng-Yuan Huang, Chih-Kai Yang, Yu-Hsiu Lin, Mei-Li Hsueh, Li-Hua Lee, and Ching-Wen Wang. 2021. "Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities" Agronomy 11, no. 9: 1805. https://doi.org/10.3390/agronomy11091805
APA StyleChen, C.-I., Lin, K.-H., Huang, M.-Y., Yang, C.-K., Lin, Y.-H., Hsueh, M.-L., Lee, L.-H., & Wang, C.-W. (2021). Photosynthetic Physiology Comparisons between No Tillage and Sod Culture of Citrus Farming in Different Seasons under Various Light Intensities. Agronomy, 11(9), 1805. https://doi.org/10.3390/agronomy11091805







