A Sustainable Reversed-Phase HPTLC Method for the Quantitative Estimation of Hesperidin in Traditional and Ultrasound-Assisted Extracts of Different Varieties of Citrus Fruit Peels and Commercial Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Chromatography
2.4. HSP Calibration Plot and Quality Control (QC) Samples
2.5. Sample Preparation for the Quantitative Analysis of HSP in TE of Citrus Fruit Peels
2.6. Sample Preparation for the Quantitative Analysis of HSP in UBE of Citrus Fruit Peels
2.7. Sample Processing for the Quantitative Analysis of HSP in TE of Commercial Tablet Dosage Forms
2.8. Sample Preparation for the Quantitative Analysis of HSP in UBE of Commercial Tablet Dosage Forms
2.9. Validation Studies
2.10. Quantitative Estimation of HSP in TE and UBE of Citrus Fruits and Commercial Formulations
2.11. Greenness Evaluation
3. Results and Discussion
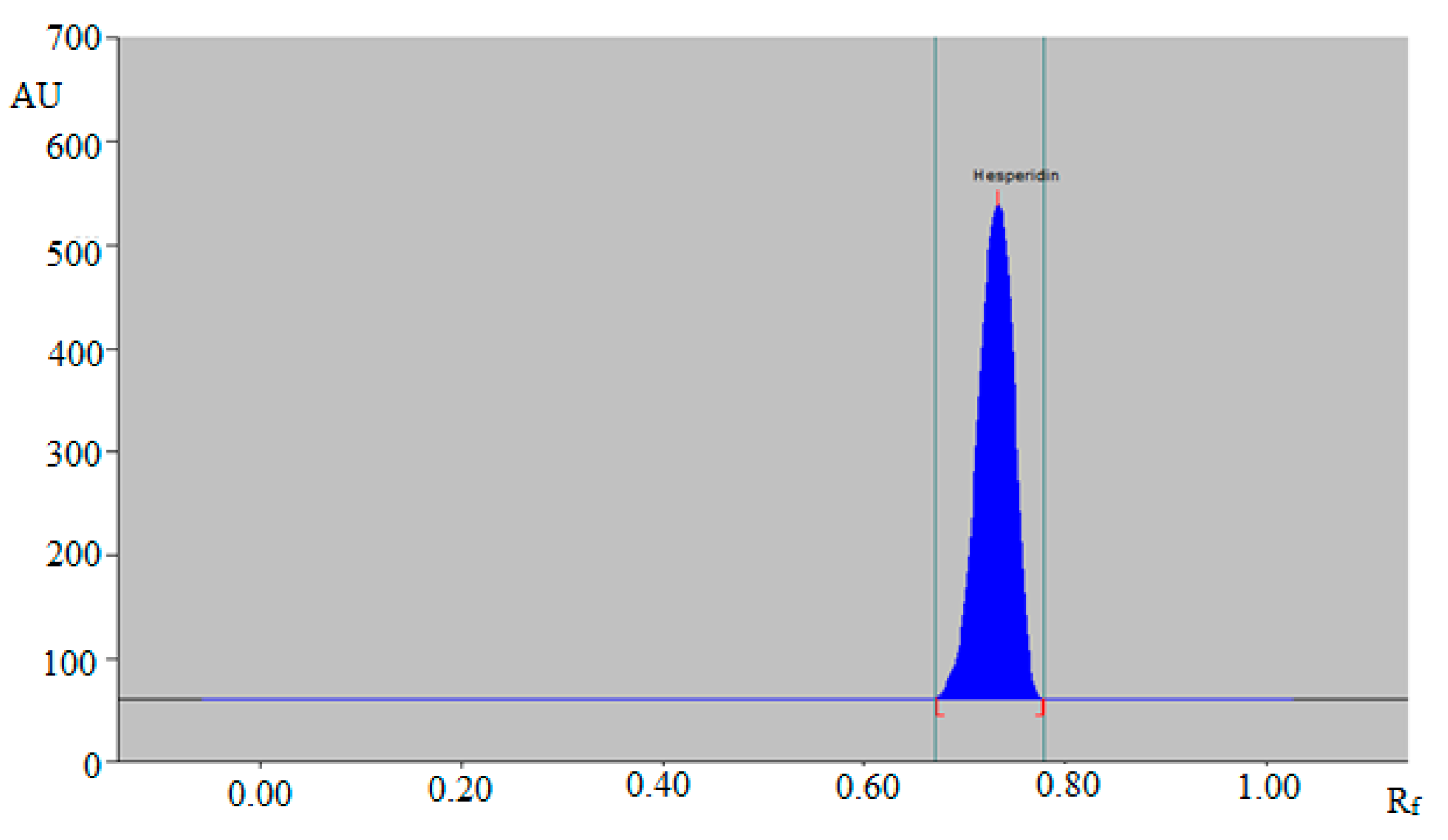
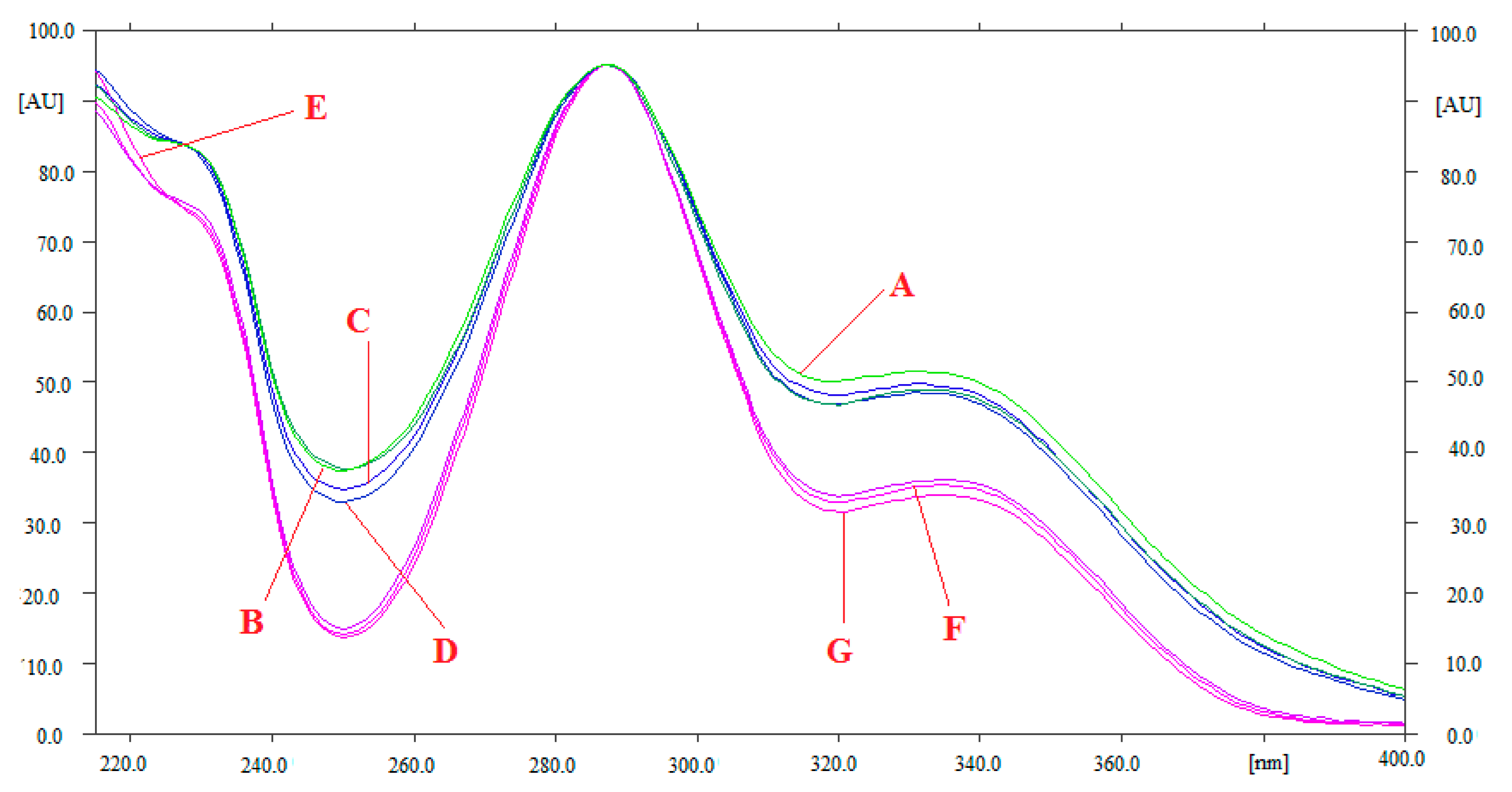
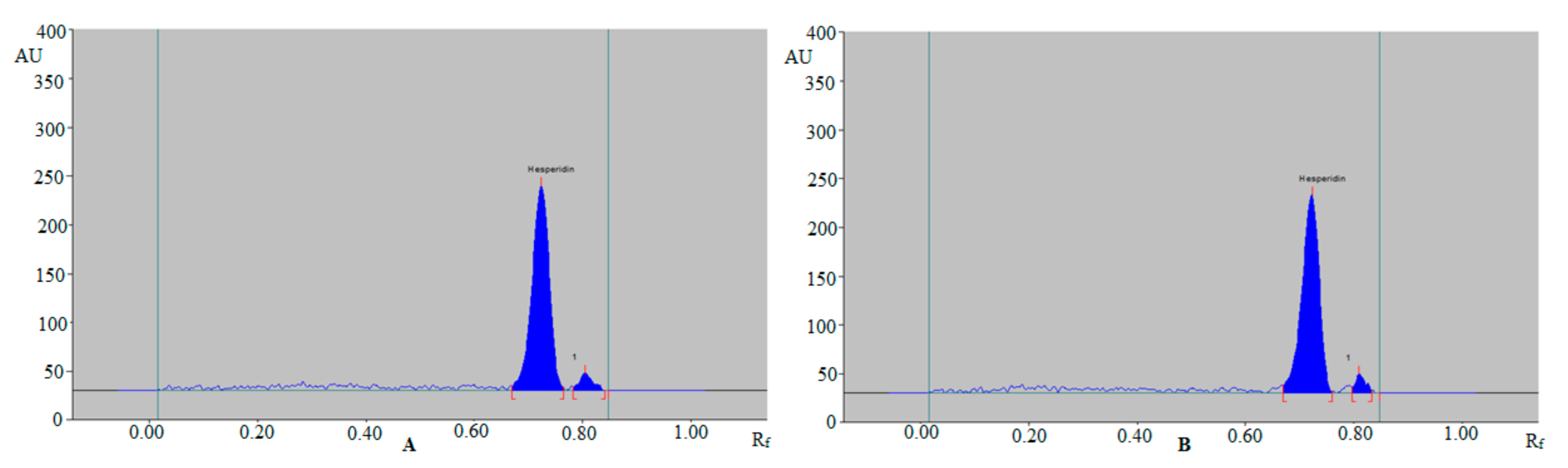
3.1. Method Development
3.2. Validation Studies
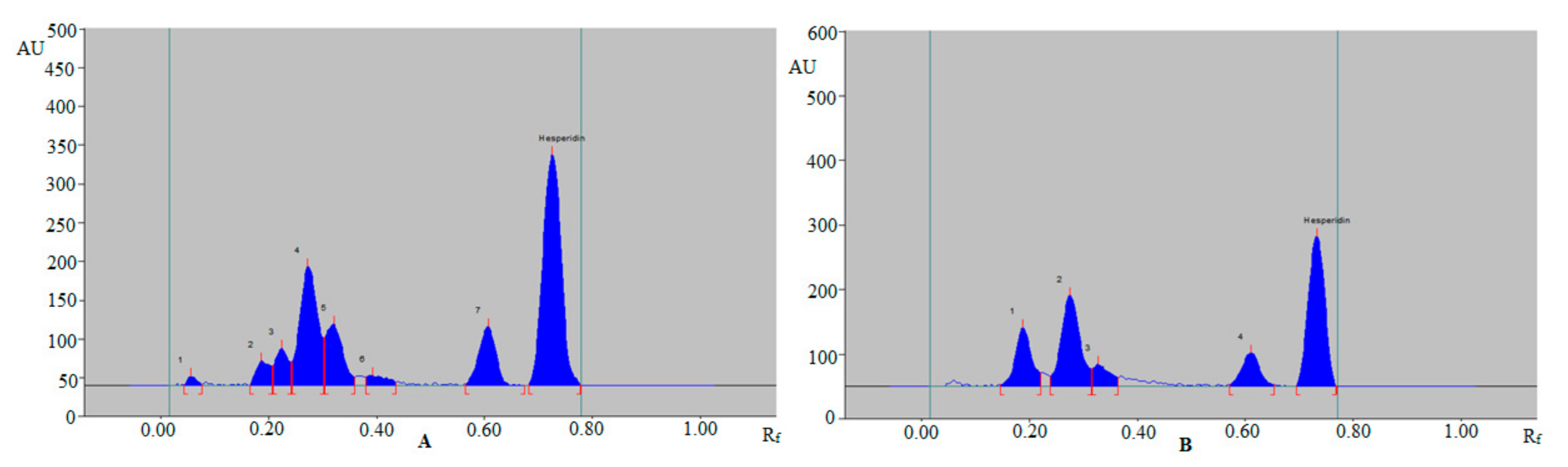
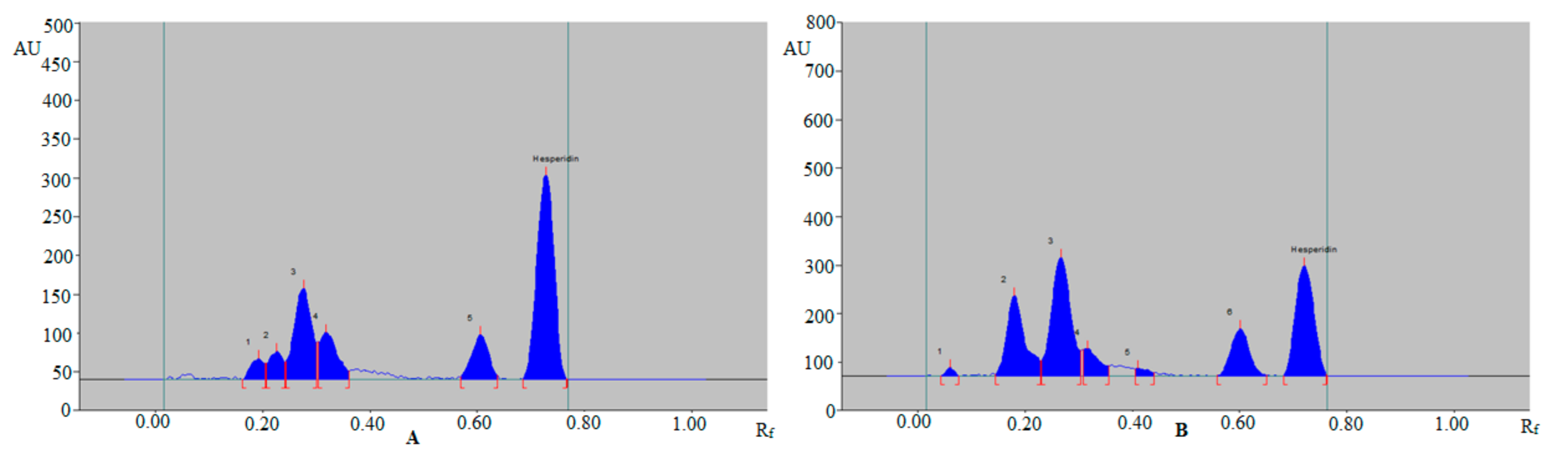
3.3. Application of Sustainable RP-HPTLC Assay for Quantitative Estimation of HSP in Various TE and UBE of Different Varieties of Citrus Fruit Peels and Commercial Formulations
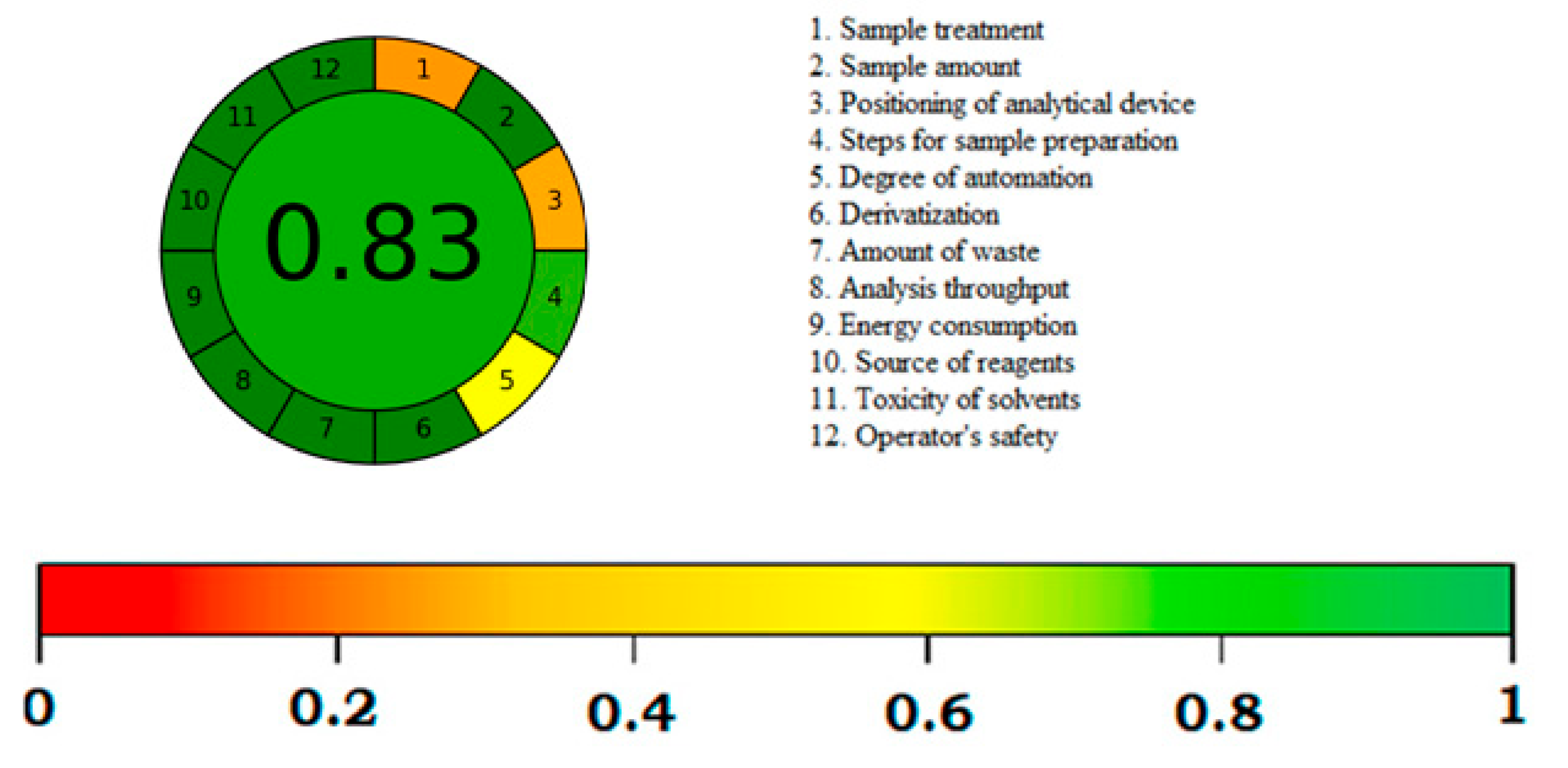
3.4. Greenness Assessment
3.5. Comparison with Reported Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J. Flavonoids in grape fruit and commercial grape fruit juices: Concentration, distribution, and potential health benefits. Proc. Fla. State Hort. Soc. 2007, 120, 288–294. [Google Scholar]
- Jokic, S.; Safranko, S.; Jakovljevic, M.; Cikos, A.M.; Kajic, N.; Kolarevic, F.; Babic, J.; Molnar, M. Sustainable green procedure for extraction of hesperidin from selected Croatian mandarin peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Saidani Tounsi, M. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric Food Chem. 2005, 15, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): Anti-inflammatory and analgesic activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Crespo, M.E.; Gálvez, J.; Cruz, T.; Ocete, M.A.; Zarzuelo, A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999, 65, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Emim, J.A.; Oliveira, A.B.; Lapa, A.J. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussesquinone, in rats and mice. J. Pharm. Pharmacol. 1994, 46, 118–122. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, S.H.; Park, S.H.; Park, E.K.; Kim, D.H. Antiallergic activity of hesperidin is activated by intestinal microflora. Pharmacology 2004, 71, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.; Singla, A.K. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother. Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2011, 49, 276–282. [Google Scholar] [CrossRef]
- Srilatha, D.; Nasare, M.; Nagasandhya, B.; Prasad, V.; Diwan, P. Development and validation of spectrophotometric method for simultaneous estimation of hesperidin and diosmin in the pharmaceutical dosage form. ISRN Spectroscop. 2013, 2013, E534830. [Google Scholar] [CrossRef] [Green Version]
- Goren, R.; Monselise, S.P. Determination of hesperidin in dry matter of citrus tissues by ultra-violet spectrophotometry method. J. Assoc. Off. Agric. Chem. 1964, 47, 677–681. [Google Scholar]
- El-Shafae, A.M.; El-Domiaty, M.M. Improved LC methods for the determination of diosmin and/ or hesperidin in plant extracts and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2001, 26, 539–545. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Xu, G.; Liu, D. Ultrasound-assisted extraction of hesperidin from penggan (Citrus reticuleta) peel. Ultrason. Sonochem. 2008, 15, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N. Qualitative and quantitative estimation of hesperidin in peel and juice of citrus fruits by RP-HPLC method growing in Kurdistan region/Iraq. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 220–224. [Google Scholar]
- Pereira, R.M.S.; Lopez, B.G.C.; Diniz, S.N.; Antunes, A.A.; Garcia, D.M.; Oliveira, C.R.; Marcucci, M.C. Quantification of flavonoids Brazilian orange peels and industrial orange juice processing wastes. Agric. Sci. 2017, 8, 631–644. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, T.; Khan, M.R.; Shabbir, M.A.; Zia, M.A. Phytochemical profiling and HPLC quantification of citrus peel from different varieties. Prog. Nutr. 2017, 20, S279–S288. [Google Scholar]
- Al-Jaber, H.I.; Shakya, A.K.; Elagbar, Z.A. Phytochemical profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia heirosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ 2020, 8, E9769. [Google Scholar] [CrossRef]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wele, A.; Paolini, J. LC-MS/MS analysis of flavonoid compounds from Zanthoxylum zanthoxyloids extracts and their antioxidant activities. Nat. Prod. Comm. 2017, 12, 1865–1868. [Google Scholar]
- Lee, J.T.; Pao, L.H.; Hsieh, C.D.; Huang, P.W.; Hu, H.Y.P. Development and validation of an LC-MS/MS method for simultaneous quantification of hesperidin and hesperetin in rat plasma for pharmacokinetic studies. Anal. Methods 2017, 9, 3329–3337. [Google Scholar] [CrossRef]
- Mandal, P.; Dan, S.; Chakraborty, S.; Ghosh, B.; Saha, C.; Khanam, J.; Pal, T.K. Simultaneous determination and quantitation of diosmetin and hesperetin in human plasma by liquid chromatographic mass spectrometry with an application to pharmacokinetic studies. J. Chromatogr. Sci. 2019, 57, 451–461. [Google Scholar] [CrossRef]
- Takiyama, M.; Matsumoto, T.; Watanabe, J. LC-MS/MS detection of citrus unshiu peel-derived flavonoids in the plasma and brain after oral administration of yokukansankachimpihange in rats. Xenobiotica 2019, 49, 1494–1503. [Google Scholar] [CrossRef]
- Araujo-Leon, J.A.; Ortiz-Andrade, R.; Vera-Sanchez, R.A.; Oney-Montalvo, J.E.; Coral-Martinez, T.I.; Cantillo-Ciau, Z. Development and optimization of a high sensitivity LC-MS/MS method for the determination of hesperidin and naringenin in rat plasma: Pharmacokinetic approach. Molecules 2020, 25, 4241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Y.; Shan, M.; Yu, S.; Cheng, F.; Chen, P.; Bao, B.; Cao, Y.; Zhang, L.; Wu, Q. Simultaneous determination of three active flavonoids in rat plasma by HPLC-MS/MS: Application to a comparative pharmacokinetic investigation after oral administration Schizonepeta tenuifolia aqueous extract with and without its volatile oil. Acta Chroomatogr 2020. [CrossRef]
- Wang, W.; Zhao, L.; Huang, H.; Yao, J.; Zhou, L.; Wang, D.; Qiu, X. Development of an ultra-high performance liquid chromatography method for simultaneous determination of six active compounds in Fructus aurantii and rat plasma and its application to a comparative pharmacokinetic study in rats administered with different doses. J. Anal. Methods Chem. 2018, 2018, E7579136. [Google Scholar]
- Mohamed, D.; Tawakkol, S.M. Fluorimetric determination of diosmin and hesperidin in combined dosage forms and in plasma through complex formation with terbium. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Alam, A.; Anwer, M.K.; Alqasoumi, S.I. Quantitative estimation of hesperidin in different varieties of citrus peels. Asian Pac. J. Trop. Biomed. 2014, 4, 262–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, P.; Shakeel, F.; Alqarni, M.H.; Foudah, A.I.; Faiyazuddin, M.; Alshehri, S. Rapid, sensitive, and sustainable reversed-phase HPTLC method in comparison to the normal-phase HPTLC for the determination of pterostilbene in capsule dosage form. Processes 2021, 9, 1305. [Google Scholar] [CrossRef]
- Alam, P.; Ezzeldin, E.; Iqbal, M.; Anwer, M.K.; Mostafa, G.A.E.; Alqarni, M.H.; Foudah, A.I.; Shakeel, F. Ecofriendly densitometric RP-HPTLC method for determination of rivaroxaban in nanoparticle formulations using green solvents. RSC Adv. 2020, 10, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Iqbal, M.; Ezzeldin, E.; Khalil, N.Y.; Foudah, A.I.; Alqarni, M.H.; Shakeel, F. Simple and accurate HPTLC-densitometry method for quantification of delafloxacin (a novel fluoroquinolone antibiotic) in plasma samples: Application to pharmacokinetic study in rats. Antibiotics 2020, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.A.; Elmansi, H.; Fathy, M.E. Green RP-HPLC method for simultaneous determination of moxifloxacin combinations: Investigation of the greenness for the proposed method. Microchem. J. 2019, 148, 151–161. [Google Scholar] [CrossRef]
- Abou-Taleb, N.H.; Al-Enany, N.M.; El-Sherbiny, D.T.; El-Subbagh, H.I. Digitally enhanced thin layer chromatography for simultaneous determination of norfloxacin tinidazole with the aid of Taguchi orthogonal array and desirability function approach: Greenness assessment by analytical eco-scale. J. Sep. Sci. 2020, 43, 1195–1202. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, E105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Koscielniak, P. What color is your method? Adaptation of the RGB additive color model to analytical method evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Validation of Analytical Procedures–Text and Methodology. In Proceedings of the International Conference on Harmonization (ICH), Q2 (R1), Geneva, Switzerland, 11–13 October 2005. [Google Scholar]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, E105960. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| Linearity range (ng band−1) | 20–2000 |
| Regression equation | y = 34.463x − 112.940 |
| R2 | 0.9995 |
| Slope ± SD | 34.463 ± 1.870 |
| Intercept ± SD | 112.940 ± 4.420 |
| Standard error of slope | 0.76 |
| Standard error of intercept | 1.80 |
| 95% confidence interval of slope | 31.17–37.74 |
| 95% confidence interval of intercept | 105.17–120.70 |
| LOD ± SD (ng band−1) | 7.02 ± 0.28 |
| LOQ ± SD (ng band−1) | 21.06 ± 0.84 |
| Parameters | Value |
|---|---|
| Rf | 0.73 ± 0.03 |
| As | 1.05 ± 0.04 |
| N m−1 | 5154 ± 3.11 |
| Conc. (ng band−1) | Conc. Found (ng band−1) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| 60 | 61.04 ± 0.51 | 101.73 | 0.83 |
| 800 | 810.42 ± 4.13 | 101.30 | 0.50 |
| 2000 | 1984.31 ± 6.62 | 99.21 | 0.33 |
| Conc. (ng band−1) | Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng band−1) ± SD | Standard Error | CV (%) | Conc. (ng band−1) ± SD | Standard Error | CV (%) | |
| 60 | 58.84 ± 0.43 | 0.17 | 0.73 | 59.54 ± 0.48 | 0.19 | 0.80 |
| 800 | 794.81 ± 3.81 | 1.55 | 0.47 | 812.41 ± 4.02 | 1.64 | 0.49 |
| 2000 | 2016.15 ± 6.61 | 2.69 | 0.32 | 1983.54 ± 7.17 | 2.92 | 0.36 |
| Conc. (ng band−1) | Mobile Phase Composition (Ethanol/Water) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | Level | Conc. (ng band−1) ± SD | % CV | Rf | |
| 52:48 | +2.0 | 784.18 ± 4.51 | 0.57 | 0.72 | ||
| 800 | 50:50 | 50:50 | 0.0 | 804.54 ± 4.73 | 0.58 | 0.73 |
| 48:52 | −2.0 | 821.34 ± 5.10 | 0.62 | 0.74 | ||
| Samples | Traditional Extraction | Ultrasound-Based Extraction |
|---|---|---|
| Amount of HSP (% w w−1) | ||
| Grapefruit peels (Citrus paradise) | 8.26 ± 0.42 | 11.41 ± 0.51 |
| Mosambi peels (Citrus limetta) | 6.94 ± 0.33 | 8.86 ± 0.48 |
| Lemon peels (Citrus lemon) | 5.90 ± 0.31 | 7.98 ± 0.45 |
| Orange peels (Citrus sinensis) | 6.81 ± 0.28 | 8.64 ± 0.50 |
| Formulation A | 5.31 ± 0.25 | 6.72 ± 0.35 |
| Formulation A | 5.55 ± 0.27 | 6.92 ± 0.38 |
| Analytical Method | Linearity Range | Accuracy (% Recovery) | Precision (% CV) | Ref. |
|---|---|---|---|---|
| HPLC | 20–100 (µg mL−1) | 89.70–107.00 | 0.44–1.80 | [16] |
| LC-MS | 0.25–2.50 (µg mL−1) | 89.10–114.90 | 4.40–8.70 | [20] |
| Spectrophotometry | 5.00–50.00 (µg mL−1) | 98.66–101.00 | 0.05–0.21 | [12] |
| Fluorimetry | 3.86 × 10−6–1.64 × 10−5 (mol) | 97.04–98.34 | 0.48–0.71 | [27] |
| Normal-phase HPTLC | 100–800 (ng band−1) | 98.55–99.38 | 0.76–1.20 | [28] |
| RP-HPTLC | 20–2000 (ng band−1) | 99.21–101.73 | 0.32–0.80 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foudah, A.I.; Shakeel, F.; Alam, P.; Alqarni, M.H.; Abdel-Kader, M.S.; Alshehri, S. A Sustainable Reversed-Phase HPTLC Method for the Quantitative Estimation of Hesperidin in Traditional and Ultrasound-Assisted Extracts of Different Varieties of Citrus Fruit Peels and Commercial Tablets. Agronomy 2021, 11, 1744. https://doi.org/10.3390/agronomy11091744
Foudah AI, Shakeel F, Alam P, Alqarni MH, Abdel-Kader MS, Alshehri S. A Sustainable Reversed-Phase HPTLC Method for the Quantitative Estimation of Hesperidin in Traditional and Ultrasound-Assisted Extracts of Different Varieties of Citrus Fruit Peels and Commercial Tablets. Agronomy. 2021; 11(9):1744. https://doi.org/10.3390/agronomy11091744
Chicago/Turabian StyleFoudah, Ahmed I., Faiyaz Shakeel, Prawez Alam, Mohammed H. Alqarni, Maged S. Abdel-Kader, and Sultan Alshehri. 2021. "A Sustainable Reversed-Phase HPTLC Method for the Quantitative Estimation of Hesperidin in Traditional and Ultrasound-Assisted Extracts of Different Varieties of Citrus Fruit Peels and Commercial Tablets" Agronomy 11, no. 9: 1744. https://doi.org/10.3390/agronomy11091744
APA StyleFoudah, A. I., Shakeel, F., Alam, P., Alqarni, M. H., Abdel-Kader, M. S., & Alshehri, S. (2021). A Sustainable Reversed-Phase HPTLC Method for the Quantitative Estimation of Hesperidin in Traditional and Ultrasound-Assisted Extracts of Different Varieties of Citrus Fruit Peels and Commercial Tablets. Agronomy, 11(9), 1744. https://doi.org/10.3390/agronomy11091744








