Glucose Induces Thylakoid Formation and Upregulates Green Pigment Contents in Complete Dark Culture of the Angiosperm Pachiramacrocarpa
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials, Cultural Practices, and Glucose Treatments
2.2. Determination of Total Green Pigments
2.3. Ultrastructure of the Thylakoid System
3. Results
3.1. Color of P. macrocarpa Leaves under Dark Culture
3.2. Green Ratio of Newly Developed Leaves of P. macrocarpa Plants Grown in Darkness
3.3. Green Pigments in Pre-Illuminated Mature and Young Leaves of P. macrocarpa
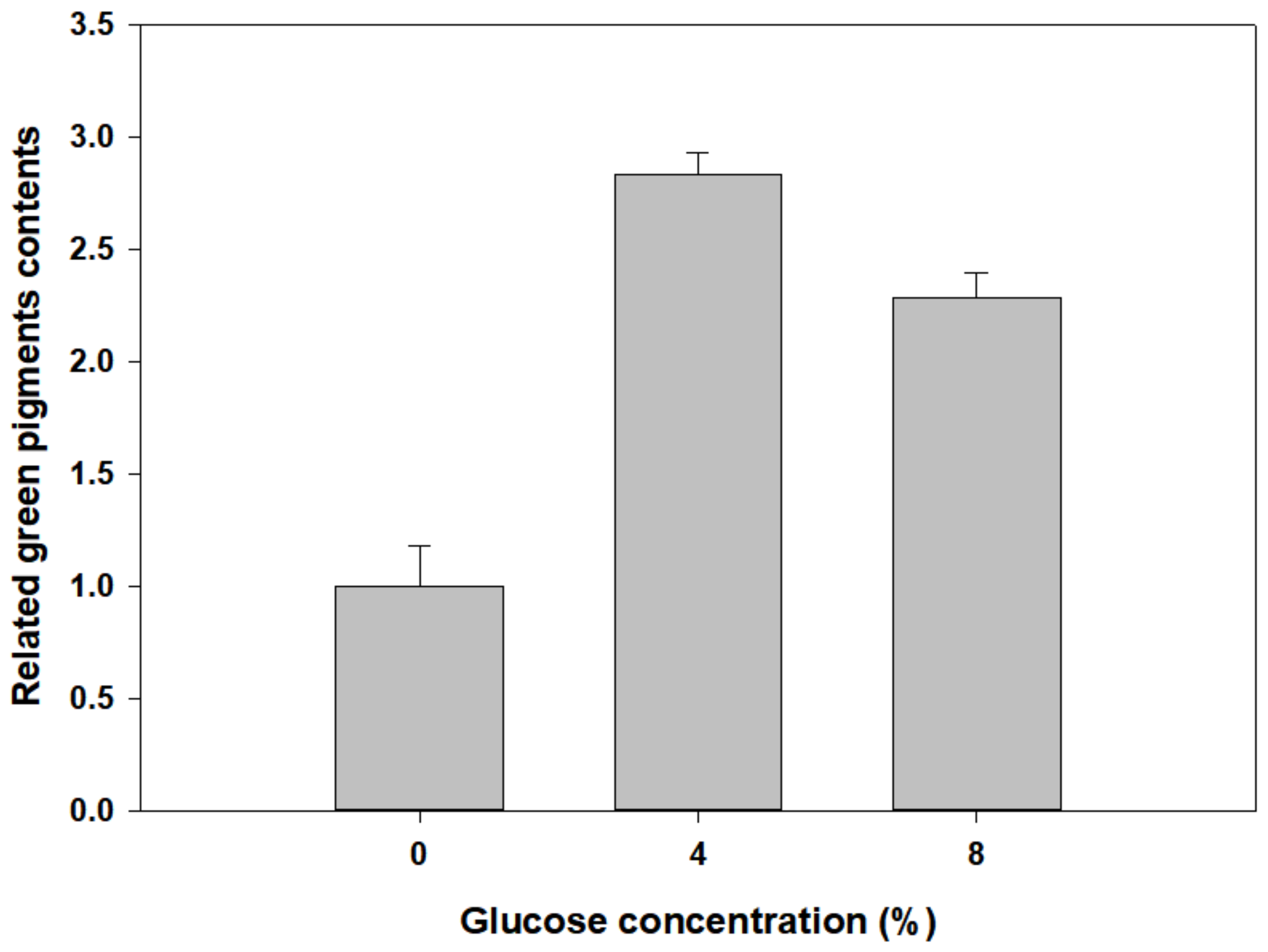
3.4. Green Pigment Contents of Newly Developed Leaves of P. macrocarpa
3.5. Budding Ratios of Newly Developed Leaves of P. macrocarpa in Dark Culture
3.6. Ultrastructure and Morphology of the Thylakoid System in Mesophyll Cells from Newly Developed Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| Chl | Chlorophyll |
| Chlide | Chlorophyllide |
| ChlP | Chloroplast |
| CLH | Chlorophyllase |
| DPOR | Dark protochlorophyllide oxidoreductase |
| LPOR | Light-dependent Pchlide oxidoreductase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Pchlide | Protochlorophyllide |
| PLB | Prolamellar body |
| PmCLH | Pachira macrocarpa CLH |
| PYL8 | Pyrabactin resistance-like 8 |
| SDS | Sodium dodecylsulfate |
| TEM | Transmission electronic microscopy |
References
- Heyes, D.J.; Ruban, A.V.; Hunter, C.N. Protochlorophyllide oxidoreductase: “Dark” reactions of a light-driven enzyme. Biochemistry 2003, 42, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, C.; El Bakkouri, M.; Buhr, F.; Muraki, N.; Nomata, J.; Kurisu, G.; Fujita, Y.; Reinbothe, S. Chlorophyll biosynthesis: Spotlight on protochlorophyllide reduction. Trends Plant Sci. 2010, 15, 614–624. [Google Scholar] [CrossRef]
- Breznenová, K.; Demko, V.; Pavlovič, A.; Gálová, E.; Balážová, R.; Hudák, J. Light-independent accumulation of essential chlorophyll biosynthesis- and photosynthesis-related proteins in Pinus mugo and Pinus sylvestris seedlings. Photosynthetica 2010, 48, 16–22. [Google Scholar] [CrossRef]
- Masuda, T.; Takamiya, K. Novel insights into the enzymology, regulation and physiological functions of light-dependent protochlorophyllide oxidoreductase in angiosperms. Photosynth. Res. 2004, 81, 1–29. [Google Scholar] [CrossRef]
- Schoefs, B.; Franck, F. Protochlorophyllide reduction: Mechanisms and evolution. Photochem. Photobiol. 2003, 78, 543–557. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Häusler, R.E.; Heinrichs, L.; Schmitz, J.; Flügge, U. How sugars might coordinate chloroplast and nuclear gene expression during acclimation to high light intensities. Mol. Plant 2014, 7, 1121–1137. [Google Scholar] [CrossRef]
- Lee, H.N.; Lee, K.H.; Kim, C.S. Abscisic acid receptor PYRABACTIN RESISTANCE-LIKE 8, PYL8, is involved in glucose response and dark-induced leaf senescence in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 463, 24–28. [Google Scholar] [CrossRef]
- Heinrichs, L.; Schmitz, J.; Flügge, U.I.; Häusler, R.E. The mysterious rescue of adg1-1/tpt-2—An Arabidopsis thaliana double mutant impaired in acclimation to high light—By exogenously supplied sugars. Front. Plant Sci. 2012, 3, 265. [Google Scholar] [CrossRef][Green Version]
- Packer, N.; Adamson, H. Incorporation of 5-aminolevulinic acid into chlorophyll in darkness in barley. Physiol Plant 1986, 68, 222–230. [Google Scholar] [CrossRef]
- Walmsley, J.; Adamson, H. Gabaculine inhibition of chlorophyll synthesis in light and darkness in intact barley (Hordeum vulgare) seedlings. Plant Sci. 1990, 68, 65–70. [Google Scholar] [CrossRef]
- Chen, C.M.; Chao, P.Y.; Huang, M.Y.; Yang, J.H.; Lin, K.H.; Yang, C.M. Chlorophyllase activity in green and non-green tissues of variegated plants. S. Afr. J Bot. 2012, 81, 44–49. [Google Scholar] [CrossRef]
- Chen, C.M.; Yang, J.H.; Liu, C.H.; Lin, K.H.; Yang, C.M. Molecular, structural, and phylogenetic characterization of two chlorophyllase isoforms in Pachira macrocarpa. Plant Syst. Evol. 2014, 300, 633–643. [Google Scholar] [CrossRef]
- Johnson, C.M.; Stout, P.R.; Broyer, T.C.; Carlton, A.B. Comparative chlorine requirements of different plant species. Plant Soil 1957, 8, 337–353. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedelman, P.E. Determination of accurate extraction and simultaneously equation for assaying chlorophyll a and b extracted with different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Rook, F.; Hadingham, S.A.; Bevan, M.W. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 2006, 29, 426–434. [Google Scholar] [CrossRef]
- Humplík, J.F.; Turečková, V.; Fellner, M.; Bergougnoux, V. Spatio-temporal changes in endogenous abscisic acid contents during etiolated growth and photomorphogenesis in tomato seedlings. Plant Signal Behav. 2015, 8, e1039213. [Google Scholar] [CrossRef]
- Arenas-Huertero, F.; Arroyo, A.; Zhou, L.; Sheen, J.; León, P. Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 2000, 14, 2085–2096. [Google Scholar]
- Matsoukas, I.G. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front. Genet. 2014, 5, 218. [Google Scholar] [CrossRef]
- Eichacker, L.; Paulsen, H.; Rüdiger, W. Synthesis of chlorophyll a regulates translation of chlorophyll a apoproteins P700, CP47, CP43 and D2 in barley etioplasts. Eur. J. Biochem. 1992, 205, 17–24. [Google Scholar] [CrossRef]
- Katalin, S.; Beáta, V.; Éva, H.; Béla, B. Etiolation symptoms in sunflower (Helianthus annuus) cotyledons partially covered by the pericarp of the achene. Ann. Bot. 2007, 99, 857–867. [Google Scholar]
- Solymosi, K.; Bóka, K.; Böddi, B. Transient etiolation: Protochlorophyll(ide) and chlorophyll forms in differentiating plastids of closed and breaking leaf buds of horse chestnut (Aesculus hippocastanum). Tree Physiol. 2006, 26, 1087–1096. [Google Scholar] [CrossRef]
- Laetsch, W.M.; Price, I. Development of the dimorphic chloroplasts of sugar cane. Am. J. Bot. 1969, 56, 77–87. [Google Scholar] [CrossRef]
- Brzezowski, P.; Richter, A.S.; Grimm, B. Regulation and function of tetrapyrrole biosynthesis in plants and algae. Biochim. Biophys. Acta 2015, 1847, 968–985. [Google Scholar] [CrossRef]
- Pribil, M.; Labs, M.; Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014, 65, 1955–1972. [Google Scholar] [CrossRef]
- Rast, A.; Heinz, S.; Nickelsen, J. Biogenesis of thylakoid membranes. Biochim. Biophys. Acta. 2015, 1847, 821–830. [Google Scholar] [CrossRef]
- Sundqvist, C.; Dahlin, C. With chlorophyll pigments from prolamellar bodies to light-harvesting complexes. Physiol. Plant 1997, 100, 48–759. [Google Scholar] [CrossRef]
- Philippar, K.; Geis, T.; Ilkavets, I.; Oster, U.; Schwenkert, S.; Meurer, J.; Soll, J. Chloroplast biogenesis: The use of mutants to study the etioplast-chloroplast transition. Proc. Natl. Acad. Sci. USA 2007, 104, 678–683. [Google Scholar] [CrossRef]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem. Sci. 2001, 26, 310–317. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.C. Sugars as signaling molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Q.; Xiong, H.; Wang, J.; Chen, S.; Yang, Z.; Dai, S. Proteomic insight into the response of Arabidopsis chloroplasts to darkness. PLoS ONE 2016, 11, e0154235. [Google Scholar] [CrossRef]
- Chi, W.; Sun, X.; Zhang, L. Intracellular signaling from plastid to nucleus. Annu. Rev. Plant Biol. 2013, 64, 559–582. [Google Scholar] [CrossRef]
- Nott, A.; Jung, H.S.; Koussevitzky, S.; Chory, J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006, 57, 739–759. [Google Scholar] [CrossRef]
- Woodson, J.D.; Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohnishi, A.; Sasaki, D.; Fujii, S.; Iwase, A.; Sugimoto, K.; Masuda, T.; Wada, H. Shoot removal induces chloroplast development in roots via cytokinin signaling. Plant Physiol. 2017, 173, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Neff, M.M.; Hong, S.W.; Zhang, H.; Deng, X.W.; Xiong, L. Integration of light and abscisic acid signaling during seed germination and early seedling development. Proc. Natl. Acad. Sci. USA 2008, 105, 4495–4500. [Google Scholar] [PubMed]
- Gangappa, S.N.; Botto, J.F. The multifaceted roles of HY5 in plant growth and development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chi, W.; Sun, X.; Feng, P.; Guo, H.; Li, J.; Lin, R.; Lu, C.; Wang, H.; Leister, D.; et al. Convergence of light and chloroplast signals for de-etiolation through ABI4–HY5 and COP1. Nat. Plants 2016, 2, 16066. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-C.; Lin, K.-H.; Huang, M.-Y.; Yang, C.-M. Glucose Induces Thylakoid Formation and Upregulates Green Pigment Contents in Complete Dark Culture of the Angiosperm Pachiramacrocarpa. Agronomy 2021, 11, 1746. https://doi.org/10.3390/agronomy11091746
Lee T-C, Lin K-H, Huang M-Y, Yang C-M. Glucose Induces Thylakoid Formation and Upregulates Green Pigment Contents in Complete Dark Culture of the Angiosperm Pachiramacrocarpa. Agronomy. 2021; 11(9):1746. https://doi.org/10.3390/agronomy11091746
Chicago/Turabian StyleLee, Tzan-Chain, Kuan-Hung Lin, Meng-Yuan Huang, and Chi-Ming Yang. 2021. "Glucose Induces Thylakoid Formation and Upregulates Green Pigment Contents in Complete Dark Culture of the Angiosperm Pachiramacrocarpa" Agronomy 11, no. 9: 1746. https://doi.org/10.3390/agronomy11091746
APA StyleLee, T.-C., Lin, K.-H., Huang, M.-Y., & Yang, C.-M. (2021). Glucose Induces Thylakoid Formation and Upregulates Green Pigment Contents in Complete Dark Culture of the Angiosperm Pachiramacrocarpa. Agronomy, 11(9), 1746. https://doi.org/10.3390/agronomy11091746







