Abstract
Brassica napus L. is a vegetable oil crop, commonly known as rapeseed (or canola). It is widely used as a source of oil and protein for food and industrial applications, but also as a remedy, and in a field of attraction or as an ornament due to its diverse flower colors. Every part of rapeseed is useful, even the waste, which could be used to feed animals, or recycled. In this review, the use of rapeseed in these applications is presented, starting with the preparation of oil and protein from the seeds, before their release in the market, to the utilization of natural unprocessed rapeseed. Progress in rapeseed exploitation for food, remedy, energy source, and industrial applications are analyzed to show variability in diverse findings, to provide insights and progressive descriptions of rapeseed usage to other scholars. Moreover, advancements in breeding for rapeseed improvement were described. In the future, strategies could be developed or improved to avoid or decrease crop losses, but also to increase interest in propagating the valuable traits of rapeseed.
Keywords:
rapeseed oil; rapeseed protein; processing; utilization; food; remediation; ornament; genetic improvement 1. Introduction
Brassicaceae comprises of many economically important species widely used as sources of oil and food, and as ornamental plants [1,2]. The youngest species, B. napus, is commonly used as an oil crop and has several common names—rapeseed, oilseed rape, and colza. A modified variant of rapeseed developed in Canada has been named “canola” or “double low” variety, for its low content in erucic acid (less than 2%) and glucosinolates (less than 30 μmol/g in meal fraction) [3]. Rapeseed originated from spontaneous hybridization between turnip rape (B. rapa) and cabbage (B. oleraceae) about 7500 years ago [4]. It is widely cultivated in many places in the world. India has been cultivating rapeseed since 4000 BC; it extended to China and Japan about 2000 years ago [5], and was naturally introduced in Europe and New Zealand where wild forms of ancestors were also found [6]. About 70 million tons (MT, yield) of rapeseed are produced per year around the world, involving 66 countries: 34 countries in Europe, 15 countries in Asia, 9 countries in America, 6 countries in Africa, and 2 countries in Oceania [7,8] (Figure 1).
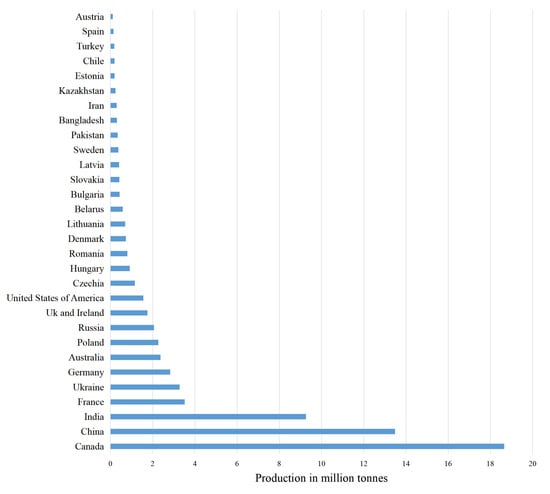
Figure 1.
Worldwide rapeseed production in some main countries in year 2019 [8].
The biggest rapeseed-producing countries in 2019/2020 were Canada (19 MT), China (13.1 MT), and India (7.7 MT); however, the European Union produced 16.83 MT of rapeseed [9]. Rapeseed production is estimated to hit 68.90 MT in 2020/2021 [10].
Rapeseed is an annual species [11]. The winter, semi–winter, and spring types differ in their cold and drought tolerances; consequently, the growing conditions are also different. Winter-type rapeseed grows well in relatively high humidity and cooler temperatures. Rapeseed cultivation needs well-drained soils with a pH ranging from 5.5 to 8.5 for optimal growth (Figure 2A). Depending on the genotype and the environment, it requires 110 to 150 days to fully grow and mature (Figure 2B). The length of mature stem varies from 120 to 150 cm (Figure 2C), and mature seeds are spherical—1.8–2.7 mm in diameter [11]—with red-brown to dark brown or black color (Figure 2D).

Figure 2.
Winter-type rapeseed: (A) Field established crop stand at a site in Huanggang, Hubei province (June 2018). (B) Blooming field in Xining, Qinghai Province (August 2018). (C) Post-harvested and dried stem and siliques. (D) Mature seeds.
Every part of rapeseed—flower, seeds, leaves, stem, and root—is used for food, remedies, cosmetics, or industrial applications. Seeds are the most important part, as they are used as oil and protein sources. Rapeseed seed oil and protein contents vary in different lines of cultivars, and other components such as glucosinolates, phenols, phytic acid, cellulose, and sugars are also found in the seeds. Known for its production of high-quality vegetable oil, rapeseed competes with other crops. It is the second-most produced oil crop in the world, after soybean, with 68.02 MT and 337.48 MT in the year 2019/2020, respectively, surpassing sunflower (53.48 MT), peanut (45.52 MT), and cottonseed (44.3 MT) [12].
Rapeseed production, as in other crops, often encounters major difficulties, because of multiple factors, such as the decrease in labor hands and farmers due to the increasing cost of labor and agricultural inputs but resulting in lower outputs, weak agricultural mechanization, yield instability due to climate variability, and weak cultivars (shatter, biotic and abiotic factors). For instance, two of the most destructive infections that weaken rapeseed crops in the world are stem rot disease, which is caused by Sclerotinia sclerotiorum; and clubroot disease, which is caused by Plasmodiophora brassicae. In China, stem rot and clubroot diseases have caused yield losses of 10–80% [13] and 20–30% [14], respectively. This review synthesizes diverse progress in the exploitation of rapeseed as biomass for food, remedy, energy source, and industrial applications. Advances in breeding for rapeseed improvement are described, and directions for future research are provided. This will provide an advanced portrait of the use of rapeseed and the current state of research for its amelioration, in order to provide insights and expose existing gaps.
2. Rapeseed Seeds Processing
Rapeseed is mainly known as a source of edible and industrial oil, as well as protein. Multiple extraction methods have been tested, and their variation affects oil and protein yield and quality, notably the usage of solvents, temperature, pressure, and the time of processing. However, some of these methods have not been tested at an industrial level. One of the most common oil extraction methods is with a solvent (mostly hexane). In brief, seeds are heated for softening, flaked to burst cell walls, and cooked to promote cell disruption, before compression to release the oil, leaving the rest of the seeds to form a protein cake. Residual oil is then extracted using the solvent, which filters the cake and removes the oil. The solvent is removed from the cake and the oil, which undergo refining and processing stages before their release in the market (Figure 3) [15,16].
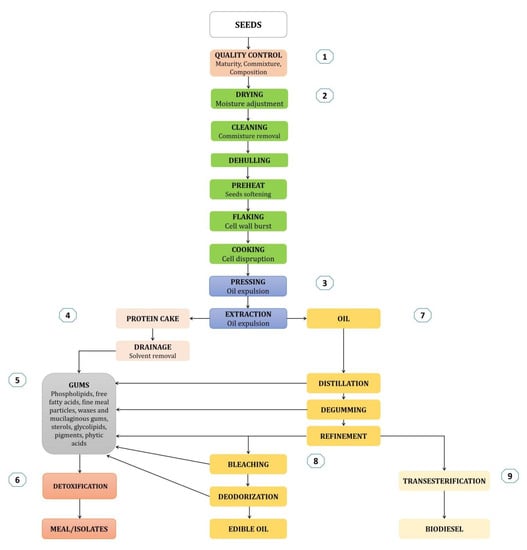
Figure 3.
From seeds to processed oil and protein. The chart was built in reference to Unger [15] and the Canola Council of Canada [16]. Each color indicates a different stage of processing: (1) quality control, (2) seed pre-treatment, (3) oil release, (4–6) protein cake processing, (7–8) edible oil refinement, (9) biodiesel preparation.
This review focuses on advancements in processing that have emerged in the last years, highlighting especially the variation in oil/protein yield and quality, from the selection of cultivar and the time of seed-sowing, to the choice of extraction method. Table 1 summarizes the rapeseed oil extraction methods, showing the advantages and limitations of each method. In a recent study that compared yield, oil, and protein from different cultivars sown at different periods of the year, it was revealed that the cultivar sown in mid-autumn displayed the best performance in grain yield, oil productivity, and oleic acid content [17].

Table 1.
Comparative study of rapeseed oil extraction methods.
2.1. Hexane Free Oil Extraction
Some alternatives to the usage of hexane in oil extraction have been proposed, due to concerns regarding the impact of rapeseed oil production and processing on human health and the environment, since beneficial antioxidants, phytosterols, and phenolic compounds might be partially lost in the extraction with hexane [40]. Other methods were reviewed by Gaber et al. [19], using enzymes (aqueous enzyme-assisted extraction), gas (carbon dioxide or propane), heat (microwave-assisted extraction), or ultrasound, which might offer more advantages: safer for human consumption, less time-consuming, better oxidative stability and shelf-life, preserved or improved beneficial oil compounds which could be reduced or removed by the solvent extraction, and even had a better taste and odor. However, these methods need to prove their performance on an industrial scale and from an economical point of view, probably the reason why solvent extraction is still maintained as one of the common extraction methods for rapeseed oil.
Confortin et al. [17] demonstrated that using hexane for oil extraction offered low cost and gave a higher oil yield, in comparison to the supercritical fluid extraction with CO2. Major oil contents are obtained during the pressing process, and hexane is only used to extract minor residual oil in the flake, and even after the extraction, the oil is separated from hexane before mixing it with the oil obtained from pressing. In addition, hexane has low toxicity and is digestible at less than 2% of total food. There is no evidence of any risk in consuming foods with a residual trace of hexane [18].
2.2. Aqueous Extraction
Aqueous extraction is a proposed method to preserve both oil and protein high quality. For instance, aqueous extraction of rapeseed oil (using a slurry composed of 1.5:10 water–kernel) gave an oil of high quality (low acid and peroxide content) and high yield (94.73%), and the water content of 25% could be easily removed by drying and no waste was left [20]. Fetzer et al. [21] investigated the effect of various parameters in aqueous extraction of protein that would affect the protein yield and found the best protein yield at a NaCl concentration of 0.25 M, with a temperature of 20 °C, and with the usage of enzyme protease. Other parameters such as solid/liquid ration, extraction time, and pH displayed no difference among tested samples, even in alkaline conditions, and one-hour treatment was preferred. pH, temperature, and salt treatments have a perceptible impact on protein yield. Gerzhova et al. [22] demonstrated that alkaline pH was better in extractability, but without salt addition. However, a pH value higher than 10 was the best condition to get the highest protein extraction yield, and salt addition improved the solubility of proteins, notably the cruciferin. Moreover, Akbari and Wu [23] suggested a treatment that consisted of an acidic washing at pH 4, an alkaline extraction at pH 12.5, an isoelectric precipitation at pH 4, and an ultrafiltration to get a maximum protein content and cruciferin and napin yield.
2.3. Heat Treatments
Heat treatments affected the final products. In fact, during the oil deodorization process, the oil is submitted to a high-temperature treatment, which promotes the reduction of linoleic acids and their transformation into trans–fatty acid isomers [24], which could extend the frying time [25]. Moreover, the refined oil might lose its beneficial nutrients, such as vitamins (notably tocopherols), essential fatty acids, and antioxidants [26,27,28], but it also had a decreased phenol, β–carotene, and oxygen radical absorbance, while the harmful compounds, such as acid, peroxide, and p–anisidine were increased [29]. Furthermore, heat could denature proteins and decrease their solubility. The usage of heat treatment is thus not beneficial to obtain high oil and protein yields with good quality, and could decrease the beneficial compounds and increase the harmful ones. This suggests that cold-pressed oil might be the best choice since no harmful solvents for extraction and no heat treatments are used. Nevertheless, it has been reported that meal from cold press extraction had a higher anti-nutritive glucosinolates level, compared to that of the solvent extraction [30].
2.4. Chemical-Free Extraction for Better Protein Quality
To avoid the usage of chemicals for the extraction and concentration of proteins, and to eliminate lignocellulosic fiber, multiple techniques were proposed [31,32,33]. For instance, dry fractionation, which consisted of a combination of mechanical separation (milling) with electrostatic (particle charge) or turbo (density) separation was reported to enrich protein and phenolic fractions from plant materials due to the lignin removal [34,35,36]. Sieving was also a proposed technique for fractionation. For instance, by using 250 to 600 μm sieve, an increase in crude protein in particles, which resulted from <250 to 355 μm sieve, and a decrease in total dietary fiber in particles, which resulted from 250 to 355 μm sieve in the meal were observed in black- and yellow-seeded B. napus and B juncea. These meals were suggested to be the replacement of soy meal in broiler chicken since no significant effect on growth performance was observed [37]. The dark color and bitter taste of isolates, which are caused by phenolic compounds, could be removed by ultrafiltration/diafiltration at pH 12 and precipitation at pH 3.5 [38], resulting in a decrease of phenolic acids and condensed tannins. Isolates might have a light color and no flavor, which are more suitable for feeding. Recently, it was discovered that the bitter taste of isolates was caused by a flavonoid compound named kaempferol 3–O–(2‴–O–Sinapoyl–β–sophoroside) at a low concentration of 3.4 μmol/L [39]. Knowing this, the bitter taste of isolates can now be removed.
2.5. Preparing Biodiesel from Rapeseed
The standardization of rapeseed oil as a fuel was first made 20 years ago [41]. Biodiesel is produced by the transesterification of animal or vegetable oils. In the presence of a catalyst, the oil reacts with alcohol, which is usually methanol [42]. The biodiesel output depends on the amount of free fatty acids, the type and ratio of alcohol, the catalyst used, and the processing time and temperature. For instance, Kai et al. [43] tested a method of producing rapeseed biodiesel with dimethyl carbonate, by using active sodium methoxide as a catalyst. The conversion rate was reported to be greater than 96% at 65 °C, with a processing time of two hours, a dimethyl carbonate and oil ratio of 3:1, and 2.0 wt% of sodium methoxide. Transesterification has been suggested to be the best procedure to produce rapeseed biodiesel because it is cheaper and less problematic for engines [44]. However, the use of a catalyst is a limitation due to its sensitivity to water, which decreases its performance, and the presence of free fatty acid in the feedstock, which promotes soap production, increasing its viscosity and complicating its purification [45,46]. Fortunately, an alternative method has been proposed in rapeseed biodiesel production, by using carboxylate ester treatments, to avoid issues related to catalyst utilization [47].
3. Seed Oil and Protein-Processing Products
Rapeseed has been cultivated since ancient times in India, and in the Middle Ages, in Europe. Due to its low cost, the oil from rapeseed was used to make soap, enlighten lamps (odorless and slow–burning), lubricate engines, and cook meals [48]. Nowadays, multiple utilizations of rapeseed have emerged and they are beneficial for health, environment, and economy.
3.1. Edible Oil
3.1.1. Biochemical Properties
Rapeseed oil is mainly composed of triacylglycerol; it contains a low content of saturated fatty acids (SFA, ~7%), compared to usual cooking oil, such as peanut (17%), olive and soybean (15%), corn (13%), and sunflower (12%) [49]. The biochemical composition of rapeseed with five other cooking oils is presented in Table 2. High SFA usually has a higher melting point and poor solubility, which leads to the formation of sediments, and then affects the oil clarity and digestibility [50]. Rapeseed oil contains high monounsaturated fatty acids (MUFA, ~59 to 62% of oleic acid), and polyunsaturated fatty acids (PUFA, ~19% of linoleic acid omega–6, and ~9–11% of alpha–linolenic acid omega–3) [49,51,52,53]. Rapeseed oil also contains low transfats, which puts it in high competition with other oil crops. Besides, high levels of vitamins are also observed, such as vitamin E (100 g of oil contains ~22 mg of alpha–tocopherol,~27 mg of gamma–tocopherol, and~1 mg of delta–tocopherol) and Vitamin K. Tocopherols are natural antioxidants and have a low evaporation and degradation rate at a high temperature [54]; it was reported that about 30 mg/100 g of vitamin E were still maintained after frying, which makes rapeseed oil much better than other vegetable oils [55]. Tocopherols could protect the PUFA in living cells, but unfortunately, its content is reduced after the oil purification, especially during the deodorization [56]. Similarly, the amount of sterols is reduced during these processes [57]. Rapeseed oil is rich in phytosterols, particularly brassicasterol, which is in high amounts in Brassica oils. The products of oxidized phytosterols were reported to be harmful since they were mutagenic and could originate free radicals, which could promote inflammation, affect metabolism, hormones activity, and cell viability at high concentrations [58,59,60]. Phenolics are also present in rapeseed oil, which are in majority of canolol (~59%) and sinapine (~2%), and other minor unidentified phenolics [61].

Table 2.
Biochemical composition of common vegetable cooking oil [49,62].
3.1.2. Health Benefits of Rapeseed Oil
The fatty acid profile and other components present in rapeseed oil might justify its beneficial impact on human health—as suitable for patients having various diseases or just for disease prevention. Rapeseed oil has a low content of SFA, a high level of MUFA (oleic acid) and PUFA (omega–3 and omega–6), and a high content of tocopherols and phytosterols. First, SFA are essential; they can elevate the lipids level in blood. However, SFA can be naturally synthesized by the human body, so a supplement intake is useless or at least should be kept at the lowest level (<10% of total calories) [63]. Fortunately, rapeseed oil contains less than 7% of SFA, which meets the recommended intake. Unsaturated fatty acids (UFA) can lower lipid levels in the blood, and then should be taken properly. It was reported that UFA could positively affect human blood lipids compared to SFA [64]. Several studies demonstrated the good effects of rich MUFA diets on human blood lipid and glucose. For instance, it could decrease the chance to have foam cells’ formation and the development of atherosclerosis, which were induced by LDL cholesterol oxidation, and then could reduce the risk of cardiovascular disease [65,66]. Moreover, rich MUFA diets were reported to be suitable for diabetic subjects since they could improve glycemic control [67,68,69,70], blood lipids [71], and lower insulin response [72,73]. It has been affirmed that rich MUFA diets are as effective as high-carbohydrates and low-fat diets in monitoring blood glucose in diabetics [74,75]. Besides, PUFA, such as α–linoleic acid (omega–3) could reduce coronary issues and the production of inflammatory eicosanoids and cytokines (tumor necrosis factors and interleukins). Rich PUFA diets are beneficial for the immune system, heart, vision, cognition, and in healing tumoral cells [76,77,78,79]. Other studies also supported this fact, such as the reduction of cardiovascular disease which was explained by the reduction of blood pressure, with an increase of the protective blood lipoprotein [80,81,82,83]. Moreover, omega–3 could protect the kidneys [84,85,86], and the brain from stroke [87]. Ingestion of omega–3 is strongly recommended to promote good health and to prevent disease [88]. Additionally, conjugated linoleic acids (omega–6) were reported to support body fat reduction and had antidiabetogenic, anticarcinogenic, antiatherogenic, and immune–modulating properties [89]. The phytosterols in rapeseed could decrease blood cholesterol [90,91,92,93] and had an anticancer ability [94]. It was recommended to consume 2 g/day of phytosterols to effectively reduce the LDL cholesterol by 10–15% [90,91]. Tocopherols might work with other compounds to boost immunity [95] and prevent cancer [96].
Following these studies, several investigations on a rapeseed-based diet confirmed the health benefits of rapeseed oil intake on blood lipid and glucose, but also on heart health, probably due to its richness in UFA. For instance, a low level of erucic acid, a high level of UFA, and phytosterols of rapeseed oil could reduce the LDL cholesterol in the blood and prevent coronary heart disease [97,98,99]. A cardiac antiarrhythmic effect was also reported [100], and an improvement of endothelial function reducing cardiovascular risks [97]. Note that the FDA has suggested eating about 19 g of rapeseed oil daily, to reduce the risk of coronary heart disease [101]. In several studies in compliance with this FDA requirement, intake of rapeseed oil reduced total LDL and apolipoproteins cholesterol levels, which indicated improved cholesterol levels with rapeseed diet compared to other diets rich in SFA [102,103,104,105,106,107]. A lower blood glucose level was also reported in diabetic subjects who consumed rapeseed oil [105,108]. These studies indicated that rapeseed oil could improve blood lipids and glucose compared to diets with high SFA. Recent years’ discoveries reinforced these findings. Indeed, the effects of high oleic acid rapeseed oil consumption resulted in improved glycemic control and an attenuated cardiovascular risk factor in type 2 diabetes subjects [109]. Additionally, Liu et al. [110] found a reduced fat mass in a high oleic acid rapeseed intake rather than in a flax/safflower oil diet, particularly in the abdominal fat, due to the reduction of blood pressure and triglycerides. Moreover, consuming more MUFA than PUFA decreased the risk of metabolic syndrome and obesity in that study. Taken together, the rapeseed oil diet has positive effects on blood lipid and glucose, on the cardiovascular system, and on supporting fat loss.
It is clear that rapeseed oil has a lot of qualities, but despite the above-mentioned benefits, some controversies have been pointed out, notably its antioxidant and bad cholesterol-reducing activities. A study demonstrated that 10% of rapeseed oil diet decreased antioxidants, and increased bad cholesterol and blood pressure, compared to soybean oil diet, which resulted in a short lifetime in rats [111]. Another study exposed a metabolic syndrome and heart disease related to rapeseed oil consumption in overweight/obese subjects: a metabolic syndrome was found in regular consumers rather than in occasional consumers [112]. Some studies demonstrated that a higher chance of heart failure, coronary artery damage, and shorter lifetime was found in humans who consumed high UFA oils compared to the ones who consumed SFA oils [113,114]. Additionally, some inflammatory markers were found to be increased while heating rapeseed oil [115]. Given the fact that the bad effects of rapeseed oil on health are less supported due to the limited number of performed studies in comparison to those that demonstrated good effects, its beneficial effects are still convincing. However, it would be safer to perform in-depth studies investigating the other side, to comprehend in which precise case it might be harmful.
3.1.3. Multiple Usages of Edible Rapeseed Oil
The main usage of rapeseed oil is as liquid oil for frying, for salads and dressing, cooking, baking, and in the formulation of shortenings. The choice of vegetable oil for these common usages depends on the flavor, nutritional value, texture, stability, cost, and availability, while avoiding transfats as possible [56]. The word “virgin oil” is often seen in the market and this kind of oil is extracted by cold-pressing and filtration. Virgin oil is qualified as the best in terms of nutritional value and economical asset (simple processing with high selling price). The frying oils quality depends on the oxidative stability, and the amount of saturated and transfats should be at the lowest. The rapeseed “high oleic acid and low linolenic acid” (HOLL) oil has a better frying performance compared to sunflower and palm oils [56,116]. HOLL oil is more refined with a light flavor and a smooth texture, and it contains ~78% of oleic acid, ~12% of linoleic acid, and ~3% of alpha–linolenic acid. It is suitable for deep-frying, recycling, and long-term storage due to its high stability and its oxidation-resistance. HOLL oil has less acrylamide, less oxidized, and toxic compounds, so that it could be re-used for as far as 10 days [56,117]. Moreover, rapeseed oil can be blended with other oils to improve physicochemical properties. It was reported that an 80:20 blend of rapeseed and olive oil, added with 20% of palm oil was a better oil combination compared to a blend with a higher ratio of olive oil, and the fatty acid profile showed low free fatty acids and high oleic acid contents, but also a low peroxide level and a high iodine value [118], which might indicate deep-frying suitability and long-time storage. Otherwise, raw oils add an extra flavor and texture for salads and mayonnaise, and rapeseed oil enhances their nutritional value.
3.2. Inedible Oil
3.2.1. Biodiesel
Rapeseed oil was reported to have a low cold point (0 °C) and pour point (−15 °C), which was much lower than that of other feedstock [119], and which has made it more suitable for biodiesel usage. Rapeseed is the favorite oil crop for biodiesel production in Europe [120], which accounted for 50 to 70% of European biodiesel production [121], as in 2008, 66% of biodiesel came from rapeseed [122]. Rapeseed biodiesel maintains a fluid property even at low temperatures and has delayed crystal formation, making it suitable for cold climates. Moreover, rapeseed has a higher oil content and a lower iodine value (less oxidation) compared to other vegetables; for instance, 127–160 and 48 gallons per acre were obtained in rapeseed and soybean, respectively [123], with 114 and 130 of iodine value, respectively [124]. Based on these pieces of evidence, rapeseed oil generates more energy, with less chance of oxidation and deposit formation that might clog fuel pumps and injectors.
One of the main factors that degrade environmental wellness is the emission of greenhouse gas (GHG) from the transport sector. Rapeseed oil can reduce GHG (up to 90%) compared to fossil diesel [125,126]. Concrete reductions of GHG are obvious in Europe and North America with rapeseed-based biofuels, as for Canada, annual GHG was reduced by 4.4 megatons [127]. Moreover, rapeseed biodiesel is biodegradable (decompose within ~30 days) [128], and accelerates the degradation of regular diesel fuels when they are blended. It is less poisonous in water, which decreases the impact of spills in sensitive areas. Rapeseed oil is also a clean alternative for fuel, which can be blended to diesel fuels for use in aviation, ship, trucks, and heavy machinery for agriculture, forestry, mining, and construction [129]. The limitations of using biodiesel as fuel are the cost (it is relatively high compared to petroleum), storage due to its vulnerability to oxidation, and its ability to degrade some materials made of elastomer and rubber in fuel distributions [130].
3.2.2. Other Industrial Applications of Rapeseed Oil
Rapeseed oils are used in making useful tools. High erucic acid rapeseed (HEAR) oil is especially used for industrial applications. HEAR was originally produced to increase the oil yield, but also to fight disease and stress [131]. Canada developed an herbicide-tolerant cultivar: a super high erucic acid cultivar with ~66% of erucic acid contents [131]. Erucic acid was subjected to a high-temperature splitting and distillation [132], to produce erucamide (slip agent for the plastic film), and not only erucic acid and its derivatives were cationic surfactants, which could be used in detergent, for laundry softness, and other household products, but they could also be used as emollient (erucyl alcohol), food emulsifier (glyceryl tribehenate), and in producing photographic material (film and paper from silver behenate) [133]. They were also widely used in pharmaceutical products, ink, paper, textile, foam, plastics, and fuel industries’ manufacturing [124]. Rapeseed oil could also be used as a dust mask in swine barns to reduce health effects [134].
HEAR is an excellent material for cosmetics fabrication, erucic acid could improve hair substantivity [133], whereas oleic acids could soften hair and protect against dryness and linoleic acid promotes hair growth and maintains a healthy scalp [135]. Rapeseed oil can be used to manufacture creams and soaps. Soaps are produced in the cold process so that all the beneficial compounds are still maintained, and light color, dense, and creamy foams are obvious during usage. While making homemade soap, rapeseed oil can be mixed with other oils such as coconut oil, or other oils that enhance the scent [136].
Rapeseed oil pesticide was developed and used to eliminate insects by irritation, including aphids, loopers, worms, caterpillars, and mites, among others. Rapeseed oil insecticide was used as a spray and in an irrigation system and had no harmful effects on humans and the environment due to its low toxicity and rapid decomposition [137,138]. The active compounds are yet to be identified in these rapeseed pesticide oils.
3.3. Rapeseed Meal
3.3.1. Meal Composition
Rapeseed meal is the residual product from seed oil extraction. According to the growth conditions, harvest, and processing, the protein content in rapeseed meals could vary from 35 to 40%. The oil and carbohydrates (sugar, starch, and fiber) contents in rapeseed meals could also vary according to the processing, with a ratio of ~1–3.5% and ~23%, respectively [139,140]. It has been revealed that the amino acid profile is suitable for animal feeding (less lysine and high methionine and cysteine) [140]. Rapeseed meal served as an excellent co-addition to other protein sources due to this richness in methionine and cysteine [141]. It also has a good source of minerals (phosphorus and selenium) and vitamins (choline, niacin, and tocopherols). The meal from the modified variant of rapeseed contains less than 30 μmol/g of glucosinolates, some minerals, and vitamins. Cultivars with low glucosinolates content emerged in the late 1960s [142], and the trait was incorporated into low erucic acid lines to generate a rapeseed cultivar containing both low erucic acid and low glucosinolates contents [143]. Anti-nutritive compounds such as glucosinolates and tannins still subsist in these rapeseed meals, but at lower levels. In fact, not only are they harmful to health, but they negatively affect the palatability and digestibility of the meal [144], and the eggs’ weight and quality in hens [145]. Fermentation and enzymatic treatments have been established to reduce these anti–nutritive compounds in rapeseed meals, and the protein value could be increased at the same time [146,147,148,149]. For instance, a fermentation coupled with enzymatic hydrolysis decreased the glucosinolates content by 30.06%, and increased the trichloroacetic acid-soluble protein content by 81.70%, compared to a fermentation process alone [149]. Actually, various double low cultivars have been developed, as presented by Hansen et al. [150], who performed a meta–analysis showing those various double low cultivars and their non–adverse effects as a meal in animal growth performance.
3.3.2. Human Food Application
Rapeseed proteins have a good balance of amino acid profiles [151]. Thus, rapeseed meal has been qualified as a good protein source for human consumption due to its nutritional value and functionality [152,153]. Rapeseed is reported to have three to four times higher proteins than rice and wheat [154]. It had better emulsifying and foaming properties in comparison with soybean [155]. Moreover, rapeseed meal could be used as a substrate for fungi to produce enzymes. For instance, Trichoderma reesi could produce xylanase, xylosidase, cellulase, and acetyl–xylan esterase, with rapeseed meal as substrate. Some modifications might be done to protein to improve functional properties, for instance, to get a gel that is 3.5 times stronger than the protein alone [156], to improve water and oil absorption, foaming, emulsification activity, and stability of hydrolysates [157]. In addition, transglutaminase was reported to enhance the hardness, elasticity, and strength of gels at 40 °C [158]. Besides, rapeseed protein hydrolysates were used in many applications, such as in inhibition of angiotensin I converting enzyme [159,160,161], as an antioxidant [152,160,162], as an anti–hypertensive agent [163], for therapeutic protein productions as in Chinese hamster ovary cells growth promoter [164] and in meat flavor production [165].
Some powder proteins supplements based on rapeseed have been developed, such as SuperteinTM and Puratein of Burcon Nutrascience [166]. The production of these isolates did not require harsh chemicals and were reported to have better organoleptic, physical, and functional properties compared to the isolates from conventional extraction [167]. Isolexx and Vitalexx of BioExx Specialty Proteins, Ltd. are other isolates used in a variety of foods and beverages at the same range as soy proteins [168], especially to assist muscle growth in bodybuilders. The capacity of rapeseed proteins (albumin and globulin fractions, and isolate) to substitute soybean protein isolate as an emulsifier was studied by Tan et al. [169]. Globulin fractions demonstrated a higher emulsifying capacity and a higher emulsifying activity index at low pH, and droplet size was smaller or comparable at pH4 and pH7. One concern in rapeseed isolate usage in the food industry is its bitter taste, which could be removed by the discovery of its factor kaempferol 3–O–(2‴–O–Sinapoyl–β–sophoroside) [39]. Another concern is the safety of rapeseed isolate for consumption and its allergy causative property. The European Food Safety Authority Panel [170] evaluated IsolexxTM, and reported that heavy adults could take about 2.2 g isolate per kilogram of body weight per day; however, allergies have been reported in subjects who had allergic reactions to mustard intake. No nutritional drawback nor toxic effects were found at the recommended use and dosage, indicating that rapeseed protein isolate is safe for human consumption.
3.3.3. Animal Fodder
Rapeseed meals can be used to feed ruminants, poultry, fish, and crustaceans. Incorporation of rapeseed meals in animal diets implies a balance in protein ratio, which consequently has an impact on palatability and feed intake, body performance, and the production of milk, meat, or eggs.
Rapeseed meals, as protein supplements, could enhance weight in cattle [171,172,173,174]. It could be used as a substitute to barley (up to 15%), as demonstrated by Damiran and McKinnon [175], but they found no significant change in the performance of steers. Besides, the milk yield was significantly increased in cattle supplemented with rapeseed meal (compared to those fed with soybean meal) [176,177], and in a combination of rapeseed meal with wheat distillers’ dried grains with soluble [178], or as a substitution for wheat [179]. Incorporating rapeseed meals in pig diet was limited to 25% [180] since they only could tolerate 2 to 2.5 μmol/g of glucosinolates [151,181]. Rapeseed meal was incorporated in wheat, soybean, or corn-based diets, and affected feed efficiency, protein digestibility, energy value, and microbial community [37], without influencing feed intake and body weight [182]. In contrast, Lee and Woyengo [183] found that adding cold–pressed rapeseed meal into corn–soybean meal basal diet could reduce daily feed intake and growth performance. Besides, Lyu et al. [184] demonstrated that a high fiber diet in pigs negatively affected nutrient digestibility and energy value, despite a period of adaptation. Thus, dehulled rapeseed meal is a better choice. It was revealed that the dehulled rapeseed meal could improve the digestibility of phosphorus but did not alter the bodyweight of pigs [185]. Otherwise, Velayudhan et al. [186] studied the impact of incorporating rapeseed meal in sows during gestation and lactation and reported that a ratio of 300 g/kg of rapeseed meal could support sows and suckling piglet performances without negative impacts on nutrient digestibility and energy value, and with a positive impact on the sows’ gut lactic acid bacteria. In contrast to pigs, sheep could tolerate high-glucosinolate meals and could be fed with rapeseed meals, with no bad effects on the consumption of the meal, the growth performance and weight of sheep, and milk production [187,188,189]. Other studies demonstrated that weight gain in sheep and goats was obvious with the addition of rapeseed meals to their diets [190,191]. Sutton [192] investigated feeding rapeseed meals to horses and no effect was found on a feed intake up to 15%. The incorporation of rapeseed meal was beneficial for rabbits’ growth [193].
In poultry, the supplementation of rapeseed meal was beneficial for ostriches [194]. In layer chicken, rapeseed meal formulated on a digestible amino acid was found to be a cost-effective diet, and it did not negatively affect the production of eggs or feed intake [195,196,197]. Broiler chickens could be fed rapeseed meal, even with a diet formulated on digestible amino acid up to 30%, improving the average daily weight gain, without any harmful effects on health and performance [198,199]. Rapeseed meal replacement in basis diets, such as soybean meal, has been studied for poultry. It was recently reported that partial replacement of rapeseed meal did not harm chickens [200,201]. Moreover, Mnisi and Mlambo [202,203] investigated this replacement in Japanese quail diet and found that growth performance, health, and meat quality were not affected at a ratio of less or equal to 12.5%. However, feed intake decreased at 17.5% of rapeseed meal. For turkey, adding 15% of raw or fermented rapeseed meal in the diet resulted in an improved final bodyweight, a stimulated antioxidant activity, a positive effect on intestinal histomorphology [204], as well as improved meat quality in terms of antioxidant contents and fatty acid profile [148]. Choosing between yellow or black seed rapeseed meal has an impact on nutritive value. Rad-Spice et al. [205] and Kozlowski et al. [206] performed analyses on broiler chicken and turkey, respectively. In broiler chicken, a gain of body weight was found to be significantly lower in the yellow seed B. napus diet, but no difference in the feed conversion ratio was found. In turkey, no difference in apparent metabolizable energy and standardized ileal amino acid digestibility was found, as well as in feed intake and bodyweight gain. These findings suggest that both black and yellow seed rapeseed meal could effectively replace soybean meal, as long as diets are formulated on a digestible amino acid basis. Reducing seed hulls is one of the focuses of rapeseed breeding. It has been proven that yellow seed cultivars (less fiber) are more digestible [207]. Digestibility could be improved by manipulation of the seed coat development through genetic engineering [208].
Fishes and crustaceans were fed rapeseed meal, alongside other diets, and no negative performance was observed [209,210,211,212,213,214]; however, the feed ratio in shrimps should not exceed 30% to avoid growth defects and feed intake reduction due to the fiber content in rapeseed meal [215]. To sum up, rapeseed meals can be incorporated in animal diets, with the right formulation. Table 3 summarizes the rapeseed meal assays performed on animals.

Table 3.
Rapeseed meal assay in animals.
3.3.4. Protein for Bioplastic Based Materials and Cosmetics Fabrication
Due to some anti-nutritional compounds that are present in meals after oil extraction, the usage of rapeseed meal in the non–edible sector has been studied and exploited. In recent years, the use of rapeseed meals to make bioplastic-based materials and cosmetics has emerged. Bandara et al. [216] reviewed the success of using rapeseed protein as a capsule for bioactive drugs delivery. Rapeseed protein could be used as an adhesive, while blended with resins and nanomaterials, but also as plastic films for packaging. Additionally, Zhang et al. [217] discussed the fabrication of polymer films from rapeseed protein, based on aqueous extraction and protein precipitation. Thermoplastic properties of rapeseed protein are palpable in the presence of water, glycerol, sorbitol, and polyethylene glycol. Moreover, the mechanical and moisture barrier properties are comparable with that of other bioplastics from plants. Taken together, the beneficial uses of rapeseed protein in manufacturing capsule and plastic packaging are reinforced.
The processing of meals for bioplastics production affects the property of the final product. For example, injection molding of the meal at 120 °C resulted in stronger samples, and the viscoelastic properties became higher with increasing temperature [218]. In addition, highly deformable green biodegradable materials were produced from rapeseed meals, and a higher protein content promoted the elongation at break and decreased tensile strength. Moreover, that tensile strength and modulus were enhanced by the addition of cellulose fiber at more than 5 wt% [219]. Besides, densification and torrefaction of rapeseed meal and hull were studied by Azargohar et al. [220]. The torrefaction aimed to increase the hydrophobicity and heating value of the pellet. They observed that increasing the rapeseed meal/water mass ratio could improve the pellets’ mechanical strength. Thus, the torrefied pellets had lower moisture adsorption, a lower oxygen content, but a higher heating value, and a higher carbon content. Porosity was also increased. The addition of alkali lignin could improve the torrefied pellets’ mechanical strength, had no effect on the relaxed density of the pellets, and increased the moisture adsorption.
Rapeseed meals could also be used for fabrication of cosmetics. The application of rapeseed hydrolysates in producing skin anti-aging formulations was reported by Rivera et al. [221], who converted the rapeseed protein-rich residues to biologically active peptides by using enzymes. After 24 h of exposure, hydrolysates were biocompatible with skin, in contrast to the non-hydrolyzed extract, which led to cells’ toxicity. The antioxidant and anti-inflammatory activities were obvious, indicating that the enzymatic treatments of rapeseed protein emerged as bioactive compounds suitable for skin.
4. Utilization of Natural Unprocessed Rapeseed
4.1. Vegetable Food, Tea, and Homeopathy
Rapeseed leaves and stems could be used as edible vegetables, or as a potherb for seasoning. Seeds could be used as condiments and as spices. The winter-type cultivar is the most cultivated rapeseed in the world, which gives it great economic potential due to the limitation of existing vegetable crops in winter [222]. Green leaves are one of the richest sources of essential minerals, protein, and vitamins [223]; they are rich in antioxidant phenolic compounds and regular consumption of leaves is recommended to prevent the risk of chronic diseases [224,225,226,227,228,229]. Rapeseed leaves are excellent vegetable diets for weight loss, due to their low–fat composition, their richness in calcium (the highest in green leafy vegetables), and their high content of vitamin C [222,230]. Vitamin C is a well–known antioxidant that boosts immunity and protects macromolecules, as oxidation is causative of several acute and chronic diseases, such as cold, asthenia, allergy, and bad cholesterol [231]. Brassica also contains a good level of folate (Vitamin B9), which is greatly needed during pregnancy to produce new cells and prevent birth defects, especially in the brain and the spine [232]. Additionally, rapeseed contains iron, carotene, and dietary fiber, which reduce lipid absorption while combined with cholesterol and cholate [233]. Phytates and tocopherols (vitamin E) are also antioxidants that are present in rapeseed [234], and phytosterols could reduce serum and low-density lipoprotein (LDL) cholesterols [235,236].
Glucosinolates are active compounds that are found mainly in cruciferous vegetables. Lots of studies have confirmed that eating cruciferous vegetables could greatly prevent cancer, due to the presence of glucosinolates that have demonstrated to have anti-cancer properties [237,238,239,240,241,242,243]. Glucosinolates are only harmful when they are processed for animal feed, because toxic products that are catalyzed by an enzyme called myrosinase could be released during the treatment [244]. Fortunately, myrosinase could be removed without affecting plant bioactive compounds, by ablating myrosin cells, which normally contain myrosinase [245]. Dietary glucosinolates cannot be directly assimilated by mammals (including humans), but are hydrolyzed into isothiocyanates and other cyanates [246], and these compounds have a strong anti-cancer ability because they are one of the best substances in vegetables that induce apoptosis [247,248,249,250]. Isocyanates’ ability to inhibit and fight against cancer was reported in several studies [237,251,252,253,254,255,256].
Rapeseed flowers could be used as infused tea [257], and the pollens could be eaten because they are beneficial for the immune system to fight against diseases such as cancer. This could be explained by the presence of steroids in pollen, which could affect cancer cells’ viability, notably in the prostate [258,259,260]. Apart from the leaves, rapeseed roots are also beneficial because of their various diuretic, anti–gout, anti–inflammatory, and anti–scurvy qualities [261]. Some recent studies have demonstrated that rapeseed has a good performance against Alzheimer’s [262] and prostate disease [263]. Thus, rapeseed is an excellent vegetable crop, it is of low cost, and has a long period of availability, so it can be used as a frequent component in the human diet.
4.2. Honey Production from Rapeseed
One hectare of land may contain about 350–700 thousand rapeseed plants, and each plant can produce more than 100 flowers [264], which offers a high potential for rapeseed in producing honey [265]. It has been estimated that one hectare of crops might produce 60–90 kg of honey [266]. About 40% of honey produced in China comes from rapeseed [267], while Canada produced ~80–96 million pounds of honey in 2015 to 2019 [268].
Honeybees are highly attracted by flowers with bright colors as in rapeseed, but also by the odor of nectars, which is due to phenylacetic and phenylpropionic acids [269]. Indeed, rapeseed and bees have a mutually beneficial relationship. Rapeseed has a long flowering period, and flowers make a good habitation for bees, but also provide high quantity and high quality of nectar [270]. Thus, bees can have good shelter and nutrition for up to one month, without the need to go far for feed. Rapeseed pollen also has a good balance of protein and fat that meet the requirements of a bee colony to reach optimum production, i.e., a crude protein value of 22% to 27%, and fat content of ~7% [271,272].
Rapeseed flowers can be pollinated by bees, and consequently, the yield is increased [273,274]. This implies that one strategy to increase the yield of rapeseed is to increase the bees’ density, already proven to be effective in a study led by Xie et al. [275], resulting in more than 30% higher seed yield, in comparison to the non–pollinated crops.
In other studies, the pollinated crops displayed higher pod numbers, seeds per pod, and seed weight [276,277,278], but bees’ density is also an important key in crop productivity [279]. The germination rate is also improved by the bees’ pollination [270,280]. In addition, seeds treated with insecticides seemed not to be harmful to the bees, because insecticides were not released in the air, and no poisonings were obvious in the fields [281]. Thus, humans too can be a part of a mutual relationship with rapeseed and bees by providing protection and needs for these two actors, and in turn, humans can get abundant honey with all its benefits.
4.3. Ornament and Field Attraction
Rapeseed flowers have been used to decorate buildings and graves in Germany, Switzerland, and Italy [281,282,283], and now they are widely used for ornamental purposes. Rapeseed is an excellent crop in horticulture—it is beautiful as indoor and outdoor decorative flowers, and as a field to visit for leisure and tourism purposes. As rapeseed has a long flowering period, its beauty can be enjoyed for an extended period of time. In addition, the creation of colorful rapeseed flowers via interspecific hybridization and intergenic hybridization resulted in acquiring white, milky white, golden yellow, orange, purple, pink, and red flowers [284,285,286]. Flower colors are due to pigments, such as carotenoids, betalains (in yellow and red flowers), and anthocyanins (in yellow, red, orange, pink, blue, and purple flowers) [287,288], which are accumulated in the vacuoles of epidermal cells, and their amount in the cells, their association with other pigments or metal ions, vacuole pH, and the thickness of the epidermal cells were proven to influence the color type and intensity [288,289,290,291]. In rapeseed, color variation is linked to cyanidin biosynthesis [286]. A recent study reported the difference in chemical constituents of rapeseed petals displaying different colors and highlighted that the highest level of flavonoids was found in red petals and the lowest was in pale white petals, whereas the richest amount of phenolic acids was found in pink and pale white petals [292]. Various colors of rapeseed flowers increase its value as decorative and landscape plants, and exploitation in the art and tourism sector creates employment and income for local people.
4.4. Agricultural Residues
Besides, after the seeds are harvested, the rapeseed straws are used for animal feed. The yield of straws is about four times higher than that of the seed yield, and straws still contain a good level of proteins, which are significantly higher than that of the legume [293]. Rapeseed crude proteins are also higher than that of wheat [294,295]. The only drawback in using straw as animal feed is the high level of fiber, which decreases digestibility and palatability. Ammonia treatments were suggested to solve this difficulty. Huang et al. [296] reported that to reduce fiber and increase protein availability, the rapeseed straw should be impregnated with water (30% of straw volume) and 3.5% of ammonia urea. Adding 40% of this mixture to cattle diet could effectively increase their weight. Rapeseed leaves could be used to feed animals—they contained more than 15% of crude proteins and promoted weight gain in animals, 800–1200 g and 150–250 g per animal per day in cattle and lambs, respectively [297]. Producing rapeseed forage is low-cost, which makes it useful for animal feed.
Rapeseed straws, hulls, and cake could be used to produce biochars [298,299,300]. Biochars are used as a mixture with composts to enhance soil property, to increase crop production [301]. In the system of soil enrichment, rapeseed agricultural waste is an excellent substrate in vermicomposting [302], and biochars of rapeseed could effectively reduce the availability and intake of heavy metals in soil [303,304,305]. In addition, the dehulled rapeseed could be used to furnish fuel pellets for energy production [300].
4.5. Phytoremediation
The environmental pollution caused by heavy metals is a major problem in agricultural development, because of soil and groundwater contamination, which are dangers for public health. Plants can be used for phytoremediation—the root can absorb cadmium (Cd), the main heavy metal that causes pollution. The absorbed Cd from the soil ascends above and is eliminated when the plant is fully grown. Using plants for bioremediation could maintain productivity and is pollution-free [306]. Several studies have demonstrated that rapeseed is an excellent crop for phytoremediation, for its performance in absorbing heavy metal [307,308,309,310,311]. For instance, Cadmium (Cd) and Zinc (Zn) could be removed at 2.4% and 1.80%, respectively, while planting rapeseed in soils polluted with those heavy metals [307]. Thus, a significant part of lead (Pb), Cd, and Zn could be accumulated in the roots of rapeseed after growth in soil rich in heavy metal contamination [308]. Similarly, Belouchrani et al. discovered that rapeseed could accumulate a high concentration of Zn, which could positively affect the root, stem, leaves size, and number, and the dry matter of rapeseed after 12 weeks of growth [309]. Moreover, areas polluted by chrome (Cr) could be cleaned with rapeseed after four months of growth. The concentration of Cr in the shoot and root of rapeseed increased, while those of the soil decreased at a level of 1% [310]. Rapeseed root fixes and accumulates most of the soil heavy metals because the rapeseed main root develops on the surface horizon, where the highest amount of heavy metals are concentrated [308]. Rapeseed has been qualified as a heavy metal hyperaccumulator [309,312], which makes it an excellent crop for phytoremediation.
Additionally, rapeseed could supplement nutrients to the soil; for instance, by using rapeseed as a green manure, rice yield was increased [313,314,315]. Moreover, rapeseed could enhance crops with growth and development with a hormone named brassinolide [316]. Cycling rapeseed cultivation with other plants is not competitive, because rapeseed is a spring/winter type crop, whereas others such as rice, corn, and soybean are summer crops. Another advantage of rapeseed manure is its ability to improve beneficial soil bacteria [317] and to alleviate or suppress the harmful effect of infections [318,319,320,321,322] and nematodes [323,324,325]. Another beneficial use of rapeseed manure is in weed management, which could increase crop yield [326,327,328,329,330]. Thus, rapeseed can be used for phytoremediation and manure to detoxify the soil and balance soil micro-organisms for the good of the crops.
5. Advancement in Breeding Aiming to Improve Rapeseed
In the past century, conventional breeding has been doing well in constantly increasing the potential yield of many crops [331,332]. However, more precise and cheaper methodologies have been invented. Increasing hybrid performance is the main goal of breeding in many crops, including rapeseed, i.e., higher biomass yield, better seed quality traits, fast growth and productiveness, more resistant to stress and disease [333,334].
5.1. Hybrid Heterotic Potential
A cross between two parental lines with valuable traits is an approach that could enhance offspring with superior qualities (heterosis). Multiple factors affect the level of heterosis, but in comparison to self–pollinated species, the cross–pollinated species display a higher level of heterosis [335,336]. Moreover, genetic diversity (GD), reproductivity of parents, adaptability, studied traits, and environmental parameters affect heterosis [335,336,337], but GD has been thought as insufficient to increase heterosis level [338]. In rapeseed, hybrid was often a result of a cross between different accession (intraspecific heterosis), which is an easy and low–cost but highly effective technique, or a cross between different subspecies (interspecific heterosis), which offers a higher level of genome stability, and is commonly used in allopolyploid species, or between different species/genus (wide hybridization heterosis) that display stronger hybrid vigor compared to the intraspecific heterosis [337]. Hybrid performances in seed-yield and yield-related traits were tested in several studies involving crosses between semi-winter and spring types [339], winter and semi-winter types [340], winter and resynthesized types [341], and resynthesized lines (i.e., lines derived from artificial resynthesis of rapeseed from the two parental species, in order to increase the genetic diversity to introduce one or more genes to improve specific traits) [342,343], half-diallel populations [344,345], and wide hybridization, which resulted from a cross between B. napus and B. oleracea [346]. For example, Radoev et al. [341] found a heterosis level of 30% for grain yield and 0.7% for kernel weight, while combining winter and resynthesized rapeseed. DH lines were developed in four locations in Germany, and 33 QTLs and many detected epistatic interactions were found. It has been affirmed that epistasis with partial dominance to over-dominance factors is responsible for the heterosis performance. Additionally, Li et al. [346] suggested that introgression of B. oleracea can be beneficial for genetic variation and heterotic potential in rapeseed since significant positive correlations were found between the introgressed B. oleracea genomic components and heterosis. To investigate the correlation between hybrid performance and genetic distance, Luo et al. [347] led studies on harvest index and found that GD was not correlated with harvest index heterosis, indicating a limited prediction of heterosis with GD in their study (SNP-based methods).
5.2. Pollination Control Systems for Hybrid Seed Production
In rapeseed, pollination control systems play important roles in heterotic applications. The pollination control systems aim to increase the vigor and productivity of the hybrid [348]. It includes the use of techniques of self-incompatibility (SI) [349], and those inducing sterility, such as cytoplasmic male sterility (CMS), genie (or nuclear) male sterility (GMS), pollen sterility induced through chemical hybridizing agents (CHAs), and pollen sterility induced through recombinant DNA (rDNA) technology [350]. However, SI is sometimes unstable and is sensitive to high temperatures, CO2 and NaCl, resulting in problems in the maintenance and propagation of alleles from parental lines. Thus, in allotetraploid rapeseed, male sterility is the only efficient approach to produce hybrid seed (reviewed by Singh et al.) [351]. Male sterility is important in hybrid breeding but also in biological safety applications, which limits the dispersion of transgenes [352]. Among the multiple successes in rapeseed male sterility application is the increased resistance to Sclerotinia sclerotiorum in Ogura CMS restorer with B. oleraceae as a donor [353]. In another study, pollen and seed fertility were improved, and glucosinolate content was reduced [354]. Moreover, better cold tolerance was observed in a male sterile hybrid by protoplast fusion of a cold-sensitive male sterile B. oleraceae and a cold-tolerant fertile B. rapa [355].
5.3. Exotic Germplasm to Enhance Heterotic Potential
A cross between an elite germplasm and an exotic germplasm is an approach that could enhance heterotic potentials. Exotic germplasm has extensive genetic diversity for important traits that could be provided to the elite germplasm, which were probably lost in the elite due to selection procedures or accession issues [356,357,358]. Unfortunately, using exotic germplasm is time-consuming and usage of Marker–Assisted Selection (MAS) is faster but inefficient for complex traits as yield, and massive linkage drags are often encountered, ensuing a gap between elite cultivars and introgressed materials [359]. In rapeseed, numerous studies have been undertaken to analyze the effect of exotic sources in breeding [350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367]. For instance, Kramer et al. [365] analyzed QTL alleles from exotic sources in hybrid seed yield of spring-type B. napus. A previous study revealed that some QTL alleles from exotic donors increased hybrid seed yield while re-evaluating the QTL in the same genetic background and hybrid combination. However, evaluation in a new genetic background resulted in no difference in exotic QTL or significantly lower seed yield in the hybrid than the spring QTL alleles, indicating that the effect of the exotic source was not predictive of other genetic backgrounds or hybrid combinations. Another study performed by Wang et al. [368] demonstrated excellent adaptability under low-nitrogen stress of a rapeseed type with introgression of exotic subgenome component from B. rapa and B. carinata; the hybrid showed strong heterosis in both vegetative and reproductive growth. Besides, Guo et al. [367] investigated eight yield traits in rapeseed, comparing resynthesized lines developed from a cross between B. oleracea L. ssp. alboglabra Bailey and B. rapa L. ssp. chinensis and campestris, and common lines, including elite bred from China and exotic spring lines from Germany and Canada. Significant differences between them were found: the resynthesized lines displayed higher siliques length, 1000–seed weight, and main inflorescence length, but shorter plant height and first branch height primary branch number, seeds per siliques and siliques density and lower number of primary branches and number of seeds per siliques, which were not found in the progenies B. oleracea and B. rapa, indicating that resynthesized lines can provide new germplasm for common lines in rapeseed breeding and gene pool enrichment.
5.4. Genomic Selection (GS)
Genomic selection (GS) is a strategy proposed initially by Meuwissen et al. [369]. GS is performed from phenotypic analysis, predicting the genetic value or the genomic estimated breeding values of an individual in a testing population, including progeny or/and those with no specific phenotypes. Knowledge of the relationship between individuals is needed to analyze genetic covariance and then to make predictions. In rapeseed, GS has been carried out in several studies to enhance important traits, such as seed oil and fatty acid contents, seed protein content, seed glucosinolates, seed acid detergent lignin and hull content, oil yield, seed yield, seedling emergence, lodging resistance, flowering time, and plant height [366,370,371,372]. For example, Li et al. [371] effectively compared different GS models using FT trait in B. napus and found high levels and no apparent difference of accuracies among the models. Moreover, Würschum et al. [370] could detect small-effect QTLs with GS, and reported medium-to-high prediction accuracies, studying yield-related traits in rapeseed. Thus, GS is an effective way to detect valuable breeding materials for complex traits such as yield.
5.5. Nested Association Mapping (NAM) and Multi–Parents Advanced Generation Intercrosses (MAGIC)
NAM is a concept initially tested in maize; it correlates phenotype to genotype, and has for purpose the dissection of complex traits genetic architecture. NAM combines the advantages of association mapping and linkage analysis, i.e., it offers the feasibility of ancient and recent recombination, the possibility of many alleles analysis, and the accessibility of high mapping resolution and statistical power, using low to moderate genetic marker density [373,374]. Unfortunately, to our knowledge, rare studies have been reported on NAM in rapeseed.
In 2013, Samans et al. [375] announced the German public–private consortium, PreBreed–Yield, which aims at genotyping and characterizing extensive NAM populations, joining new genetic variety, and which would be used as a resource for B. napus genome analysis and future breeding. The basic development of the NAM population was based on a cross between an elite winter parent and 50 different founders. For example, Shunmugam et al. [376] crossed an elite parent with 50 spring lines of B. napus to generate NAM population, to study genetic variability in growth physiology, flowering and maturation phenology, yield, seed, and leaves-associated traits. Their findings indicated positive correlations among the founders and the reference line, for leaf characteristics, gas exchange, and flowering phenology. In addition, Li et al. [377] used computer simulation to find the best statistical model for application in rapeseed and indicated that higher power of QTL detection was higher in the joint composite interval mapping (JCIM) model and DH–NAM mating design.
MAGIC population is another concept of multi–parental population. Founders are carefully chosen before population development, according to the trait of interest and it is important that founders provide originality to the population. Thus, multiple interesting alleles would be in combination to develop genotypes [378]. A study performed by Zhao et al. [379] developed MAGIC populations in rapeseed by crossing eight elite parents producing 680 lines. The traits studied were related to yield and disease resistance, and offspring displayed a significant difference in five studied traits compared to parents; also, a normal distribution of trait variation was obvious. Moreover, nine lines displayed more resistance to Sclerotinia sclerotiorum and provided higher seed yield and quality performance. The development of this MAGIC population in rapeseed allowed the authors to conclude that it is ideal for the selection of elite lines [379]. More studies should be done using these multiparental populations in rapeseed, to benefit from advantages offered by these strategies.
5.6. Genome Sequencing
Access to genome sequence opens opportunities in the knowledge of their structure and function, but also the evolution of species; it is also very important to design strategies and tools that are beneficial for crop improvement [380]. Genome sequencing allows to uncover the DNA identity of an organism and permits the comprehension of genome diversity via detection and characterization of structural variation in the genome (e.g., copy number variation). This implies that normal, abnormal (disease), and rare phenotypes could be investigated through genome sequencing [381,382,383]. Therefore, genome sequencing could serve to reveal agronomical important loci. The release of genome sequence of the winter-type cultivar “Darmor–bzh” [4] opened many opportunities in comprehension of rapeseed genome, and in the achievement of studies that aim at breeding improvement. However, a single reference has been qualified as inadequate to study the genetic profile of a species [384]. Fortunately, genome assembly of two other cultivars: a winter-type “Tapidor” [384] and a semi-winter-type “Zhongshuang11 – ZS11” [385] have been reported. Actually, a rapeseed pangenome has been established with more than 1690 accessions is available [386], which is a valuable resource to structural variation of genes associated with agronomic traits. Currently, knowledge and application of genomics could help to improve traits in the field [384]. Several pieces of research have been conducted using genome sequencing/resequencing in rapeseed, comprehending traits variation, stress, or disease resistance [387,388,389,390]. For example, Yao et al. [390] reported the whole-genome re–sequencing (WGR) and fine mapping of an orange petal color (OPC) gene (Bnpc1) in spring B. napus, using two backcrosses (BC) populations produced from OPC parent and a yellow petal color parent. One BC population was subjected to WGR with bulked segregation analysis (BSA) and exposed two major candidate intervals of Bnpc1 on C9 chromosome. The other BC population was used to build genetic and physical linkage map using SSR and InDel markers. The observation indicated Bnpc1 was located in scaffold_38 on C9, flanked by two Indel markers, which were included in the first re-sequenced candidate region on C9. Their findings might help in OPC variety development in rapeseed. Another study on Brassica genera flowering time (FT) gene variation was described by Schiessl et al. [391]—they deep-sequenced B. rapa and B. oleracea flowering regulator genes, which were homologs of 35 A. thaliana genes and were able to uncover SNP, Indel, and CNV for the FT genes network. The obtained data were compared to B. napus and this helped demonstrate rearrangement in B. napus genome, which might happen as a consequence of polyploidization. Their findings enabled understanding of the evolution and speciation of Brassica genera and added important information on FT.
5.7. Genome Editing Technologies (GETs)
GETs encompass gene manipulation within an organism, or incorporation of valuable genes into an elite germplasm, both resulting in an effective alteration of genotype and phenotype without extensive backcross [392,393]. Additionally, GETs offer a more controlled, precise, and faster strategy to induce modification of genes function, like silencing or expression enhancement, but also the insertion or deletion of genes at precise locations in a genome [394,395]. GETs promote genetic variation and include systems as Zinc finger proteins (ZNFs), Transcription activator–like effector nucleases (TALENs), and CRISPR (Clustered, regularly interspaced, short, palindromic repeats)/Cas. ZFNs and TALENs allow to knock-out or cleave specific targeted sequences by use of DNA binding motif/domain, whereas CRISPR/Cas system promotes cleavage of any target sequence by changing the sequence of a guide RNA (gRNA). CRISPR/Cas is a recent technology that offers more advantages compared to ZFNs and TALENs due to its simplicity and high effectiveness, but also in CRISPR/Cas system, mutations could be simultaneously implemented in many genes with multiple gRNAs injections. GETs are effectively used to enhance crop breeding; for example, Rodríguez–Leal et al. [396] demonstrated that CRISPR/Cas9 was effective and beneficial in quantitative variation for diverse traits—they pointed out in their findings the possibility of instantaneous selection and fixation of new alleles in non-transgenic plants, and the fine manipulation of yield components.
CRISPR/Cas9 is now extensively applied to elucidate genes functions in many species, including rapeseed, to name a few in LPAT genes [397], in WRY genes [398], TERMINAL FLOWER 1 [399]. Braatz et al. [400] applied the CRISPR/Cas9 system to ALCATRAZ (ALC) genes, which are implicated in the development of valve margin in seeds, contributing to their smash from mature fruits. Removing ALC function would enable more breakage resistance of seeds during harvest. Their findings showed stable mutations inherited in T2 generations, free of wild-type alleles, and obtained from a single-step strategy. In addition, no off-target mutations were found in the T2 generations, and whole-genome sequencing revealed the insertion of vector sequences into the genome. While testing shatter resistance in ALC knocked-out plants, it was revealed that more resistance was perceived in siliques more than 5 cm in length. Another study illustrating the usage of genome editing by CRISPR/Cas9 in B. napus was reported by Yang et al. [401]. Twelve genes related to plant development regulation were subjected to the analysis. Similarly, no off-target mutations were found in T0 generation and stable and inheritable mutations were observed in T1 generation, free of novel mutations or reversion. Findings reported by both Braatz et al. [400] and Yang et al. [401] illustrated the precision and effectiveness of using CRISPR/Cas9 in altering rapeseed genome, with high stability and heritability, to get beneficial phenotypes. To date, more than 20 genes in B. napus were modified via CRISPR/Cas9, and the editing efficiency ranged from 0–70%, with almost 100% of positive transgenic lines obtained (reviewed by He et al.) [402], which is a good reason to promote the application of this strategy to enhance rapeseed breeding.
6. Future Directions
At present, it is clear that rapeseed has high potential in satisfying human, animal, and crop demands in many aspects (Figure 4), with beneficial impacts on health, environment, and economy.
Figure 4.
Global utilization of rapeseed.
Despite several efforts furnished on rapeseed research, some remarks that need reflection are discussed below, to provide direction to future research works.
To satisfy the increasing oil demand, increases in rapeseed production are required. However, until now and based on the above-mentioned findings, only the solvent and cold-pressed extractions are used for huge productions. On one hand, the use of a solvent is cheap, but might harm health. On the other hand, the cold-pressed method gives a lower yield, but healthier oil. These were the reasons why other methods of oil and protein extraction, using non-chemical tools, have arisen, but now their performance on a great scale remains unclear, i.e., would they produce healthier and long shelf-life products? Would the yield be greater and the time of production faster? Would the cost of production be lower, or at least be well covered by the selling price? Would they be environment-friendly? Consequently, these methods should be tested at an industrial scale, to determine the yield, cost, and time of production. Most importantly, the impact on health and the environment should be deeply studied.
Consuming rapeseed oil has been reported in some studies to be beneficial and in other to be detrimental. To understand this divergence, extensive studies should be performed to detect the circumstances in which consuming rapeseed oil might be unsafe.
Having a diverse and sufficient quantity of affordable and nutritious foods is necessary to support healthy and active living. Rapeseed has been demonstrated to offer benefits when consumed as vegetables, tea, and condiments, but also to provide oil, protein meal, and honey, making it an excellent choice to complete human and animal nutrition, and to prevent or fight certain diseases. However, the presence of some anti-nutritional compounds still subsist; thus, the usage of molecular tools and application of appropriate processes and formulations might cover or relieve these limitations. Besides, more diversity in rapeseed food might be created to allow larger choices. In addition, rapeseed is rich in nutritious components and good proteins; this could be exploited to tackle famine and malnutrition in under-developed countries. All these possibilities should be studied in the future.
Inedible products from rapeseed are now occupying important places in different sectors, as described earlier, but the utility should not be limited to these findings; more possibilities should be studied, for example in producing pesticides and in manufacturing fast biodegradable products such as bags or bottles, instead of using chemical-based products that increase waste and degrade the environment. The exploitation of rapeseed in pharmaceutical and cosmetic industries should be enlarged, due to its richness in anti–cancer and antioxidants compounds.
Rapeseed contributes greatly in creating a loving environment with its beautiful diverse colors, as well as boosting some crops’ growth and healing soil from heavy metals and undesirable infections. With genetic engineering, these rapeseed qualities could be enhanced and maintained, even in environmental fluctuation.
While searching for literature on rapeseed processing and usage, gaps in the time of studies was found for some topics; this might indicate a lack of interest. In addition, evolution of the studies tends to follow the same topics, whereas other areas are still unclear. This should re-ignite the interest of researchers.
In conclusion, rapeseed is an important multifunctional crop that deserves more attention, protection, and improvement. With this review, we aimed to present rapeseed value to increase researchers’ interest, explore more about its potential and re-ignite the interest of professionals (dampened by rapeseed losses caused by various diseases and decrease in cultivation, which limit rapeseed profitability). Effectively, extensive researches have allowed exploiting, at most precise, the molecular mechanism controlling beneficial traits in rapeseed (oil, protein, yield, disease resistance). Still today, efforts are being made to optimize these valuable traits. However, some issues are encountered in rapeseed cultivation. Some countries have low agricultural mechanization levels and agricultural inputs, and increased labor costs, which decrease farmers’ motivation in rapeseed cultivation. In addition, the global economic competitiveness of rapeseed should be improved. Thus, economic and management strategies should be revised to avoid the high input/low output in rapeseed production, which might motivate farmers to cultivate the crop. Thus, the sustainability of rapeseed production could be ensured and the profitability enjoyed.
Author Contributions
Conceptualization, N.R. and M.L.; methodology, N.R. and H.L.; validation, N.R. and M.L.; formal analysis, N.R.; investigation, N.R.; resources, N.R.; data curation, N.R.; writing—original draft preparation, N.R.; writing—review and editing, M.L.; visualization, M.L.; supervision, L.Y. and M.L.; project administration, M.L.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China, grant numbers 31871656, 32072098 and 31701456, and the National Key Research and Development Program grant number 2016YFD0101300, and the APC was funded by the National Science Foundation of China, grant numbers 31871656, 32072098 and 31701456.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Canola Council of Canada. About Canola. Available online: https://www.canolacouncil.org/about-canola/ (accessed on 6 July 2021).
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, R.; Lühs, W.; Friedt, W. Oilseed Rape: Chapter 2. In Genome Mapping and Molecular Breeding in Plants 2. Oilseeds; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Shahzadi, T.; Khan, F.A.; Zafar, F.; Ismail, A.; Amin, E.; Riaz, S. An Overview of Brassica Species for Crop Improvement. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 1568–1573. [Google Scholar] [CrossRef]
- Daun, J.K. Origin, Distribution, and Production. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–28. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products (Production). Available online: http://www.fao.org/faostat/en/#search/Rapeseed%20 (accessed on 24 August 2021).
- STATISTA. Worldwide Production of Rapeseed by Country. Available online: https://www.statista.com/statistics/263930/worldwide-production-of-rapeseed-by-country (accessed on 24 August 2021).
- World Rapeseed Production. Available online: https://www.worldagriculturalproduction.com/crops/rapeseed.aspx (accessed on 6 July 2021).
- Gulden, R.H.; Warwick, S.I.; Thomas, A.G. The biology of Canadian weeds. 137. Brassica napus L. and B. rapa L. Can. J. Plant Sci. 2008, 88, 951–996. [Google Scholar] [CrossRef]
- STATISTA. Worldwide Oilseed Production since 2008. Available online: https://www.statista.com/statistics/267271/worldwide-oilseed-production-since-2008 (accessed on 8 July 2020).
- Wang, Y.; Hou, Y.; Chen, C.; Zhou, M.G. Detection of resistance in Sclerotinia sclerotiorum to carbendazim and dimethachlon in Jiangsu Province of China. Australas. Plant Pathol. 2014, 43, 307–312. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Li, X.L.; Li, H.Z. Research progress in clubroot of crucifers. Plant Prot. 2011, 37, 153–158. [Google Scholar]
- Unger, E.H. Processing. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 163–189. [Google Scholar] [CrossRef]
- Canola Council of Canada. Processing Canola Seed into Oil and Meal in Canada. Available online: https://www.canolacouncil.org/about-canola/processing/ (accessed on 6 July 2021).
- Confortin, T.C.; Todero, I.; Luft, L.; Ugalde, G.A.; Mazutti, M.A.; Oliveira, Z.B.; Bottega, E.L.; Knies, A.E.; Zabot, G.L.; Tres, M.V. Oil yields, protein contents, and cost of manufacturing of oil obtained from different hybrids and sowing dates of canola. J. Environ. Chem. Eng. 2019, 7, 102972. [Google Scholar] [CrossRef]
- Swanson, R.G. Hexane Extraction in Soyfoods Processing; Regents Professor; Department of Food Science, Washington State University: Pullman, WA, USA, 2009. [Google Scholar]
- Gaber, M.A.F.M.; Tujillo, F.J. Improving oil extraction from canola seeds by conventional and advanced methods. Food Eng. Rev. 2018, 10, 198–210. [Google Scholar] [CrossRef]
- Lv, M.; Wu, W. An advanced aqueous method of extracting rapeseed oil with high quality. J. Food Process Eng. 2018, 42, e12957. [Google Scholar] [CrossRef]
- Fetzer, A.; Herfellner, T.; Stäbler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crops Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Gerzhova, A.; Mondor, M.; Benali, M.; Aider, M. A comparative study between the electro-activation technique and conventional extraction method on the extractability, composition and physicochemical properties of canola protein concentrates and isolates. Food Biosci. 2015, 11, 56–71. [Google Scholar] [CrossRef]
- Akbari, A.; Wu, J. An integrated method of isolating napin and cruciferin from defatted canola meal. Food Sci. Technol. 2015, 64, 308–315. [Google Scholar] [CrossRef]
- Hénon, G.; Kemény, Z.; Recseg, K.; Zwobada, F.; Kovari, K. Deodorization of Vegetable Oils. Part I: Modeling the Geometrical Isomerization of Polyunsaturated Fatty Acids. J. Am. Oil Chem. Soc. 1999, 76, 73–81. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and Nutritional Quality Changes of Oil during Frying. J. Am. Oil Chem. Soc. 2009, 86, 149–156. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Aniolowska, M.; Zahran, H.; Kita, A. The effect of pan frying on thermooxidative stability of refined rapeseed oil and professional blend. J. Food Sci. Technol. 2016, 53, 712–720. [Google Scholar] [CrossRef]
- Wroniak, M.; Rękas, A. Nutritional value of cold-pressed rapeseed oil during long term storage as influenced by the type of packaging material, exposure to light & oxygen and storage temperature. J. Food Sci. Technol. 2016, 53, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, R.S.; Wang, Z.G.; Wang, B.; Yang, Y.J.; Ju, X.R.; He, R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. PLoS ONE 2019, 14, e0212879. [Google Scholar] [CrossRef]
- Raikos, V.; Neacsu, M.; Duthie, G.; Nicol, F.; Reid, M.; Cantlay, L.L.; Ranawana, V. Proteomic and glucosinolate profiling of rapeseed isolates from meals produced by different oil extraction processes. J. Food Process. Preserv. 2017, 41, e13060. [Google Scholar] [CrossRef]
- Schutyser, M.A.I.; Pelgrom, P.J.M.; van der Goot, A.J.; Boom, R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015, 45, 327–335. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C. Electrostatic Separation as an Entry into Environmentally EcoFriendly Dry Biorefining of Plant Materials. J. Chem. Eng. Process Technol. 2017, 8, 4–354. [Google Scholar] [CrossRef]
- Mayer-Laigle, C.; Barakat, A.; Barron, C.; Delenne, J.Y.; Frank, X.; Mabille, F.; Rouau, X.; Sadoudi, A.; Samson, M.F.; Lullien-Pellerin, V. DRY biorefineries: Multiscale modeling studies and innovative processing. Innov. Food Sci. Emerg. Technol. 2017, 46, 131–139. [Google Scholar] [CrossRef]
- Jafari, M.; Rajabzadeh, A.R.; Tabtabaei, S.; Marsolais, F.; Legge, R.L. Physicochemical characterization of a navy bean (Phaseolus vulgaris) protein fraction produced using a solvent-free method. Food Chem. 2016, 208, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Laguna, O.; Barakat, A.; Alhamada, H.; Durand, E.; Baréa, B.; Fine, F.; Villeneuve, P.; Citeau, M.; Dauguet, S.; Lecomte, J. Production of proteins and phenolic compounds enriched fractions from rapeseed and sunflower meals by dry fractionation processes. Ind. Crops Prod. 2018, 118, 160–172. [Google Scholar] [CrossRef]
- Kdidi, S.; Vaca-Medina, G.; Peydecastaing, J.; Oukarroum, A.; Fayoud, N.; Barakat, A. Electrostatic separation for sustainable production of rapeseed oil cake protein concentrate: Effect of mechanical disruption on protein and lignocellulosic fiber separation. Powder Technol. 2019, 344, 10–16. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; Regassa, A.; Nyachoti, C.M. Effect of high canola meal content on growth performance, nutrient digestibility and fecal bacteria in nursery pigs fed either corn or wheat based diets. Anim. Feed Sci. Technol. 2017, 231, 59–66. [Google Scholar] [CrossRef]
- Xu, L.; Diosady, L.L. Removal of phenolic compounds in the production of high-quality canola protein isolates. Food Res. 2002, 35, 23–30. [Google Scholar] [CrossRef]
- Hald, C.; Dawid, C.; Tressel, R.; Hofmann, T. Kaempferol 3-O-(2‴-O-Sinapoyl-β-sophoroside) Causes the Undesired Bitter Taste of Canola/Rapeseed Protein Isolates. J. Agric. Food Chem. 2019, 67, 372–378. [Google Scholar] [CrossRef]
- Zago, E.; Lecomte, J.; Barouh, N.; Aouf, C.; Carré, P.; Fine, F.; Villeneuve, P. Influence of rapeseed meal treatments on its total phenolic content and composition in sinapine, sinapic acid and canolol. Ind. Crops Prod. 2015, 76, 1061–1070. [Google Scholar] [CrossRef]
- Thuneke, K.; Remmele, E.; Widmann, B.; Wilharm, T. Standardisation of rapeseed oil as a fuel. In Proceedings of the 1st World Conference on Biomass for Energy and Industry, Sevilla, Spain, 5–9 June 2000. [Google Scholar]
- Vijayaraghavan, K.; Hemanathan, K. Biodiesel production from freshwater algae. Energy Fuels 2009, 23, 5448. [Google Scholar] [CrossRef]
- Kai, T.; Mak, G.L.; Wada, S.; Nakazato, T.; Takanashi, H.; Uemura, Y. Production of biodiesel fuel from canola oil with dimethyl carbonate using an active sodium methoxide catalyst prepared by crystallization. Bioresour. Technol. 2014, 163, 360–363. [Google Scholar] [CrossRef]
- Dworakowska, S.; Bednarz, S.; Bogdal, D. Production of biodiesel from rapeseed oil. In Proceedings of the 1st World Sustainability Forum, Basel, Switzerland, 1–30 November 2011. [Google Scholar]
- Ma, F.; Clements, L.D.; Hanna, M.A. The effects of catalyst, free fatty acids and water on transesterification of beef tallow. Trans. ASAE 1998, 41, 1261–1264. [Google Scholar] [CrossRef]
- Tyson, K.S.; Bozell, J.; Wallace, R.; Petersen, E.; Moens, L. Biomass Oil Analysis: Research Needs and Recommendations; NREL/TP-510-34796; National Renewable Energy Laboratory, US Department of Energy: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Goembira, F.; Matsuura, K.; Saka, S. Biodiesel production from rapeseed oil by various supercritical carboxylate esters. Fuel 2012, 97, 373–378. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. The Biology of Brassica napus L. (Canola/Rapeseed). Available online: https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/biology-documents/brassica-napus-l-/eng/1330729090093/1330729278970 (accessed on 25 August 2021).
- CanolaInfo. Comparison of Dietary Fats. Available online: https://www.canolainfo.org/quadrant/media/files/downloads/pdfs/DietaryFatChartprint2016.pdf (accessed on 6 July 2021).
- Liu, H.; Biliaderis, C.G.; Przybylski, R.; Eskin, N.A.M. Phase transitions of canola sediments. J. Am. Oil Chem. Soc. 1993, 70, 441–448. [Google Scholar] [CrossRef]
- Ackman, R.G. Rapeseed Fatty Acids—An Ideal Mixture for Health, Nutrition, and Food Use. In Canola and Rapeseed. Production, Chemistry, Nutrition and Processing Technology; Shahidi, F., Ed.; Avi Book; Van Nostrand Reinhold: New York, NY, USA, 1990; pp. 81–98. [Google Scholar] [CrossRef]
- Vecchio, A.J. High-laurate rapeseed. INFORM 1996, 7, 230–243. [Google Scholar]
- Tso, P.; Ding, S.; DeMichele, K.; Huang, Y.S. Intestinal Absorption of High γ-Linolenic Acid Canola Oil in Lymph Fistula Rats. In γ-Linolenic Acid: Recent Advances in Biotechnology and Clinical Applications; Huang, Y.-S., Ziboh, V.A., Eds.; AOCS Press: Champaign, IL, USA, 2001; pp. 321–334. [Google Scholar] [CrossRef]
- Pongracz, G. Hitzestabilitaet der tocopherol. Fat Sci. Technol. 1988, 90, 249–258. [Google Scholar]
- Matthäus, B. High oleic low linolenic rapeseed oil as alternative to common used frying oils. Qual. Nutr. Process. Process. Technol. 2006, 108, 200–211. [Google Scholar]
- Cmolik, J.; Pokorny, J.; Reblova, Z.; Svoboda, Z. Tocopherol retention in physically refined rapeseed oil as a function of deodorization temperature. Eur. J. Lipid Sci. Technol. 2008, 110, 754–759. [Google Scholar] [CrossRef]
- Rudzinska, M.; Wasowicz, E.; Uchman, W. Plant sterols in food technology. Acta Sci. Pol. Technol. Aliment. 2005, 4, 147–156. [Google Scholar]
- Ryan, E.; Chopra, J.; McCarthy, F.; Maguire, A.R.; O’Brien, N.M. Qualitative and quantitative comparison of the cytotoxic and apoptotic potential of phytosterol oxidation products with their corresponding cholesterol oxidation products. Br. J. Nutr. 2005, 94, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Hovenkamp, E.; Demonty, I.; Plat, J.; Lutjohann, D.; Mensink, R.P.; Trautwein, E.A. Biological effects of oxidized phytosterols: A review of the current knowledge. Prog. Lipid Res. 2008, 47, 37–49. [Google Scholar] [CrossRef]
- Javitt, N.B. Oxysterols: Novel biologic roles for the 21st century. Steroids 2008, 73, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wahala, K.; Heinonen, M. Processing of rapeseed oil: Effects on sinapic acid derivative content and oxidative stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 27 August 2021).
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- Galassetti, P.; Pontello, A. Dietary effects on oxidation of low-density lipoprotein and atherogenesis. Curr. Atheroscler. Rep. 2006, 8, 523–529. [Google Scholar] [CrossRef]
- Louheranta, A.M.; Sarkkinen, E.S.; Vidgren, H.M.; Schwab, U.S.; Uusitupa, M.I. Association of the fatty acid profile of serum lipids with glucose and insulin metabolism during 2 fat-modified diets in subjects with impaired glucose tolerance. Am. J. Clin. Nutr. 2002, 76, 331–337. [Google Scholar] [CrossRef][Green Version]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef]
- Due, A.; Larsen, T.M.; Hermansen, K.; Stender, S.; Holst, J.J.; Toubro, S.; Martinussen, T.; Astrup, A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am. J. Clin. Nutr. 2008, 87, 855–862. [Google Scholar] [CrossRef]
- Riserus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Salar, A.; Faghih, S.; Pishdad, G.R. Rice bran oil and canola oil improve blood lipids compared to sunflower oil in women with type 2 diabetes: A randomized, single-blind, controlled trial. J. Clin. Lipidol. 2016, 10, 299–305. [Google Scholar] [CrossRef]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nälsén, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001, 44, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Adams-Huet, B.; Brinkley, L.; Grundy, S.M.; Garg, A. Lipid, glycemic, and insulin responses to meals rich in saturated, cis-monounsaturated, and polyunsaturated (n-3 and n-6) fatty acids in subjects with type 2 diabetes. Diabetes Care 2007, 30, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Bonanome, A.; Grundy, S.M.; Zhang, Z.J.; Unger, R.H. Comparison of a high-carbohydrate diet with a high-monounsaturated-fat diet in patients with non-insulin-dependent diabetes-mellitus. N. Engl. J. Med. 1988, 319, 829–834. [Google Scholar] [CrossRef]
- Campbell, L.V.; Marmot, P.E.; Dyer, J.A.; Borkman, M.; Storlien, L.H. The high-monounsaturated fat diet as a practical alternative for NIDDM. Diabetes Care 1994, 17, 177–182. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, e20–e30. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr. Cancer 2007, 57, 177–183. [Google Scholar] [CrossRef]
- Duda, M.K.; O’Shea, K.M.; Stanley, W.C. Omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: Mechanisms and clinical potential. Cardiovasc. Res. 2009, 84, 33–41. [Google Scholar] [CrossRef]
- Fetterman, J.J.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health-Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Wolk, A.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am. J. Clin. Nutr. 1999, 69, 890–897. [Google Scholar] [CrossRef]
- Albert, C.M.; Oh, K.; Whang, W.; Manson, J.E.; Chae, C.U.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 2005, 112, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Ascherio, A.; Hu, F.B.; Stampfer, M.J.; Willett, W.C.; Siscovick, D.S.; Rimm, E.B. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 2005, 111, 157–164. [Google Scholar] [CrossRef]
- Sioen, I.; Hacquebard, M.; Hick, G.; Maindiaux, V.; Larondelle, Y.; Carpentier, Y.A.; De Henauw, S. Effect of ALA-enriched food supply on cardiovascular risk factors in males. Lipids 2009, 44, 603–611. [Google Scholar] [CrossRef]
- Hall, A.V.; Parbtani, A.; Clark, W.F.; Spanner, E.; Keeney, M.; Chin-Yee, I.; Philbrick, D.J.; Holub, B.J. Abrogation of MRL/lpr lupus nephritis by dietary flaxseed. Am. J. Kidney Dis. 1993, 22, 326–332. [Google Scholar] [CrossRef]
- Clark, W.F.; Parbtani, A.; Huff, M.W.; Spanner, E.; de Salis, H.; Chin-Yee, I.; Philbrick, D.J.; Holub, B.J. Flaxseed: A potential treatment for lupus nephritis. Kidney Int. 1995, 48, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, D.; Bankovic-Calic, N.; Peng, C.Y.; Ogborn, M.R.; Aukema, H.M. Dietary flax oil during pregnancy and lactation retards disease progression in rat off spring with inherited kidney disease. Pediatr. Res. 2006, 60, 729–733. [Google Scholar] [CrossRef][Green Version]
- Nguemeni, C.; Delplanque, B.; Rovère, C.; Simon-Rousseau, N.; Gandin, C.; Agnani, G.; Nahon, J.L.; Heurteaux, C.; Blondeau, N. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol. Res. 2010, 61, 226–233. [Google Scholar] [CrossRef]
- Whelan, J.; Rust, C. Innovative dietary sources of n-3 fatty acids. Ann. Rev. Nutr. 2006, 26, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Rainer, L.; Heiss, C.J. Conjugated linoleic acid: Health implications and effects on body composition. J. Am. Diet. Assoc. 2004, 104, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.A.; Garg, M.L. Beyond blood lipids: Phytosterols, statins and omega-3 polyunsaturated fatty acid therapy for hyperlipidemia. J. Nutr. Biochem. 2009, 20, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Simonen, P. Phytosterols, Phytostanols, and Lipoprotein Metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; Koppenol, W.P.; de Jong, A.; Hiemstra, H.; Vermeer, M.A.; Noakes, M.; Luscombe-Marsh, N.D. Plant sterols lower LDL-cholesterol and triglycerides in dyslipidemic individuals with or at risk of developing type 2 diabetes; a randomized, double-blind, placebo-controlled study. Nutr. Diabetes 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Woyengo, T.A.; Ramprasath, V.R.; Jones, P.J. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Pae, M.; Dao, M.C.; Smith, D.; Meydani, S.N.; Wu, D. Dietary Supplementation with Tocotrienols Enhances Immune Function in C57BL/6 Mice. J. Nutr. 2010, 140, 1335–1341. [Google Scholar] [CrossRef]
- Bhatia, E.; Doddivenaka, C.; Zhang, X.; Bommareddy, A.; Krishnan, P.; Matthees, D.P. Chemopreventive effects of dietary canola oil on colon cancer development. Nutr. Cancer 2011, 63, 242–247. [Google Scholar] [CrossRef]
- Stricker, H.; Duchini, F.; Facchini, M.; Mombelli, G. Canola oil decreases cholesterol and improves endothelial function in patients with peripheral arterial occlusive disease—A pilot study. Artery Res. 2008, 2, 67–73. [Google Scholar] [CrossRef]
- Palomaki, A.; Pohjantasahti-Maaroos, H.; Wallenius, M.; Kankkunen, P.; Aro, H.; Husgafvel, S.; Pihlava, J.M.; Oksanen, K. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis. 2010, 9, 137. [Google Scholar] [CrossRef]
- Iggman, D.; Gustafsson, I.B.; Berglund, L.; Vessby, B.; Marckmann, P.; Riserus, U. Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: A randomized controlled study. J. Intern. Med. 2011, 270, 356–364. [Google Scholar] [CrossRef]
- McLennan, P.L.; Dallimore, J.A. Dietary canola oil modifies myocardial fatty acids and inhibits cardiac arrhythmias in rats. J. Nutr. 1995, 125, 1003–1009. [Google Scholar] [CrossRef]
- FDA. Qualified Health Claims: Letter of Enforcement Discretion—Unsaturated Fatty Acids from Canola Oil and Reduced Risk of Coronary Heart Disease; Docket No. 2006Q-0091; U.S. Food and Drug Administration: Washington, DC, USA, 2009. Available online: http://www.fda.gov/Food/LabelingNutrition/LabelClaims/QualifiedHealthClaims/ucm072958.htm (accessed on 22 June 2019).
- Baudet, M.F.; Jacotot, B. Dietary fats and lecithin-cholesterol acyltransferase activity in healthy humans. Ann. Nutr. Metab. 1988, 32, 352–359. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Ausman, L.M.; Carrasco, W.; Jenner, J.L.; Gualtieri, L.J.; Goldin, B.R.; Ordovas, J.M.; Schaefer, E.J. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler. Thromb. 1993, 13, 1533–1542. [Google Scholar] [CrossRef]
- Matheson, B.; Walker, K.Z.; Taylor, D.M.; Peterkin, R.; Lugg, D.; O’Dea, K. Effect on serum lipids of monounsaturated oil and margarine in the diet of an Antarctic Expedition. Am. J. Clin. Nutr. 1996, 63, 933–938. [Google Scholar] [CrossRef]
- Sarkkinen, E.S.; Uusitupa, M.I.; Gylling, H.; Miettinen, T.A. Fat-modified diets influence serum concentrations of cholesterol precursors and plant sterols in hypercholesterolemic subjects. Metabolism 1998, 47, 744–750. [Google Scholar] [CrossRef]
- Becker, C.C.; Lund, P.; Holmer, G.; Jensen, H.; Sandstrom, B. Effects of butter oil blends withincreased concentrations of stearic, oleic and linolenic acid on blood lipids in young adults. Eur. J. Clin. Nutr. 1999, 53, 535–541. [Google Scholar] [CrossRef][Green Version]
- Kratz, M.; Cullen, P.; Kannenberg, F.; Kassner, A.; Fobker, M.; Abuja, P.M.; Assmann, G.; Wahrburg, U. Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur. J. Clin. Nutr. 2002, 56, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M.; Schwab, U.; Makimattila, S.; Karhapaa, P.; Sarkkinen, E.; Maliranta, H.; Agren, J.; Penttilä, I. Effects of two high-fat diets with different fatty acid compositions on glucose and lipid metabolism in healthy young women. Am. J. Clin. Nutr. 1994, 59, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Faulkner, D.; Augustin, L.S.A.; Mitchell, S.; Ireland, C.; Srichaikul, K.; Mirrahimi, A.; Chiavaroli, L.; et al. Effect of Lowering the Glycemic Load With Canola Oil on Glycemic Control and Cardiovascular Risk Factors: A Randomized Controlled Trial. Diabetes Care 2014, 37, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kris-Etherton, P.M.; West, S.G.; Lamarche, B.; Jenkins, D.J.A.; Fleming, J.A. Effects of Canola and High-Oleic Acid Canola Oils on Abdominal Fat Mass in Individuals with Central Obesity. Obesity 2016, 24, 2261–2268. [Google Scholar] [CrossRef]
- Papazzo, A.; Conlan, X.; Lexis, L.; Lewandowski, P. The effect of short-term canola oil ingestion on oxidative stress in the vasculature of stroke-prone spontaneously hypertensive rats. Lipids Health Dis. 2011, 10, 180. [Google Scholar] [CrossRef]
- Sun, Y.; Magnussen, C.G.; Dwyer, T.; Oddy, W.H.; Venn, A.J.; Smith, K.J. Cross-Sectional Associations between Dietary Fat-Related Behaviors and Continuous Metabolic Syndrome Score among Young Australian Adults. Nutrients 2018, 10, 972. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [PubMed]
- Hamley, S. The effect of replacing saturated fat with mostly n-6 polyunsaturated fat on coronary heart disease: A meta-analysis of randomised controlled trials. Nutr. J. 2017, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Mboma, J.; Leblanc, N.; Angers, P.; Rocher, A.; Vigor, C.; Oger, C. Effects of Cyclic Fatty Acid Monomers from Heated Vegetable Oil on Markers of Inflammation and Oxidative Stress in Male Wistar Rats. J. Agric. Food Chem. 2018, 66, 7172–7180. [Google Scholar] [CrossRef]
- Przybylski, R.; Eskin, N.A.M. Oil Composition and Properties. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 189–227. [Google Scholar] [CrossRef]
- Przybylski, R.; Gruczynska, E.; Aladedunye, F. Performance of regular and modified canola and soybean oils in rotational frying. J. Am. Oil Chem. Soc. 2013, 90, 1271–1280. [Google Scholar] [CrossRef]
- Roiaini, M.; Ardiannie, T.; Norhayati, H. Physicochemical properties of canola oil, olive oil and palm olein blends. Int. Food Res. J. 2015, 22, 1228–1234. [Google Scholar]
- Peterson, C.L.; Reece, D.L.; Hammond, B.L.; Thompson, J.; Beck, S.M. Processing, characterization, and performance of eight fuels from lipids. Appl. Eng. Agric. 1997, 13, 71–79. [Google Scholar] [CrossRef]
- Boland, M. Rapeseed. Agricultural Marketing Resource Center. 2012. Available online: http://www.agmrc.org/commodities__products/grains__oilseeds/rapeseed (accessed on 17 October 2014).
- Iveta, Z.; Eva, C. The utilization of rapeseed for biofuels production in the EU. Visegr. J. Bioecon. Sustain. Dev. 2013, 1, 11–14. [Google Scholar] [CrossRef]
- Hamelinck, C.; Koper, M.; Berndes, G.; Englund, O.; Diaz-Chavez, R.; Kunen, E.; Walden, D. Biofuels Baseline 2008; The Netherlands. 2011. Available online: http://www.ecofys.com/files/files/ecofys_2011_biofuels_baseline(2008).pdf (accessed on 19 August 2019).
- Pahl, G. Biodiesel: Growing a New Energy Economy; Chelsea Green Publishing Company: Chelsea, VT, USA, 2008. [Google Scholar]
- Aukema, H.; Campbell, L. Oil nutrition and utilization. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 245–280. [Google Scholar] [CrossRef]
- O’Connor, D. Lifecycle Analysis Canola Biodiesel; (S&T) 2 Consultants Inc.: Winnipeg, MB, Canada, 2011; p. 22. Available online: http://www.canolacouncil.org/media/504977/don_o_connor.pdf (accessed on 22 August 2019).
- Finco, A.; Bentivoglio, D.; Nijkamp, P. Integrated evaluation of biofuel production options in agriculture: An exploration of sustainable policy scenarios. Int. J. Foresight Innov. Policy 2012, 8, 173–188. [Google Scholar] [CrossRef]
- Wolinetz, M.; Hein, M. Biofuels in Canada 2017, Tracking Biofuel Consumption, Feedstocks and Avoided Greenhouse Gas Emissions; Navius Research Inc.: Vancouver, BC, Canada, 2017; Volume 15, p. 22. [Google Scholar]
- CRFA. Environmental Benefits of Biodiesel; Canadian Renewable Fuels Association: Ottawa, ON, Canada, 2010; Available online: http://www.greenfuels.org (accessed on 20 July 2019).
- Health Canada. Human Health Risk Assessment for Biodiesel Production, Distribution and Use in Canada. Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/human-health-risk-assessment-biodiesel-production-distribution-use-canada.html (accessed on 22 September 2019).
- Bio Cube Corporation. Available online: https://biocubeco.com/faqs/will-biodiesel-damage-seals-and-other-components/ (accessed on 28 June 2019).
- Zelmer, C.D.; McVetty, P.B.E. Industrial Products. In Biology and Breeding of Crucifers; Gupta, S.K., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 343–360. [Google Scholar]
- Sonntag, N.O.V. Separation of Fatty Acids. In Fatty Acids; Pryde, E.H., Ed.; AOCS Press: Champaign, IL, USA, 1979; pp. 125–156. [Google Scholar]
- Leonard, C. Sources and commercial applications of high erucic vegetable oils. Lipid Technol. 1994, 6, 79–83. [Google Scholar]
- Senthilselvan, A.; Zhang, Y.; Dosman, J.A.; Barber, E.M.; Holfeld, L.E.; Kirychuk, S.P.; Cormier, Y.; Hurst, T.S.; Rhodes, C.S. Positive human health effects of dust suppression with canola oil in swine barns. Am. J. Respir. Crit. Care Med. 1997, 156, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Frolicious. Benefits of Omega 3, 6, 9 Fatty Acids for Hair. Available online: https://www.frolicious.de/2016/01/31/benefits-of-omega-3-6-9-fatty-acids-for-hair/ (accessed on 15 November 2019).
- Lush Retail Ltd. Rapeseed Oil; Coconut Oil. Available online: https://www.lush.com/uk/en/i/rapeseed-oil-coconut-oil (accessed on 10 June 2020).
- Biopesticides and Pollution Prevention Division. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-011332_25-Nov-09.pdf (accessed on 30 October 2019).
- Thurston County Health Department. Rapeseed Oil. Available online: https://www.co.thurston.wa.us/health/ehipm/pdf_insect/insecticide%20actives/rapeseedOil.pdf (accessed on 7 May 2019).
- Arntfield, S.D.; Hickling, D. Meal Nutrition and Utilization. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–312. [Google Scholar] [CrossRef]
- Newkirk, R. Meal Nutrient Composition. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 229–244. [Google Scholar] [CrossRef]
- Barthet, V.J.; Daun, J.K. Seed Morphology, Composition, and Quality. In Canola; Elsevier: Amsterdam, The Netherlands, 2011; pp. 119–163. [Google Scholar] [CrossRef]
- Downey, R.K.; Craig, B.M.; Young, C.G. Breeding rapeseed for oil and meal quality. JAOCS 1969, 46, 121. [Google Scholar] [CrossRef]
- Stefansson, B.R. Rapeseed breeding in Canada. Chapter 4. In The Story of Rapeseed in Western Canada; Saskatchewan Wheat Pool: Regina, SK, Canada, 1974. [Google Scholar]
- Clandinin, D.R. Rapeseed oil meal studies: 4. Effect of sinapine, the bitter substance in rapeseed oil meal, on the growth of chickens. Poult. Sci. 1961, 40, 484–487. [Google Scholar] [CrossRef]
- Zhu, L.P.; Wang, J.P.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Su, Z.W.; Xuan, Y.; Applegate, T.J.; Zhang, K.Y. The effects of varieties and levels of rapeseed expeller cake on egg production performance, egg quality, nutrient digestibility, and duodenum morphology in laying hens. Poult. Sci. 2019, 98, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Pan, J.; Xie, R.; Qin, J.; He, R.; Ma, H. Screening of strains used for producing rapeseed peptide and degradation of glucosinolates by solid-state fermentation. Sci. Technol. Food Ind. 2015, 36, 164–168. [Google Scholar] [CrossRef]
- Sun, L.; Liu, P.; Zhuo, W.; Fang, X.; Fan, H.; Wu, H. Production of high protein and polypeptide rapeseed meal by microbial fermentation. China Oils Fats 2017, 42, 94–98. [Google Scholar]
- Drazbo, A.; Kozlowski, K.; Ognik, K.; Zaworska, A.; Jankowski, J. The effect of raw and fermented rapeseed cake on growth performance, carcass traits, and breast meat quality in turkey. Poult. Sci. 2019, 98, 6161–6169. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Li, L.; Liu, J.; Liu, C.L.; Fu, J.J.; Xiao, X.J.; Wang, G.; Wang, J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020, 85, 340–348. [Google Scholar] [CrossRef]
- Hansen, J.O.; Overland, M.; Skrede, A.; Anderson, D.M.; Collins, S.A. A meta-analysis of the effects of dietary canola/double low rapeseed meal on growth performance of weanling and growing-finishing pigs. Anim. Feed Sci. Technol. 2020, 259, 114302. [Google Scholar] [CrossRef]
- Pastuszewska, P.; Ochtabinska, A.; Morawski, A. A note on the nutritional adequacy of stock diets for laboratory rats and mice. J. Anim. Feed Sci. 2000, 9, 533–542. [Google Scholar] [CrossRef]
- Aider, M.; Barbana, C. Canola proteins: Composition, extraction, functional properties, bioactivity, applications as a food ingredient and allergenicity—A practical and critical review. Trends Food Sci. Technol. 2011, 22, 21–39. [Google Scholar] [CrossRef]
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Canola proteins for human consumption: Extraction, profile, and functional properties. J. Food Sci. 2011, 76, R16–R28. [Google Scholar] [CrossRef] [PubMed]
- Fu, T. Rapeseed quality improvement. Crop Res. 2007, 21, 159–162. [Google Scholar]
- Khattab, R.Y.; Arntfield, S.D. Functional properties of raw and processed canola meal. LWT Food Sci. Technol. 2009, 42, 1119–1124. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Arntfield, S.D. Rheological characteristics of commercial canola protein isolate-κ carrageenan systems. Food Hydrocoll. 2004, 18, 419–427. [Google Scholar] [CrossRef]
- Vioque, J.; Sanchez-Vioque, R.; Clements, A.; Pedroche, J.; Millan, F. Partially hydrolyzed rapeseed protein isolates with improved functional properties. J. Am. Oil Chem. Soc. 2000, 77, 447–450. [Google Scholar] [CrossRef]
- Pinterits, A.; Arntfield, S.D. The effect of limited proteolysis on canola protein gelation. Food Chem. 2007, 102, 1337–1343. [Google Scholar] [CrossRef]
- Wu, J.; Muir, A.D. Comparative structural, emulsifying, and biological properties of 2 major canola proteins, cruciferin and napin. J. Food Sci. 2008, 73, C210–C216. [Google Scholar] [CrossRef] [PubMed]
- Yoshie-Stark, Y.; Wada, Y.; Wasche, A. Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem. 2008, 107, 32–39. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Muir, A.D. Production of angiotensin I-converting enzyme inhibitory peptides from defatted canola meal. Bioresour. Technol. 2009, 100, 5283–5287. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Haczk, M.; Shahidi, R. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Marczak, E.D.; Usui, H.; Fujita, H.; Yang, Y.; Yokoo, M.; Lipkowski, A.W.; Yoshikawa, M. New antihypertensive peptides isolated from rapeseed. Peptides 2003, 24, 791–798. [Google Scholar] [CrossRef]
- Chabanon, G.; Alves da Costa, L.; Farges, B.; Harscoat, C.; Chenu, S.; Goergen, J.L.; Marc, A.; Marc, I.; Chevalot, I. Influence of the rapeseed hydrolysis process on CHO cell growth. Bioresour. Technol. 2008, 99, 7143–7151. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tian, S.; Small, D.M. Generation of meat-like flavourings from enzymatic hydrolysates of proteins from Brassica sp. Food Chem. 2010, 119, 167–172. [Google Scholar] [CrossRef]
- Burcon. Canola Protein. Available online: https://burcon.live-website.com/products/canola-proteins/ (accessed on 6 July 2021).
- Schweizer, M.; Segall, K.; Medina, S.; Willardsen, R.; Tergesen, J. Rapeseed/canola protein isolates for use in the food industry. Qual. Nutr. Process. Process. Technol. 2007, 160–162. [Google Scholar]
- Helm, A.G. Safety Assessment of Rapeseed Protein (IsolexxTM); Food Safety Authority of Ireland: Dublin, Ireland, 2012; pp. 1–7. Available online: https://www.fsai.ie/uploadedFiles/Science_and_Health/Novel_Foods/Applications/2012%20Rapeseed%20protein.pdf (accessed on 22 July 2019).
- Tan, S.H.; Mailer, R.J.; Blanchard, C.L.; Agboola, S.O. Emulsifying properties of proteins extracted from Australian canola meal. LWT-Food Sci. Technol. 2014, 57, 376–382. [Google Scholar] [CrossRef]
- European Food Safety Authority Panel. Scientific Opinion on the safety of “rapeseed protein isolate” as a Novel Food ingredient. EFSA Panel Diet. Prod. Nutr. Allerg. NDA 2013, 11, 3420. [Google Scholar] [CrossRef]
- Petit, H.V.; Veira, D.M. Effect of post-weaning protein supplementation of beef steers fed grass silage on performance during the finishing phase, and carcass quality. Can. J. Anim. Sci. 1994, 74, 699–701. [Google Scholar] [CrossRef]
- Patterson, H.H.; Whittier, J.C.; Rittenhouse, L.R.; Schutz, D.N. Performance of beef cows receiving cull beans, sunflower meal, and canola meal as protein supplements while grazing native winter range in Eastern Colorado. J. Anim. Sci. 1999, 77, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Xu, L.; Li, C.; Beauchemin, K.A. Effects of supplemental canola meal and various types of distillers’ grains on growth performance of backgrounded steers. Can. J. Anim. Sci. 2013, 93, 281–286. [Google Scholar] [CrossRef]
- Paula, E.M.; Broderick, G.A.; Faciola, A.P. Effects of replacing soybean meal with canola meal for lactating dairy cows fed 3 different ratios of alfalfa to corn silage. J. Dairy Sci. 2020, 103, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Damiran, D.; McKinnon, J.J. Evaluation of wheat-based dried distillers grains with solubles or canola meal derived from Brassica napus seed as an energy source for feedlot steers. Transl. Anim. Sci. 2018, 2 (Suppl. S1), S139–S144. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, P.; Hetta, M.; Swensson, C. Evaluation of canola meal as a protein supplement for dairy cows: A review and a meta-analysis. Can. J. Anim. Sci. 2011, 91, 529–543. [Google Scholar] [CrossRef]
- Martineau, R.; Ouellet, D.R.; Lapierre, H. Feeding canola meal to dairy cows: A meta-analysis on lactational responses. J. Dairy Sci. 2013, 96, 1701–1714. [Google Scholar] [CrossRef]
- Chibisa, G.E.; Christensen, D.A.; Mutsvangwa, T. Replacing canola meal as the major protein source with wheat dried distillers’ grains alters omasal fatty acid flow and milk fatty acid composition in dairy cows. Can. J. Anim. Sci. 2013, 93, 137–147. [Google Scholar] [CrossRef][Green Version]
- Auldist, M.J.; Marett, L.C.; Greenwood, J.S.; Wright, M.M.; Hannah, M.; Jacobs, J.L.; Wales, W.J. Replacing wheat with canola meal in a partial mixed ration increases the milk production of cows grazing at a restricted pasture allowance in spring. Anim. Prod. Sci. 2014, 54, 869–878. [Google Scholar] [CrossRef]
- Brand, T.S.; Brandt, D.A.; Cruywagen, C.W. Utilisation of growing-finishing pig diets containing high levels of solvent or expeller oil extracted canola meal. N. Z. J. Agric. Res. 2001, 44, 31–35. [Google Scholar] [CrossRef][Green Version]
- Schone, F.; Rudolph, B.; Kirchheim, U.; Knapp, G. Counteracting the negative effects of rapeseed and rapeseed press cake in pig diets. Br. J. Nutr. 1997, 78, 947–962. [Google Scholar] [CrossRef]
- Do, S.H.; Kim, B.O.; Fang, L.H.; You, D.H.; Hong, J.S.; Kim, Y.Y. Various levels of rapeseed meal in weaning pig diets from weaning to finishing periods. AJAS 2017, 30, 1292–1302. [Google Scholar] [CrossRef]
- Lee, J.W.; Woyengo, T.A. Growth performance, organ weights, and blood parameters of nursery pigs fed diets containing increasing levels of cold-pressed canola cake. J. Anim. Sci. 2018, 96, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.Q.; Huang, C.F.; Li, Y.K.; Li, P.L.; Liu, H.; Chen, Y.F.; Li, D.F.; Lai, C.H. Adaptation duration for net energy determination of high fiber diets in growing pigs. Anim. Feed Sci. Technol. 2018, 241, 15–26. [Google Scholar] [CrossRef]
- Mejicanos, G.A.; Kim, J.W.; Nyachoti, C.M. Tail-end dehulling of canola meal improves apparent and total tract digestibility of phosphorus when fed to growing pigs. J. Anim. Sci. 2018, 96, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, D.E.; Hossain, M.M.; Regassa, A.; Nyachoti, C.M. Effect of canola meal inclusion as a major protein source in gestation and lactation sow diets with or without enzymes on reproductive performance, milk composition, fecal bacterial profile and nutrient digestibility. Anim. Feed Sci. Technol. 2018, 241, 141–150. [Google Scholar] [CrossRef]
- Vincent, I.C.; Williams, H.L.; Hill, R. Feeding British rapeseed meals to pregnant and lactating ewes. Anim. Prod. 1988, 47, 283–289. [Google Scholar] [CrossRef]
- Vincent, I.C.; Thompson, J.; Hill, R. The voluntary feed intake and weight gain of lambs given concentrate feeds containing rapeseed meal with a range of glucosinolate contents. Anim. Prod. 1990, 50, 587. [Google Scholar] [CrossRef]
- Hill, R. Rapeseed meal in the diets of ruminants. Nutr. Abs. Rev. B 1991, 61, 139–155. [Google Scholar]
- Reis, P.J.; Tunks, D.A.; Munro, S.G. Effects of the infusion of amino acids into the abomasum of sheep, with emphasis on the relative value of methionine, cysteine and homocysteine for wool growth. J. Agric. Sci. 1990, 114, 59–68. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Sykes, J.M.; Bignell, W.C. Influence of lupins and canola supplements on plasma amino acids, wool fibre diameter and liveweight in genetically divergent first cross Merino lambs. In Proceedings of the World Congress on Fats and Oils, Sydney, Australia, 27–30 September 2009. [Google Scholar]
- Sutton, E.I. Canola Meal for Other Species. Horses. Feed Intake and Performance. In Canola Nutritionist Manual; Canola Council of Canada: Winnipeg, MB, Canada, 1988. [Google Scholar]
- Lebas, F.; Colin, M. Utilization of rapeseed oil meal in growing rabbit feeding. Effect of dehulling. Ann. Zootech. 1977, 26, 93–97. [Google Scholar] [CrossRef]
- Brand, T.S.; De Brabander, L.; Van Schalkwyk, S.J.; Pfister, B.; Hays, J.P. The true metabolisable energy content of canola oilcake meal and full-fat rapeseed seed for ostriches (Struthio camelus). Br. Poult. Sci. 2000, 41, 201–203. [Google Scholar] [CrossRef]
- Badshah, A.; Aurang, Z.; Nizakat, B.; Sajjad, A.; Chaudry, M.A.; Sattar, A. Utilization of rapeseed meal/cake in poultry feed. Part II. Effect of incorporating higher levels of rapeseed cake in poultry diet on laying performance of brown-egg layer. Pak. J. Sci. Ind. Res. 2001, 44, 171–174. [Google Scholar]
- Marcu, N.; Banto, E.; Sut-Gherman, M.; Dinea, M.; Ludu, O.; Ceghezi, J. The effect of soybean meal substitution with rape meal in laying hens nutrition. Bull. Univ. Stiint. Agric. Med. Vet. Cluj-Napoca Ser. Zooteh. Biotehnol. 2004, 60, 138–142. [Google Scholar]
- Perez-Maldonado, R.A.; Barram, K.M. Evaluation of Australian Canola Meal for Production and Egg Quality in Two Layer Strains. In Proceedings of the 16th Australian Poultry Science Symposium, Sydney, Australia, 14–16 February 2004; pp. 171–174. [Google Scholar]
- Newkirk, R.W.; Classen, H.L. The effects of toasting canola meal on body weight, feed conversion efficiency, and mortality in broiler chickens. Poult. Sci. 2002, 81, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.R.; Devegowda, G.; Khosravinia, H. Effects of enzyme addition to broiler diets containing varying levels of double zero rapeseed meal. Asian-Aust. J. Anim. Sci. 2006, 19, 1354–1360. [Google Scholar] [CrossRef]
- Manyeula, F.; Mlambo, V.; Marume, U.; Sebola, N.A. Nutrient digestibility, haemo-biochemical parameters and growth performance of an indigenous chicken strain fed canola meal-containing diets. Trop. Anim. Health Prod. 2019, 51, 2343–2350. [Google Scholar] [CrossRef]
- Manyeula, F.; Mlambo, V.; Marume, U.; Sebola, N.A. Partial replacement of soybean products with canola meal in indigenous chicken diets: Size of internal organs, carcass characteristics and breast meat quality. Poult. Sci. 2020, 99, 256–262. [Google Scholar] [CrossRef]
- Mnisi, C.M.; Mlambo, V. Growth performance, haematology, serum biochemistry and meat quality characteristics of Japanese quail (Coturnix coturnix japonica) fed canola meal-based diets. Anim. Nutr. 2018, 4, 37–43. [Google Scholar] [CrossRef]
- Mnisi, C.M.; Mlambo, V. Protease treatment of canola meal-containing Japanese quail diets: Effect on physiological parameters and meat quality traits. J. Appl. Anim. Res. 2018, 46, 1389–1394. [Google Scholar] [CrossRef]
- Drażbo, A.; Ognik, K.; Zaworska, A.; Ferenc, K.; Jankowski, J. The effect of raw and fermented rapeseed cake on the metabolic parameters, immune status, and intestinal morphology of turkeys. Poult. Sci. 2018, 97, 3910–3920. [Google Scholar] [CrossRef]
- Rad-Spicea, M.; Rogiewicza, A.; Jankowskib, J.; Slominski, B.A. Yellow-seeded B. napus and B. juncea canola. Part 1. Nutritive value of the meal for broiler chickens. Anim. Feed Sci. Technol. 2018, 240, 66–77. [Google Scholar] [CrossRef]
- Kozlowskia, K.; Mikulskia, D.; Rogiewiczc, A.; Zdunczykb, Z.; Rad-Spicec, M.; Jeroch, H.; Jankowskia, J.; Slominskic, B.A. Yellow-seeded B. napus and B. juncea canola. Part 2. Nutritive value of the meal for turkeys. Anim. Feed Sci. Technol. 2018, 240, 102–116. [Google Scholar] [CrossRef]
- Bell, J.M. Factors affecting the nutritional value of canola meal: A review. Can. J. Anim. Sci. 1993, 73, 679–697. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Transcriptome analysis of secondary-wall-enriched seed coat tissues of canola (Brassica napus L.). Plant Cell Rep. 2010, 29, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.A.; Dosanjh, B.S.; Little, M.; Roy, R.J.J.; McBride, J.R. Potential for including Canola Products (Meal and Oil) in Diets for Oreochromis mossambicus × O. aureus Hybrids. In Proceedings of the Third International Symposium on Feeding and Nutrition in Fish, Toba, Japan, 28 August–1 September 1989; pp. 301–314. [Google Scholar]
- Lim, C.; Beames, R.M.; Eales, J.G.; Prendergast, A.F.; McLeese, J.M.; Shearer, K.D.; Higgs, D.A. Nutritive values of low and high fibre canola meals for shrimp. Aquacult. Nutr. 1997, 3, 269–279. [Google Scholar] [CrossRef]
- Abdul-Aziz, G.M.; El-Nady, M.A.; Shalaby, A.S.; Mahmoud, S.H. Partial substitution of soybean meal protein by different plant protein sources in diets for Nile tilapia fingerlings. Bull. Fac. Agric. Univ. Cairo 1999, 50, 189–202. [Google Scholar] [CrossRef]
- Glencross, B. Pilot Assessment of the Potential for Canola Meal and Oil Use in Aquaculture Feeds; Final Report for the Grains Research and Development Corporation. Fisheries Research Contract Report No. 5; Department of Fisheries, Western Australia: North Beach, Australia, 2003. [Google Scholar]
- Xu, W.J.; Jin, J.Y.; Zou, T.; Han, D.; Liu, H.K.; Zhu, X.M. Growth, feed utilization and metabolic responses of three gibel carp (Carassius gibelio) strains to fishmeal and plant protein-based diets. Aquac. Nutr. 2018, 25, 319–332. [Google Scholar] [CrossRef]
- Von Danwitz, A.; Schulz, C. Effects of dietary rapeseed glucosinolates, sinapic acid and phytic acid on feed intake, growth performance and fish health in turbot (Psetta maxima L.). Aquaculture 2020, 516, 734624. [Google Scholar] [CrossRef]
- Lim, C.; Klesius, P.H.; Higgs, D.A. Substitution of canoila meal for soybean meal in diets for channel catfish, Ictalurus punctatus. J. World Aquac. Soc. 1998, 29, 161–168. [Google Scholar] [CrossRef]
- Bandara, N.; Akbari, A.; Esparza, Y.; Wu, J. Canola Protein: A Promising Protein Source for Delivery, Adhesive, and Material Applications. J. Am. Oil Chem. Soc. 2018, 95, 1075–1090. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Rempel, C. Processing and characteristics of canola protein-based biodegradable packaging: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 475–485. [Google Scholar] [CrossRef]
- Delgado, M.; Felix, M.; Bengoechea, C. Development of bioplastic materials: From rapeseed oil industry by products to added-value biodegradable biocomposite materials. Ind. Crops Prod. 2018, 125, 401–407. [Google Scholar] [CrossRef]
- Li, S.; Ball, B.; Donner, E.; Thompson, M.R.; Rempel, C.; Liu, Q. Mechanical properties of green canola meal composites and reinforcement with cellulose fibers. Polym. Bull. 2019, 76, 1257–1275. [Google Scholar] [CrossRef]
- Azargohar, R.; Soleimani, M.; Nosran, S.; Bond, T.; Karunakaran, C.; Dalai, A.K.; Tabil, L.G. Thermo-physical characterization of torrefied fuel pellet from co-pelletization of canola hulls and meal. Ind. Crops Prod. 2019, 128, 424–435. [Google Scholar] [CrossRef]
- Rivera, D.; Rommi, K.; Fernandes, M.M.; Lantto, R.; Tzanov, T. Biocompounds from rapeseed oil industry co-stream as active ingredients for skin care applications. Int. J. Cosmet. Sci. 2015, 37, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, P.; Shi, H.; Zhou, Y.; Huang, H.; Tang, S. Preliminary results of yield, income and nutrition of double low rapeseed (Brassica napus L.) cultivated for oilseed vegetable-dual-purpose. Chin. Agric. Sci. Bull. 2009, 25, 224–227. [Google Scholar]
- Martinez, S.; Losada, P.; Franco, I.; Carballo, J. Protein, amino acid, ash and mineral contents in Brassica spp. Grown in northwest Spain. Int. J. Food Sci. Technol. 2010, 46, 146–153. [Google Scholar] [CrossRef]
- Van Duyn, M.A.; Pivonka, E. Overview of health benefits of fruit and vegetable consumption for the dietetics professional. Selective literature. J. Am. Diet. Assoc. 2000, 100, 1511–1521. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and chemical value of Amaranthus hybridus L. leaves from Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar] [CrossRef]
- Boffetta, P.; Couto, E.; Wichmann, J.; Ferrari, P.; Trichopoulos, D.; Bueno-de-Mesquita, H.B.; van Duijnhoven, F.J.; Büchner, F.L.; Key, T.; Boeing, H.; et al. Fruit and vegetable intake and overall cancer risk in the european prospective investigation into cancer and nutrition (EPIC). JNCI-J. Natl. Cancer Inst. 2010, 102, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K. Vegetables and Fruit in the Prevention of Chronic Age-Related Diseases. In Molecular Basis of Nutrition and Aging; Molecular Nutrition; Academic Press: Cambridge, MA, USA, 2016; pp. 707–722. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesonga, J.; Schreiner, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Analysis of the tumoral cytotoxicity of green tea-infusions enriched with broccoli. Food Chem. 2012, 132, 1197–1206. [Google Scholar] [CrossRef]
- Michels, A.J.; Frei, B.; Vitamin, C. Biochemical, Physiological, and Molecular Aspects of Human Nutrition; Stipanuk, M.H., Caudill, M.A., Eds.; Elsevier/Saunders: St. Louis, MO, USA, 2012; pp. 626–654. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D. Chapter Five—Recent Developments in Folate Nutrition. Adv. Food Nutr. Res. 2018, 83, 195–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Wang, C.; Yu, G. Research on functional properties and modification of dietary fiber. Grain Process. 2010, 35, 57–59. [Google Scholar]
- Khattab, R.; Goldberg, E.; Lin, L.; Thiyam, U. Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 2010, 122, 1266–1272. [Google Scholar] [CrossRef]
- Ling, W.H.; Jones, P.J.H. Dietary phytosterols: A review of metabolism, benefits, and side effects. Life Sci. 1995, 57, 195–206. [Google Scholar] [CrossRef]
- Venkatramesh, M.; Karunanandaa, B.; Sun, B.; Grunter, C.A.; Boddupalli, S.; Kishore, G.M. Expression of a streptomyces 3-hydroxysteroid oxidase gene in oilseeds for converting phytosterols to phytostanols. Phytochemistry 2003, 62, 39–46. [Google Scholar] [CrossRef]
- Verhoeven, D.T.; Verhagen, H.; Goldbohm, R.A.; van den Brandt, P.A.; van Poppel, G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 1997, 103, 79–129. [Google Scholar] [CrossRef]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–382. [Google Scholar] [CrossRef]
- Yu, X.; Xu, X.; Teng, G. Inhibition effect of glucosinolates on tumour cells. Sci. Technol. Food Ind. 2010, 1, 165–167. [Google Scholar]
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Jeffery, E.H. Cancer prevention with Brassica vegetables. Acta Hortic. 2014, 1106, 143–146. [Google Scholar] [CrossRef]
- Traka, M.H. Health Benefits of Glucosinolates. In Advances in Botanical Research; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 80, pp. 247–279. [Google Scholar] [CrossRef]
- Ho, C.L.; Tan, H.Q.; Chua, K.J.; Kang, A.; Lim, K.H.; Ling, K.L.; Yew, W.S.; Lee, Y.S.; Thiery, J.P.; Chang, M.W. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018, 2, 27–37. [Google Scholar] [CrossRef]
- Mawson, R.; Heaney, R.K.; Zdunczyk, Z.; Kozlowska, H. Rapeseed meal-glucosinolates and their antinutritional effects 2. Flavor and palatability. Nahrung-Food 1993, 37, 336–344. [Google Scholar] [CrossRef]
- Borgen, B.H.; Tangstad, O.P.; Ahuja, I.; Rossiter, J.T.; Bones, A.M. Removing the mustard oil bomb from seeds: Transgenic ablation of myrosin cells in oilseed rape (Brassica napus) produces mineless seeds. J. Exp. Bot. 2010, 61, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef] [PubMed]
- Herman-Antosiewicz, A.; Johnson, D.E.; Singh, S.V. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006, 66, 5828–5835. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, L.; Mao, J.; Huang, G.; Yuan, H. Study on extraction of sulforaphane from broccoli. J. Food Sci. Biotechnol. 2009, 28, 647–652. [Google Scholar]
- Kalpana, D.P.D.; Gayathri, R.; Gunassekaran, G.R.; Murugan, S.; Sakthisekaran, D. Apoptotic role of natural isothiocyanate from broccoli (Brassica oleracea italica) in experimental chemical lung carcinogenesis. Pharm. Biol. 2013, 51, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Abdull Razis, A.F. Apoptosis as a Mechanism of the Cancer Chemopreventive Activity of Glucosinolates: A Review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [CrossRef]
- Lampe, J.W.; Peterson, S. Brassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetables. J. Nutr. 2002, 132, 2991–2994. [Google Scholar] [CrossRef]
- Hwang, E.S.; Jeffery, E.H. Evaluation of urinary N-acetyl cysteinyl allyl isothiocyanate as a biomarker for intake and bioactivity of Brussels sprouts. Food Chem. Toxicol. 2003, 41, 1817–1825. [Google Scholar] [CrossRef]
- Ramirez, M.C.; Singletary, K. Regulation of estrogen receptor alpha expression in human breast cancer cells by sulforaphane. J. Nutr. Biochem. 2009, 20, 195–200. [Google Scholar] [CrossRef]
- Pawlik, A.; Wiczk, A.; Kaczynska, A.; Antosiewicz, J.; Herman Antosiewicz, A. Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur. J. Nutr. 2013, 52, 1949–1958. [Google Scholar] [CrossRef]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Kołodziejski, D.; Koss-Mikołajczyk, I.; Abdi, A.Y.; Jacob, C.; Bartoszek, A. Chemical Aspects of Biological Activity of Isothiocyanates and Indoles, the Products of Glucosinolate Decomposition. Curr. Pharm. Des. 2019, 25, 1717–1728. [Google Scholar] [CrossRef]
- Lim, Y.H.; Chun, J.H.; Lee, K.T.; Hong, S.T.; Lee, Y.H.; Kim, S.J. Changes in Composition and Content of Flavonoids by Processing Type in Rapeseed (Brassica napus) Flowers. Korean Soc. Environ. Agric. 2017, 36, 7–16. [Google Scholar] [CrossRef]
- Wu, L.H.; Luo, Y.M.; Xing, X.R.; Christie, P. EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agric. Ecosyst. Environ. 2004, 102, 307–318. [Google Scholar] [CrossRef]
- Feng, H.; Jiao, S.; Tang, Y.; Tang, P.; Liu, J. Advance studying of rape pollen. Food Ind. 2011, 9, 108–111. [Google Scholar]
- Zhu, C.; Du, L.; Gao, Y.; Qian, L.; Gu, L. Suppression effect of Brassica campestris pollen extract on the growth of prostate cancer cell in vitro. Zhejiang J. Integr. Tradit. Chin. West. Med. 2011, 21, 84–86. [Google Scholar]
- Saeidnia, S.; Gohari, A.R. Importance of Brassica napus as a medicinal food plant. J. Med. Plant Res. 2012, 6, 2700–2703. [Google Scholar] [CrossRef]
- Kaur, S.; Shri, R. Comparative study of pharmacognostic parameters, antioxidant and anticholinesterase potential of Brassica napus and Brassica oleracea var. Acephala. IJPSR 2018, 9, 3718–3724. [Google Scholar] [CrossRef]
- Kujawski, R.; Baraniak, J.; Kania, M.; Bartkowiak-Wieczorek, J.; Bogacz, A.; Ożarowski, M.; Cichocki, M.; Spoz, E.; Mikolajczak, P.L. Diet based on oil of seeds of Brassica napus. Implications for the prevention and treatment of prostate diseases. Herba Polonica 2015, 60, 78–88. [Google Scholar] [CrossRef]
- Mussury, R.M.; Fernandes, W.D. Studies of the floral biology and reproductive system of Brassica napus L. (Cruciferae). Braz. Arch. Biol. Technol. 2000, 43, 111–115. [Google Scholar] [CrossRef]
- Bertazzini, M.; Forlani, G. Intraspecific Variability of Floral Nectar Volume and Composition in Rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 288. [Google Scholar] [CrossRef]
- Latifundist Media. Top 10 Rapeseed Producing Countries in 2019. Available online: https://latifundist.com/en/rating/top-10-proizvoditelej-rapsa-v-2019-godu (accessed on 26 August 2021).
- Delifoods. Rapeseed Honey. Available online: https://delimfg.com/natural-honey/rapeseed-honey/ (accessed on 26 August 2021).
- Crops and Horticulture Division Agriculture and Agri-Food Canada. Statistical Overview of the Canadian Honey and Bee Industry. 2019. Available online: https://multimedia.agr.gc.ca/pack/pdf/honey_miel_2019-eng.pdf (accessed on 26 August 2021).
- Ruisinger, B.; Schieberle, P. Characterization of the key aroma compounds in rape honey by means of the molecular sensory science concept. J. Agric. Food Chem. 2012, 60, 4186–4194. [Google Scholar] [CrossRef]
- Kevan, P.G.; Lee, H.; Shuel, R.W. Sugar ratios in nectars of varieties of canola (Brassica napus). J. Apic. Res. 1991, 30, 99–102. [Google Scholar] [CrossRef]
- Stace, P. Protein Content and Amino Acid Profiles of Honey Bee-Collected Pollens; Bees ‘N Trees Consultants: Lismore, Australia, 1996. [Google Scholar] [CrossRef]
- Somerville, D.C. Nutritional Value of Bee Collected Pollens; RIRDC Publication No. 01/047; Rural Industries Research and Development Corporation, New South Wales Agriculture: New South Wales, Australia, 2001. [Google Scholar]
- Rosa, A.S.; Blochtein, B.; Ferreira, N.R.; Witter, S. Apis mellifera (Hymenoptera: Apidae) as a potential Brassica napus pollinator (cv. Hyola 432) (Brassicaceae), in Southern Brazil. Braz. J. Biol. 2010, 70, 1075–1081. [Google Scholar] [CrossRef]
- Rosa, A.S.; Blochtein, B.; Lima, D.K. Honey bee contribution to canola pollination in Southern Brazil. Sci. Agric. 2011, 68, 255–259. [Google Scholar] [CrossRef][Green Version]
- Xie, L.; Xu, B.; Sun, Y.; Zhao, F.; Yin, J.; Geng, Y. The effect of pollination by honeybees on yield of oilseed rape and fatty acid composition of rapeseed. J. Bee 2011, 4, 41–43. [Google Scholar]
- Steffan-Dewenter, I. Seed set of male-sterile and male-fertile oilseed rape (Brassica napus) in relation to pollinator density. Apidologie 2003, 34, 227–235. [Google Scholar] [CrossRef][Green Version]
- Sabbahi, R.; de Oliveira, D.; Marceau, J. Influence of honey bee (Hymenoptera: Apidae) density on the production of canola (Crucifera: Brassicacae). J. Econ. Entomol. 2005, 98, 367–372. [Google Scholar] [CrossRef]
- Durán, X.A.; Ulloa, R.B.; Carrillo, J.A.; Contreras, J.L.; Bastidas, M.T. Evaluation of yield component traits of honeybee-pollinated (Apis mellifera L.) rapeseed canola (Brassica napus L.). Chil. J. Agric. Res. 2010, 70, 309–314. [Google Scholar] [CrossRef]
- Rollin, O.; Garibaldi, L.A. Impacts of honeybee density on crop yield: A meta-analysis. J. Appl. Ecol. 2017, 56, 1152–1163. [Google Scholar] [CrossRef]
- Pernal, S.F.; Currie, R.W. Nectar quality in open-pollinated, pol CMS hybrid, and dominant SI hybrid oilseed summer rape. Can. J. Plant Sci. 1998, 78, 79–89. [Google Scholar] [CrossRef]
- Cutler, C.; Scott-Dupree, C.D. Exposure to Clothianidin Seed-Treated Canola Has No Long-Term Impact on Honey Bees. J. Econ. Entomol. 2007, 100, 765–772. [Google Scholar] [CrossRef]
- Fussel, G.E. History of cale (Brassica sp.). Nature 1995, 176, 48–51. [Google Scholar] [CrossRef]
- Baranyk, P.; Fábry, A. History of the Rapeseed (Brassica napus L.) Growing and Breeding from Middle Age Europe to Canberra. International Rapeseed Congress. 1999. Available online: http://www.regional.org.au/au/gcirc/4/374.htm (accessed on 28 September 2019).
- Cook, S.M.; Skellern, M.P.; Döring, T.F.; Pickett, J.A. Red oilseed rape? The potential for manipulation of petal colour in control strategies for the pollen beetle (Meligethes aeneus). Arthropod-Plant Interact. 2013, 7, 249–258. [Google Scholar] [CrossRef]
- Mushtaq, M.A.; Pan, Q.; Chen, D.; Zhang, Q.; Ge, X.; Li, Z. Comparative Leaves Transcriptome Analysis Emphasizing on Accumulation of Anthocyanins in Brassica: Molecular Regulation and Potential Interaction with Photosynthesis. Front. Plant Sci. 2016, 7, 311. [Google Scholar] [CrossRef]
- Fu, W.; Chen, D.; Pan, Q.; Li, F.; Zhao, Z.; Ge, X.; Li, Z. Production of red-flowered oilseed rape via the ectopic expression of Orychophragmus violaceus OvPAP2. Plant Biotechnol. J. 2018, 16, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Weiss, M.R. Floral color change: A widespread functional convergence. Am. J. Bot. 1995, 82, 167–185. [Google Scholar] [CrossRef]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Deng, J.; Chen, S.; Yin, X.J.; Wang, K.; Liu, Y.L.; Li, S.H.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.W.; Wang, S.X.; Jia, L.D.; Zhu, M.C.; Yang, J.; Zhou, B.J.; Yin, J.M.; Lu, K.; Wang, R.; Li, J.N.; et al. Identification and Characterization of Major Constituents in Different-Colored Rapeseed Petals by UPLC-HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Tu, J.; Zhang, Y.; Chen, C.; Yang, Q.; Ten, H.; Niu, J.; Yi, J. Investigation and utilization of cropping forage rape after wheat in northwest area of China. Sci. Technol. West China 2004, 6, 4–7. [Google Scholar]
- Bhuiyan, K.H.; Hossain, M.M.; Bari, M.N.; Khanam, M.R. Identification of bee plants and analysis of honey collected from different plant sources. Pak. J. Biol. Sci. 2002, 5, 1199–1201. [Google Scholar] [CrossRef]
- Khan, T.S.; Mubeen, U. Wheat straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Huang, R. Effect of Crushed and Ammoniated Rape Straw on Feeding Weining Yellow Cattle. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2013. [Google Scholar]
- Ayres, L.; Clements, B. Forage Brassicas—Quality Crops for Livestock Production; Agfact P2. 1. 13; NSW Agriculture: New South Wales, Australia, 2002; pp. 1–3. [Google Scholar]
- Karaosmanoglu, F.; Isigigur-Ergundenler, A.; Sever, A. Biochar from the straw-stalk of rapeseed plant. Energy Fuels 2000, 14, 336–339. [Google Scholar] [CrossRef]
- Ozcimen, D.; Karaosmanoglu, F. Production and characterization of bio-oil and biochar from rapeseed cake. Renew. Energy 2004, 29, 779–787. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kang, K.; Bond, T.; Dalai, A.K.; Kozinski, J.A. Effects of bio-additives on the physicochemical properties and mechanical behavior of canola hull fuel pellets. Renew. Energy 2019, 132, 296–307. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.; Cayuela, L.; Asuncion, R.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2017, 247, 1155–1164. [Google Scholar] [CrossRef]
- Raza, S.T.; Bo, Z.; Ali, Z.; Liang, T.J. Vermicomposting by Eisenia fetida is a Sustainable and Eco-Friendly Technology for Better Nutrient Recovery and Organic Waste Management in Upland Areas of China. Pak. J. Zool. 2019, 51, 1027–1034. [Google Scholar] [CrossRef]
- Salam, A.; Bashir, S.; Khan, I.; Hu, H.Q. Two years impacts of rapeseed residue and rice straw biochar on Pb and Cu immobilization and revegetation of naturally co-contaminated soil. Appl. Geochem. 2019, 105, 97–104. [Google Scholar] [CrossRef]
- Salam, A.; Bashir, S.; Khan, I.; Rizwan, M.S.; Chhajro, M.A.; Feng, X.W.; Zhu, J.; Hu, H. Biochars immobilize lead and copper in naturally contaminated soil. Environ. Eng. Sci. 2018, 35, 1349–1360. [Google Scholar] [CrossRef]
- Salam, A.; Shaheen, S.M.; Bashir, S.; Khan, I.; Wang, J.X.; Rinklebe, J.; Rehman, F.U.; Hu, H. Rice straw- and rapeseed residue-derived biochars affect the geochemical fractions and phytoavailability of Cu and Pb to maize in a contaminated soil under different moisture content. J. Environ. Manag. 2019, 237, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.W.; Gordon, A.H.; Lomax, J.A.; Chesson, A. Composition and rumen degradability of straw from three varieties of oilseed rape before and after alkali, hydrothermal and oxidative treatment. J. Sci. Food Agric. 1987, 41, 1–15. [Google Scholar] [CrossRef]
- Caraiman, P.; Pohontu, C.; Soreanu, G.; Macoveanu, M.; Cretescu, I. Optimization process of cadmium and zinc removal from soil by phytoremediation using Brassica napus and Triticales sp. Environ. Eng. Manag. J. 2012, 11, 271–278. [Google Scholar] [CrossRef]
- Angelova, V.R.; Ivanova, R.I.; Todorov, J.M.; Ivanov, K. Potential of rapeseed (Brassica napus L.) for phytoremediation of soils contaminated with heavy metals. J. Environ. Prot. ecol. 2017, 18, 468–478. [Google Scholar]
- Belouchrani, A.S.; Mameri, N.; Abdi, N.; Hocine, G.; Lounici, H.; Drouiche, N. Phytoremediation of soil contaminated with Zn using canola (Brassica napus L). Ecol. Eng. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Adiloglu, S.; Sume, M.T.S.A. Chrome (Cr) pollution in agricultural areas improvement by phytoremediation method with canola (Brassica napus L.) plant growing. J. Essent. Oil Bear. Plants 2015, 18, 1180–1186. [Google Scholar] [CrossRef]
- Gasco, G.; Álvarez, M.L.; Paz-Ferreiro, J.; Mendez, A. Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Riotinto (Spain). Chemosphere 2019, 231, 562–570. [Google Scholar] [CrossRef]
- Dhiman, S.S.; Selvaraj, C.; Li, J.; Singh, R.; Zhao, X.; Kim, D.; Kim, D.; Kim, J.Y.; Kang, Y.C.; Lee, J.K. Phytoremediation of metal-contaminated soils by the hyperaccumulator canola (Brassica napus L.) and the use of its biomass for ethanol production. Fuel 2016, 183, 107–114. [Google Scholar] [CrossRef]
- Gao, J.; Cao, W.; Li, D.; Xu, M.; Zeng, X.; Nie, J.; Zhang, W. Effects of long-term double-rice and green manure rotation on rice yield and soil organic matter in paddy field. Acta Ecol. Sin. 2011, 31, 4542–4548. [Google Scholar]
- Liu, Q.; Dai, Z.; Zhou, X.; Wang, Z.; Cong, R. Effects of rapeseed straw returning on increment of yield and supplying capacity of potassium in rice. Hubei Agric. Sci. 2014, 53, 5112–5115. [Google Scholar]
- Zhou, Y.; Wu, W.; Xu, Y.; Chen, G.; Xu, R.; Zhang, L. Effects of returned rapeseed straw on soil fertility and yield of subsequent rice. J. Yangzhou Univ. 2015, 36, 53–58. [Google Scholar]
- Grove, M.; Spencer, G.; Rohwedder, W.; Mandava, N.; Worley, J.F.; Warthen, J.D., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C., Jr. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Bernard, E.; Larkin, R.P.; Tavantzis, S.; Erich, M.S.; Alyokhin, A.; Sewell, G.; Lannan, A.; Gross, S.D. Compost, rapeseed rotation, and biocontrol agents significantly impact soil microbial communities in organic and conventional potato production systems. Appl. Soil Ecol. 2012, 52, 29–41. [Google Scholar] [CrossRef]
- Williams-Woodward, J.L.; Pfleger, F.L.; Fritz, A.V.; Allmaras, R.R. Green manures of oat, rape and sweet corn for reducing common root rot in pea (Pisum sativum) caused by Aphanomyces euteiches. Plant Soil 1997, 188, 43–48. [Google Scholar] [CrossRef]
- Ojaghian, M.R.; Cui, Z.Q.; Xie, G.L.; Li, B.; Zhang, J.Z. Brassica green manure rotation crops reduce potato stem rot caused by Sclerotinia sclerotium. Australas. Plant Pathol. 2012, 41, 347–349. [Google Scholar] [CrossRef]
- Cochran, K.A.; Rothrock, C.S. Brassica green manure amendments for management of Rhizoctonia solani in two annual ornamental crops in the field. Hortscience 2015, 50, 555–558. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Ren, P.; Zhao, S.; Wang, H.; Li, Y. Effects of different brassica green manure on growth and yield of tobacco. J. Henan Agric. Sci. 2017, 46, 52–76. [Google Scholar]
- Larkin, R.P.; Lynch, R.P. Use and effects of different brassica and other rotation crops on soil borne diseases and yield of potato. Horticulturae 2018, 4, 37. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Santo, G.S.; Wilson, J.H.; Hang, A.N. Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. Plant Dis. 1993, 77, 42–46. [Google Scholar] [CrossRef]
- Mojtahedi, H.; Santo, G.S.; Hang, A.N.; Wilson, J.H. Suppression of root-knot nematode populations with selected rapeseed cultivars as green manure. J. Nematol. 1991, 23, 170–174. [Google Scholar]
- Riga, E.; Mojtahedi, H.; Ingham, R.E.; McGuire, A.M. Green manure amendments and management of root knot nematodes on potato in the Pacific Northwest of USA. Nematol. Monogr. Perspect. 2003, 2, 151–158. [Google Scholar]
- Boydston, R.A.; Hang, A. Rapeseed (Brassica napus) green manure crop suppresses weeds in potato (Solanum tuberosum). Weed Technol. 1995, 9, 669–675. [Google Scholar] [CrossRef]
- Mennan, H.; Ngouajio, M. Effect of brassica cover crops and hazelnut husk mulch on weed control in hazelnut orchards. Hortitechnology 2012, 22, 99–105. [Google Scholar] [CrossRef]
- Lemerle, D.; Luckett, D.J.; Lockley, P.; Koetz, E.; Wu, H.W. Competitive ability of Australian canola (Brassica napus) genotypes for weed management. Crop Pasture Sci. 2014, 65, 1300–1310. [Google Scholar] [CrossRef]
- Madsen, H.; Talgre, L.; Eremeev, V.; Alaru, M.; Kauer, K.; Luik, A. Do green manures as winter cover crops impact the weediness and crop yield in an organic crop rotation? Biol. Agric. Hortic. 2016, 32, 182–191. [Google Scholar] [CrossRef]
- Mesbah, A.; Nilahyane, A.; Ghimire, B.; Beck, L.; Ghimire, R. Efficacy of cover crops on weed suppression, wheat yield, and water conservation in winter wheat-sorghum-fallow. Crop Sci. 2019, 59, 1745–1752. [Google Scholar] [CrossRef]
- Borlaug, N.E.; Dowswell, C.R. Feeding a world of ten billion people: A 21st century challenge. In Proceedings of the International Congress in the Wake of the Double Helix: From the Green Revolution to the Gene Revolution; Iowa State Press: Ames, IA, USA, 2003; pp. 27–31. [Google Scholar] [CrossRef]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar] [CrossRef]
- Kalloo, G.; Rai, M.; Singh, M.; Kumar, S. Heterosis in Crop Plants; Researchco Book Centre: New Delhi, India, 2006. [Google Scholar]
- Riddle, N.C.; Birchler, J.A. Comparative analysis of inbred and hybrid maize at the diploid and tetraploid levels. Theor. Appl. Genet. 2008, 116, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, G.; Jiang, G.; Zhang, H. Utilization of crop heterosis: A review. Euphytica 2014, 197, 161. [Google Scholar] [CrossRef]
- Munaro, E.M.; Eyherabide, G.H.; D’Andrea, K.E.; Cirilo, A.G.; Otegui, M.E. Heterosis x environment interaction in maize: What drives heterosis for grain yield? Field Crops Res. 2011, 124, 441–449. [Google Scholar] [CrossRef]
- Kaeppler, S.M. Heterosis: Many genes, many mechanisms—End the search for an undiscovered unifying theory. Int. Sch. Res. Not. Bot. 2012, 682824. [Google Scholar] [CrossRef]
- Qian, W.; Sass, O.; Meng, J.; Li, M.; Frauen, M.; Jung, C. Heterotic patterns in rapeseed (Brassica napus L.): I. Crosses between spring and Chinese semi-winter lines. Theor. Appl. Genet. 2007, 115, 27–34. [Google Scholar] [CrossRef]
- Qian, W.; Li, Q.; Noack, J.; Sass, O.; Meng, J.; Frauen, M.; Jung, C. Heterotic patterns in rapeseed (Brassica napus L.): II. Crosses between European winter and Chinese semi-winter lines. Plant Breed. 2009, 128, 466–470. [Google Scholar] [CrossRef]
- Radoev, M.; Becker, H.C.; Ecke, W. Genetic analysis of heterosis for yield and yield components in rapeseed (Brassica napus L.) by quantitative trait locus mapping. Genetics 2008, 179, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.C.; Engqvist, G.M.; Karlsson, B. Comparison of rapeseed cultivars and resynthesized lines based on allozyme and RFLP markers. Theor. Appl. Genet. 1995, 91, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Girke, A.; Schierholt, A.; Becker, H.C. Extending the rapeseed gene pool with resynthesized Brassica napus II: Heterosis. Theor. Appl. Genet. 2012, 124, 1017. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Dehghani, H.; Alizadeh, B.; Mohghaddam, M. Genetic Analysis of Oil Yield, Seed Yield, and Yield Components in Rapeseed Using Additive Main Effects and Multiplicative Interaction Biplots. Agron. J. 2010, 102, 1361–1368. [Google Scholar] [CrossRef]
- Tian, H.Y.; Channa, S.A.; Hu, S.W. Heterotic Grouping and the Heterotic Pattern among Chinese Rapeseed (Brassica napus L.) Accessions. Agron. J. 2015, 107, 1321–1330. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Q.; Mei, J.; Zhang, Y.; Li, J.; Li, Z.; Ge, X.; Xiong, Z.; Huang, Y.; Qian, Y. Improvement of Brassica napus via interspecific hybridization between B. napus and B. oleracea. Mol. Breed. 2014, 34, 1955. [Google Scholar] [CrossRef]
- Luo, X.; Ma, C.; Yi, B.; Tu, J.; Shen, J.; Fu, T. Genetic distance revealed by genomic single nucleotide polymorphisms and their relationships with harvest index heterotic traits in rapeseed (Brassica napus L.). Euphytica 2016, 209, 41. [Google Scholar] [CrossRef]
- Shivanna, K.; Sawhney, V. Pollen biology and pollen biotechnology: An introduction. In Pollen Biotechnology for Crop Production and Improvement; Knox, R., Shivanna, K., Sawhney, V., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 1–12. [Google Scholar] [CrossRef]
- Shivanna, K.R. Self-incompatibility. In Pollen Biology and Biotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Shivanna, K.R. Pollen Sterility. In Pollen Biology and Biotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar] [CrossRef]
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Behera, T.K. Current understanding of male sterility systems in vegetable Brassicas and their exploitation in hybrid breeding. Plant Reprod. 2019, 32, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Engelke, T.; Hirsche, J.; Roitsch, T. Metabolically engineered male sterility in rapeseed (Brassica napus L.). Theor. Appl. Genet. 2011, 122, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ding, Y.; Chen, Z.; Mei, J.; Qian, W. Improvement of the resistance against Sclerotinia sclerotiorum in Ogu CMS restorer in Brassica napus using wild B. oleracea as donor. Sci. Agric. Sin. 2020, 53, 1950–1958. [Google Scholar]
- Li, P.; Kang, L.; Wang, A.; Cui, C.; Jiang, L.; Guo, S.; Ge, X.; Li, Z. Development of a fertility restorer for inap CMS (Isatis indigotica) Brassica napus through genetic introgression of one alien addition. Front. Plant Sci. 2019, 10, 257. [Google Scholar] [CrossRef]
- Heath, D.W.; Earle, E.D. Synthesis of Ogura male sterile rapeseed (Brassica napus L.) with cold tolerance by protoplast fusion and effects of atrazine resistance on seed yield. Plant Cell Rep. 1996, 15, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Kilian, B.; Graner, A. NGS technologies for analyzing germplasm diversity in genebanks. Brief. Funct. Genomics 2012, 11, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, S.; Gardner, C.; Lübberstedt, T. Emerging Avenues for Utilization of Exotic Germplasm. Trends Plant Sci. 2017, 22, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Gorjanc, G.; Jenko, J.; Hearne, S.J.; Hickey, J.M. Initiating maize pre-breeding programs using genomic selection to harness polygenic variation from landrace populations. BMC Genomics 2016, 17, 30. [Google Scholar] [CrossRef]
- Seyis, F.; Snowdon, R.J.; Lühs, W.; Friedt, W. Molecular characterisation of novel resynthesised rapeseed (Brassica napus L.) lines and analysis of their genetic diversity in comparison to spring rapeseed cultivars. Plant Breed. 2003, 122, 473–478. [Google Scholar] [CrossRef]
- Quijada, P.A.; Udall, J.A.; Lambert, B.; Osborn, T.C. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 1. Identification of genomic regions from winter germplasm. Theor. Appl. Genet. 2006, 113, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Udall, J.A.; Quijada, P.A.; Lambert, B.; Osborn, T.C. Quantitative trait analysis of seed yield and other complex traits in hybrid spring rapeseed (Brassica napus L.): 2. Identification of alleles from unadapted germplasm. Theor. Appl. Genet. 2006, 113, 597–609. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Munir, I.; Ali, W.; Swati, Z.A.; Khattak, M.S.; Sohail, Q.; Sohail, Q.; Khan, I. Assessment of Genetic Diversity of Local and Exotic Brassica napus Germplasm. Pak. J. Biol. Sci. 2007, 10, 2490–2494. [Google Scholar] [CrossRef]
- Cowling, W.A.; Buirchell, B.J.; Falk, D.E. A model for incorporating novel alleles from the primary gene pool into elite crop breeding programs while reselecting major genes for domestication or adaptation. Crop Pasture Sci. 2009, 60, 1009–1015. [Google Scholar] [CrossRef]
- Kramer, C.C.; Polewicz, H.; Osborn, T.C. Evaluation of QTL alleles from exotic sources for hybrid seed yield in the original and different genetic backgrounds of spring-type Brassica napus L. Mol. Breed. 2009, 24, 419431. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Long, Y.; Liu, L.; Zhao, Y.; Tian, J.; Zhao, W.; Li, B.; Chen, L.; Chao, H.; et al. Dynamic and comparative QTL analysis for plant height in different developmental stages of Brassica napus L. Theor. Appl. Genet. 2015, 128, 1175–1192. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, J.; Wang, X.; Han, X.; Wei, B.; Wang, J.; Li, B.; Yu, H.; Huang, Q.; Gu, H.; et al. The WRKY Transcription Factor WRKY71/EXB1 Controls Shoot Branching by Transcriptionally Regulating RAX Genes in Arabidopsis. Plant Cell 2015, 27, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Ding, G.D.; Xu, F.S.; Cai, H.M.; Zou, J.; Ye, X.S. Genotype differences in photosynthetic characteristics and nitrogen efficiency of new-type oilseed rape responding to low nitrogen stress. J. Agric. Sci. 2015, 153, 1–14. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Abel, S.; Zhao, Y. Potential of genomic selection in rapeseed (Brassica napus L.) breeding. Plant Breed. 2014, 133, 45–51. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.H.; Liu, W.C.; Zhang, X.W.; Lu, Y.T. Ethylene Inhibits Root Elongation during Alkaline Stress through AUXIN1 and Associated Changes in Auxin Accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhao, Y.; Liu, P.; Shi, L.; Wang, X.; Wang, M.; Meng, J.; Reif, J.C. Seed Quality Traits Can Be Predicted with High Accuracy in Brassica napus Using Genomic Data. PLoS ONE 2016, 11, e0166624. [Google Scholar] [CrossRef]
- Yu, J.; Holland, J.B.; McMullen, M.D.; Buckler, E.S. Genetic Design and Statistical Power of Nested Association Mapping in Maize. Genetics 2008, 178, 539–551. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.D.; Kresovich, S.; Villeda, H.S.; Bradbury, P.; Li, H.; Sun, Q.; Flint-Garcia, S.; Thornsberry, J.; Acharya, C.; Bottoms, C.; et al. Genetic properties of the maize nested association mapping population. Science 2009, 325, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Samans, B.; Snowdon, R.J.; Scholz, U.; Weise, S.; von Wiren, N.; Ordon, F.; Scholz, U.; Weise, S.; von Wirén, N.; Nagel, K.A.; et al. PreBreed-Yield: Population Genomics Resources for Nested Association Mapping and Precision Breeding in Winter Oilseed Rape. In Proceedings of the International Plant and Animal Genome Conference XXI, San Diego, CA, USA, 12–16 January 2013. [Google Scholar] [CrossRef]
- Shunmugam, A.S.K.; Soolanayakanahally, R.; Yang, L.; Horner, K.; Higgins, E.; Lewis, M.; Parkin, I.; Vail, S.; Robinson, S. Characterizing growth physiology, flowering phenology, yield components and seed quality attributes in founder lines of a spring Brassica napus Nested Association Mapping population. In Proceedings of the 14th International Rapeseed Congress, Saskatoon, SK, Canada, 5–9 July 2015. [Google Scholar]
- Li, J.; Bus, A.; Spamer, V.; Stich, B. Comparison of statistical models for nested association mapping in rapeseed (Brassica napus L.) through computer simulations. BMC Plant Biol. 2016, 16, 26. [Google Scholar] [CrossRef]
- Huang, B.E.; Verbyla, K.L.; Verbyla, A.P.; Raghavan, C.; Singh, V.K.; Gaur, P.; Leung, H.; Varshney, R.K.; Cavanagh, C.R. MAGIC populations in crops: Current status and future prospects. Theor. Appl. Genet. 2015, 128, 999. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, H.; Wang, X.; Li, X. Construction and application potential of MAGIC population on genetic breeding of rapeseed (Brassica napus L.). Chin. J. Oil Crop Sci. 2017, 39, 145–151. [Google Scholar]
- Feuillet, C.; Leach, J.E.; Rogers, J.; Schnable, P.S.; Eversole, K. Crop genome sequencing: Lessons and rationales. Trends Plant Sci. 2011, 16, 77–88. [Google Scholar] [CrossRef]
- Bentley, D.R. Whole-genome re-sequencing. Curr. Opin. Genet. Dev. 2006, 16, 545–552. [Google Scholar] [CrossRef]
- Stratton, M. Genome resequencing and genetic Variation. Nat. Biotechnol. 2008, 26, 65–66. [Google Scholar] [CrossRef]
- Ng, P.C.; Kirkness, E.F. Whole Genome Sequencing. Methods Mol. Biol. 2010, 628, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.E.; Hurgobin, B.; Golicz, A.A.; Chan, C.K.; Yuan, Y.; Lee, H.; Renton, M.; Meng, J.; Li, R.; Long, Y.; et al. Assembly and comparison of two closely related Brassica napus genomes. Plant Biotechnol. J. 2017, 15, 1602–1610. [Google Scholar] [CrossRef]
- Sun, F.; Fan, G.; Hu, Q.; Zhou, Y.; Guan, M.; Tong, C.; Li, J.; Du, D.; Qi, C.; Jiang, L.; et al. The high-quality genome of Brassica napus cultivar ‘ZS11’ reveals the introgression history in semi-winter morphotype. Plant J. 2017, 92, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Liu, D.X.; Xie, W.Z.; Yang, Q.Y.; Chen, L.L. BnPIR: Brassica napus pan-genome information resource for 1689 accessions. Plant Biotechnol. J. 2021, 19, 412–414. [Google Scholar] [CrossRef]
- Huang, S.; Deng, L.; Guan, M.; Li, J.; Lu, K.; Wang, H.; Fu, D.; Mason, A.S.; Liu, S.; Hua, W. Identification of genome-wide single nucleotide polymorphisms in allopolyploid crop Brassica napus. BMC Genom. 2013, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhou, T.; Ding, G.; Yang, Q.; Shi, L.; Xu, F. Physiological, genomic and transcriptional diversity in responses to boron deficiency in rapeseed genotypes. J. Exp. Bot. 2016, 67, 5769–5784. [Google Scholar] [CrossRef]
- Li, K.; Yao, Y.; Xiao, L.; Zhao, Z.; Guo, S.; Fu, Z.; Du, D. Fine mapping of the Brassica napus Bnsdt1 gene associated with determinate growth habit. Theor. Appl. Genet. 2018, 131, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, K.; Liu, H.; Duncan, R.W.; Guo, S.; Xiao, L.; Du, D. Whole-genome re-sequencing and fine mapping of an orange petal color gene (Bnpc1) in spring Brassica napus L. to a 151-kb region. Euphytica 2017, 213, 165. [Google Scholar] [CrossRef]
- Schiessl, S.V.; Huettel, B.; Kuehn, D.; Reinhardt, R.; Snowdon, R.J. Flowering Time Gene Variation in Brassica Species Shows Evolutionary Principles. Front. Plant Sci. 2017, 8, 1742. [Google Scholar] [CrossRef]
- Liu, S.; Fan, C.; Li, J.; Cai, G.; Yang, Q.; Wu, J.; Yi, X.; Zhang, C.; Zhou, Y. A genome wide association study reveals novel elite allelic variations in seed oil content of Brassica napus. Theor. Appl. Genet. 2016, 129, 1203–1215. [Google Scholar] [CrossRef]
- Zhang, K.; Raboanatahiry, N.; Zhu, B.; Li, M. Progress in Genome Editing Technology and Its Application in Plants. Front. Plant Sci. 2017, 8, 177. [Google Scholar] [CrossRef]
- Schiml, S.; Fauser, F.; Puchta, H. The CRISPR/Cas system can be used as nuclease for in planta gene targeting and as paired nickases for directed mutagenesis in Arabidopsis resulting in heritable progeny. Plant J. 2014, 80, 1139–1150. [Google Scholar] [CrossRef]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crops Food 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering Quantitative Trait Variation for Crop Improvement by Genome Editing. Cell 2017, 171, 470–480. [Google Scholar] [CrossRef]
- Zhang, K.; Nie, L.; Cheng, Q.; Yin, Y.; Chen, K.; Qi, F.; Zou, D.; Liu, H.; Zhao, W.; Wang, B.; et al. Effective editing for lysophosphatidic acid acyltransferase 2/5 in allotetraploid rapeseed (Brassica napus L.) using CRISPR-Cas9 system. Biotechnol. Biofuels 2019, 12, 225. [Google Scholar] [CrossRef]
- Sun, Q.; Lin, L.; Liu, D.; Wu, D.; Fang, Y.; Wu, J.; Wang, Y. CRISPR/Cas9-Mediated Multiplex Genome Editing of the BnWRKY11 and BnWRKY70 Genes in Brassica napus L. Int. J. Mol. Sci. 2018, 19, 2716. [Google Scholar] [CrossRef]
- Sriboon, S.; Li, H.; Guo, C.; Senkhamwong, T.; Dai, C.; Liu, K. Knock-out of TERMINAL FLOWER 1 genes altered flowering time and plant architecture in Brassica napus. BMC Genet. 2020, 21, 52. [Google Scholar] [CrossRef]
- Braatz, J.; Harloff, H.J.; Mascher, M.; Stein, N.; Himmelbach, A.; Jung, C. CRISPR-Cas9 Targeted Mutagenesis Leads to Simultaneous Modification of Different Homoeologous Gene Copies in Polyploid Oilseed Rape (Brassica napus). Plant Physiol. 2017, 174, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, J.J.; Tang, T.; Liu, K.D.; Dai, C. CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci. Rep. 2017, 7, 7489. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, K.; Tang, M.; Zhou, W.; Chen, L.; Chen, Z.; Li, M. CRISPR-based genome editing technology and its applications in oil crops. Oil Crop Sci. 2021, 6, 105–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).