Abstract
Irregular rooting of rosemary stem cuttings, causing differences in either stem maturation or responses to growth conditions, restricts uniform production. Here, rooting efficiency of apical, middle, and basal cuttings from rosemary stems was evaluated by controlling light conditions to prevent irregular rooting. The types of light applied to the cuttings were natural sunlight (NSL), fluorescent, red, and blue (BL) light. Among these light sources, BL significantly induced root growth of not only basal cuttings, but also apical and middle cuttings, whereas NSL induced poor root formation in apical and middle cuttings. In particular, the roots of apical cuttings exposed to BL grew twice as fast as those exposed to other types of light. The overexpression of BL-induced IAA synthetic genes confirmed the rooting patterns. IAA synthetic genes were significantly upregulated by BL in the apical and middle cuttings. Irradiating with 50 μmol photons m−2 s−1 BL resulted in similar root production levels among the cutting positions with high biomass, guaranteeing the successful production of uniform cuttings. Thus, the application of proper high-intensity BL promoted healthy, similar-quality rosemary cuttings among stem cutting positions.
1. Introduction
Rosmarinus officinalis L. (rosemary), a perennial woody plant of the Lamiaceae family, is native to the Mediterranean region. It has narrow, needle-like green leaves that are widely used in beverages, spices, and fragrant essential oils. The leaves and their extracts are also used as natural remedies because of their herbal properties, such as anti-inflammatory, antioxidant, immunostimulatory, and antimicrobial effects [1]. Therefore, rosemary is positively valued in food and medicinal industries.
Annual herbs are generally reproduced using seeds. Rosemary can be propagated by seed, but stem cuttings are preferred in commercial settings [2], partly because rosemary plants require more than four years to bloom. However, the cutting quality is not uniform, and the unstable cutting supply, coupled with low rooting ability, makes it difficult to use cutting propagation for mass production. The selection of appropriate stem parts for cuttings reduces the number of samples, making cutting propagation more expensive than seed propagation [3]. Improving the rooting ability of rosemary cuttings will reduce costs and allow mass production. In most tree species, adventitious rooting ability is enhanced as cuttings become lignified [4]. Carbohydrates and some root-promoting substances appear to affect the growth of lignified cuttings [4]. In some woody plants, the chemical compositions of cuttings from distinct shoot parts are different [5,6]. Consequently, the rooting abilities of stem cuttings vary depending on their site of origin on the mother stem. Various treatments have been applied to induce rapid and regular rooting in woody plants, such as hormones and reasonable humidity levels and temperatures [7,8,9,10]. Recently, the correlation between light quality and root formation in cuttings has been intensively studied [7,11].
Light affects plant morphology, including adventitious root formation, through photoreceptors, depending on the light quality. Blue light (BL) and red light (RL) have been widely investigated because they are highly absorbed by photoreceptors [12]. BL is perceived by cryptochrome, which inhibits the elongation of young seedlings [13], phototropin, which is involved in phototropism [14], and zeaxanthin, which controls stomatal movement [15]. Lim and Eom [11] reported that BL hastens root formation in basil cuttings. RL, mostly recognized by phytochromes, induces seed germination and the synthesis of pigments, including chlorophyll [16]. Furthermore, RL leads to hypocotyl elongation and cotyledon deployment [17].
The content of endogenous auxins, such as indole-3-acetic acid (IAA), is a key factor for adventitious rooting. IAA is synthesized in apical stems and young leaves and transported through the phloem to basal organs due to auxin polarity [18,19]. IAA accumulation induces both cell division and expansion and thereby promotes root initiation and elongation [20]. IAA is synthesized by signaling pathways where Trp acts as a precursor [21]. The shows abbreviations for the names of compounds, enzymes, and genes related to IAA biosynthesis are in the back matter.
Irregular rooting depending on where cuttings originate from a mother stem reduces cutting efficiency. Therefore, this research was conducted to determine whether light conditions could contribute to regular adventitious root formation regardless of cutting part in rosemary cuttings. Cutting quality, shoot growth, and adventitious rooting of apical, middle, and basal rosemary cuttings were measured. In addition, the relative expression levels of IAA biosynthetic genes were investigated.
2. Materials and Methods
2.1. Plant Materials
Two stock plants were purchased from a commercial market (N 37°30′52.7″, E 127°08′21.6″, Seoul, Korea) in March 2016. They were grown under natural sunlight (NSL) for 4 years in a greenhouse at Kyung Hee University (N 37°14′36.0″, E 127°04′52.6″, Yongin, Korea), with a mild climate of 24 ± 5 °C and 70% ± 5% humidity. In June 2020, cuttings were collected and divided according to their positions on the stem: apical (soft green wood), middle (semi-hardened wood), and basal (hardened wood) (Figure 1A). The cuttings were 8-cm long. Four to six leaves from the bottom part of each cutting were detached for ground setting. Each cutting was initially weighed to enable measurements of biomass increase. For the experiment with exogenous auxin treatment, the cutting surface was coated with commercial rooting powder containing 0.4% 1-naphthaleneacetic acid (NAA) (Rooton, ISK Bioscience, Seoul, Korea). All cuttings were planted in 72-well plug trays (37 × 40 × 50 mm; Bumnong Co., Ltd., Jeongeup, Korea) containing horticultural soil (Baroker, Seoul Bio Co., Ltd., Eumseong, Korea).
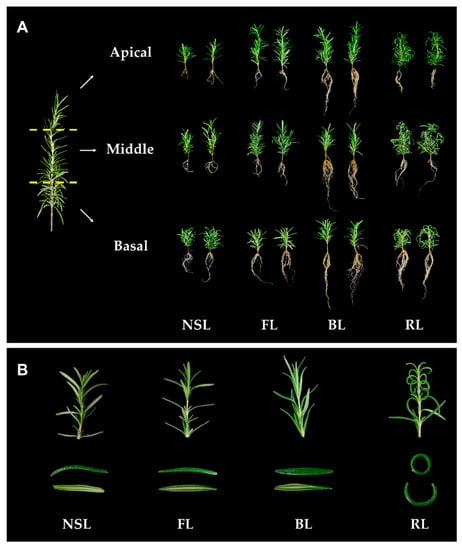
Figure 1.
(A) Morphological differences in apical, middle, and basal rosemary cuttings irradiated by different light sources—natural sunlight (NSL), fluorescent lamp light (FL), blue light (BL), and red light (RL)—at 4 weeks. (B) Morphological changes of young leaves on apical cuttings.
2.2. Light Irradiation and Growth Conditions
The light sources used in the greenhouse were NSL, fluorescent lamp light (FL), RL (625 nm), and BL (460 nm). Single wavelengths (BL and RL) were produced by light-emitting diodes (P5II model supplying 3.3 V and 1 W per module; Seoul Semiconductor Co., Seoul, Korea). The rosemary cuttings in plug trays were placed under one of the different light sources. The culture chambers were maintained at 25 ± 2 °C and 85% ± 5% humidity with 16 h of light per day. The irradiation intensity on the surface of the top-positioned leaves was controlled at 30 or 50 μmol photons m−2 s−1 (PPFD; photon flux density). Sample collection and measurements, including cutting growth and chlorophyll content, were conducted every 2 weeks for 6 weeks. Data collected between 4 and 6 weeks were mainly evaluated to determine cutting quality.
2.3. Determination of Cutting Quality
Each treatment consisted of three replicates with eight individual cuttings per replicate. To evaluate the effects of light on cutting propagation, the fresh weight of shoots and roots was assessed to quantify their growth. Shoot growth was determined using the difference between the weight of harvested cuttings containing newly grown shoots and that of the initial cuttings. Root fresh weight was determined by harvesting the adventitious roots from the cuttings.
2.4. qRT-PCR Analysis
Fresh leaf tissues attached to stems were separately collected from apical, middle, and basal rosemary cuttings cultivated under 30 PPFD FL or BL. The tissues were rapidly frozen in liquid nitrogen and stored at −80 °C until use. The frozen tissues were ground using a mortar and pestle, liquid nitrogen, and RNaseZap (Thermo Fisher, Waltham, MA, USA). Total RNA was extracted using a Spectrum Plant Total RNA Kit (STRN50, Sigma-Aldrich, MO, USA). During extraction, DNase (Sigma-Aldrich) was used to digest any contaminating DNA. First-strand cDNA was synthesized using oligo (dT) primers with SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen, MA, USA). qRT-PCR was conducted using the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with iTaq Universal SYBR Green Supermix (Bio-Rad). Each reaction was performed in a total volume of 20 μL containing 10 μL SYBR Green Supermix, 2 μL of cDNA, 4 μL of each primer, and 4 μL ddH2O. All reactions were conducted in triplicate, and the UBQ9 gene (AT5G37640) was selected as an internal control. The qRT-PCR cycling conditions were as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s. The reaction products were verified using melt-curve analysis. The cycle threshold values were measured using CFX Manager (Bio-Rad), and relative gene expression levels were calculated using the 2−ΔΔCt method.
2.5. De Novo Assembly and Investigation of IAA Biosynthetic Genes
Because of the absence of a reference-grade genome for R. officinalis, de novo transcriptome assembly is needed for gene expression analysis. Raw transcriptome sequencing data were searched for and downloaded from the National Center for Biotechnology Information (NCBI) Short Read Archive (SRA), under the accession codes SRR11477339, SRR5150701, SRR210810, and SRR210807. The data for RNA paired-end reads were assembled using the Trinity software v2.8.4 (https://github.com/trinityrnaseq/trinityrnaseq) with the -trimmomatic options to remove adaptors, poly N-containing low-quality reads, and reads shorter than 40 bp and -min_kmer_cov set to 2. All other parameters were set to their default values. The assembled transcripts were analyzed with TransDecoder v5.5.0 (https://github.com/TransDecoder/TransDecoder) to identify open reading frames. The Arabidopsis protein database (TAIR10) and Pfam database were integrated for successful prediction (Table S1). Finally, R. officinalis IAA biosynthetic intermediate genes similar to Arabidopsis IAA biosynthetic pathway-related protein sequences were searched for using the BLASTP algorithm (Tables S2 and S3).
2.6. Statistical Analysis
All data are presented as the mean ± SE from three replicates (n = 8). Statistical analysis was conducted using the SAS software (SAS version 9.3, SAS Institute Inc., Cary, NC, USA). All data were subjected to ANOVA, and significantly different means were determined using Tukey’s honestly significant difference (HSD) test at the 5% level.
3. Results and Discussion
3.1. Shoot and Root Growth Differences among Stem Positions under Different Light Qualities
The shoot growth of positional stem cuttings exposed to different light qualities for six weeks is shown in Figure 2A–D. Shoot growth among the positional stem cuttings differed significantly during the nursery period. The basal cuttings exhibited the highest shoot fresh weight. As shown in Figure 1, the apical cuttings developed terminal shoots. However, the terminal shoots mostly contained young and immature leaves, whose maximum net photosynthetic rate was lower than that of mature and old leaves [22]. Therefore, the basal cuttings, containing mature and old leaves, accumulated the largest amount of shoot biomass. The shoots grew better under artificial light (FL, RL, and BL) than NSL. Shoot fresh weight rapidly increased over the first two weeks under NSL. After the initial increase, the shoot weight of NSL-treated cuttings did not show a significant increase from two to six weeks. However, the shoot fresh weight continually increased under artificial light after the initial increase. In particular, exposure to BL and FL, which also contained blue wavelength light, resulted in greater shoot biomass accumulation than exposure to RL. After six weeks of cultivation, the shoot fresh weight of apical cuttings irradiated with BL, FL, and RL was 1.107, 1.092, and 0.852 g per cutting, respectively.
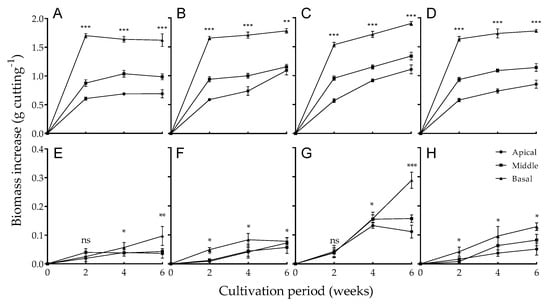
Figure 2.
Biomass increase of (A–D) shoots and (E–H) roots among apical, middle, and basal rosemary cuttings irradiated with (A,E) natural sunlight, (B,F) fluorescent lamp light, (C,G) blue light, and (D,H) red light for six weeks. Error bars indicate the mean ± SE from three replicates (n = 8). Asterisks over data points indicate significant differences at p < 0.05 (*), <0.01 (**), and <0.001 (***); not significant; ns. There were statistical differences in the increase of shoot fresh weight among cutting positions (p < 0.001), and in the increase of root fresh weight among light conditions (p < 0.01) and culture period (p < 0.01).
As shown in Figure 1B, the young, but not mature, leaves on apical cuttings showed different morphological changes depending on the light quality. BL induced the expansion and elongation of the leaves. Cuttings grown under FL and NSL exhibited similar patterns, although they occurred less frequently than under BL. Under RL, young leaves curled and did not develop normally. Different types of light had similar effects on Arabidopsis leaf morphology; Christie [23] described leaf expansion during cultivation under BL conditions. Downward curling and spiral growth occurred under RL exposure [24]. Excessively curled leaves have reduced sunlight incidence on their surfaces [25], which decreases their photosynthetic efficiency [26]. Therefore, we hypothesized that a decrease in photosynthetic efficiency adversely affects biomass accumulation in cuttings with curled leaves induced by RL irradiation.
The fresh weight of newly emerged roots was measured to evaluate the rooting abilities of stem cuttings grown under different light qualities (Figure 2E–H). In the early period of cultivation, no significant correlation between rooting ability and cutting position was observed under NSL (Figure 2E). Differences in rooting ability among cutting positions began after four weeks of cultivation and were distinctly observed at six weeks. Basal cuttings exhibited more efficient rooting than the other cuttings. The root growth of basal cuttings was 2.7-fold greater than that of apical cuttings under NSL at six weeks. Certain plant species might have better root formation when basal cuttings are used. In the case of African blackwood, cuttings of the basal position showed stronger rooting ability than those of the apical position [27]. Similarly, Populus spp. showed better rooting ability in basal cuttings than other cutting positions [28]. Basal cuttings in rosemary have strong rooting ability; however, in the conventional rosemary cutting industry (for either markets or home gardens), the apical parts of stems are preferentially used. The preference for apical cuttings is likely due to the aesthetic appeal of green shoot parts rather than the ability to form roots, as well as to conserve mother plants. However, fast root formation should be considered in a relatively large-scale cutting industry with respect to production cost.
Among the artificial lights, FL and RL did not enhance rooting in the apical and middle positions at two weeks of cultivation. Conversely, BL induced rapid root formation in apical and middle cuttings at the early stage of cultivation (Figure 2F–H). After four weeks of BL exposure, root growth significantly increased regardless of cutting position, and the cuttings presented numerous roots (Figure 1A and Figure 2G). The root fresh weights of both apical and middle stem cuttings under BL were almost two-fold greater than those of cuttings grown under other light treatments. However, the rooting abilities of cuttings are likely dependent on light quality preferences and may vary among plant species. Moon et al. [29] reported that the rooting of Pterygocalyx volubilis shoots was enhanced under RL, but inhibited under BL. Additionally, RL had a positive effect on adventitious root initiation in de-rooted Picea abies seedlings [30]. Other studies have reported that BL stimulates root formation in Betula pendula [31], Ocimum bacilicum [11], and Chrysanthemum [7] cuttings.
3.2. RNA Expression of IAA Biosynthetic Genes in Rosemary Cuttings
Figure 3 shows the expression of genes related to IAA biosynthesis under FL and BL treatment in apical, middle, and basal cuttings of rosemary. The relative gene expression levels are presented as fold changes against the gene expression values of initial cuttings immediately after separation from stock plants. The BL treatment showed higher levels of most IAA biosynthetic genes in different cutting positions than the other treatments. The total expression levels of genes in apical and middle cuttings were five-fold higher than those in basal cuttings. The expression of four upstream genes (TAA1, CYP, SUR1, and UGT) and one downstream gene (NIT1) was significantly increased in the apical and middle cuttings (Figure 3B). The upstream genes contribute to synthesis of intermediate compounds, such as IAOx, IPyA, and IG [32,33,34,35]. They also upregulate downstream gene expression and promote the synthesis of the end product [36,37,38,39]. Therefore, BL irradiation of rosemary cuttings contributes to significant root induction in apical and middle cuttings through active auxin biosynthesis. Although the gene expression levels were the lowest in basal cuttings among cutting positions, gene expression was higher in the BL treatment than in the FL treatment. In addition, root formation of basal cuttings under BL was the best among the positions. The relatively small increase in auxin-biosynthesis gene expression may indicate that the rooting establishment in basal cuttings was particularly fast, and gene expression had already increased in the earlier period compared with the apical and middle cuttings.
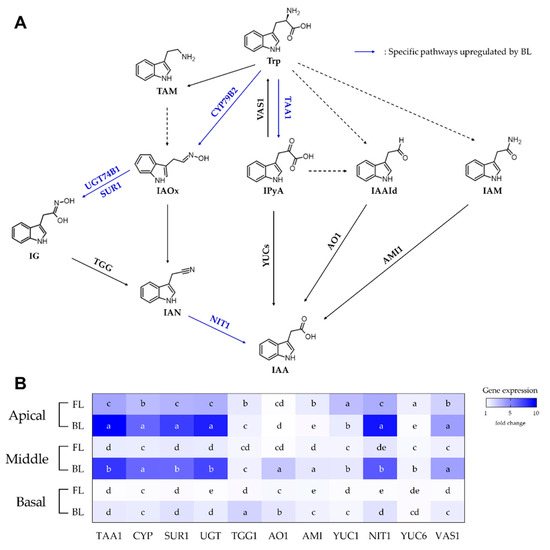
Figure 3.
(A) Tryptophan (Trp)-dependent pathways for indole-3-acetic acid (IAA) biosynthesis in plants and (B) relative gene expression levels in apical, middle, and basal positions of rosemary cuttings treated with fluorescent lamp light (FL) or blue light (BL) for 7 days. Solid and dashed arrows indicate processes for which genes are known and unknown, respectively. Blue arrows indicate pathways specifically upregulated in BL-treated rosemary cuttings. Alphabets in heatmap mean statistical differences in gene expression among treatments within the same gene (p < 0.05).
3.3. Effects of NAA Treatment on Root Growth under Different Light Qualities
The effects of both light quality and NAA treatment on the root growth of rosemary cuttings are shown in Figure 4. Under NSL, the root fresh weight increased with NAA treatment in all cutting positions. In particular, the root fresh weight of apical and middle cuttings was improved with NAA treatment by 2.7- and 2.8-fold, respectively (p < 0.001). FL + NAA treatment also increased root fresh weight, but the difference was not significant. Exposure to a single wavelength (BL or RL) + NAA treatment only increased the root fresh weight of the basal cuttings. BL + NAA treatment significantly increased the root fresh weight (1.5-fold higher) compared with BL treatment (without NAA) in basal cuttings (p < 0.05). The accumulation of carbohydrates and some root-promoting substances in lignified stems improves rooting capacity compared with semi-hardened and non-lignified green stems [4,40,41,42]. Therefore, an increase of root biomass was observed in basal rosemary cuttings regardless of light conditions (Figure 4). In particular, the treatment with BL and NAA was more effective at increasing root biomass than BL treatment without NAA, as reported by Gil et al. [7]. NAA is an exogenous auxin that induces adventitious roots in soft green stems [39]. Therefore, the apical and middle cuttings with less lignification, compared with basal cuttings, may have responded sensitively to the NAA treatment and increased their root fresh weight. The positive effects on root fresh weight increase by NAA treatment were higher under NSL than under artificial light. Thus, NAA treatment is recommended when using NSL to propagate rosemary stem cuttings.
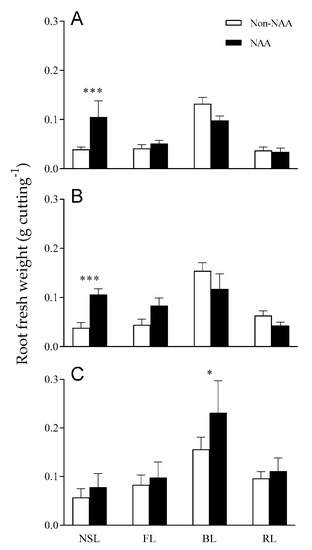
Figure 4.
NAA treatment effects on root growth of (A) apical-, (B) middle-, and (C) basal-position rosemary cuttings treated natural sunlight (NSL), fluorescent lamp light (FL), blue light (BL), and red light (RL) for 4 weeks. Error bars indicate the mean ± SE from three replications (n = 8). Asterisks over bars indicate significant differences between non-NAA and NAA treatments at p < 0.05 (*) and <0.001 (***).
3.4. Effects of Light Intensity on Rosemary Cuttings
As shown in Figure 5, higher intensities of light were associated with a slight increase in shoot growth in most cuttings. Among light sources, 50 PPFD BL and RL significantly induced shoot growth compared with FL; however, no difference was observed in shoot growth among light sources within the same light intensity.
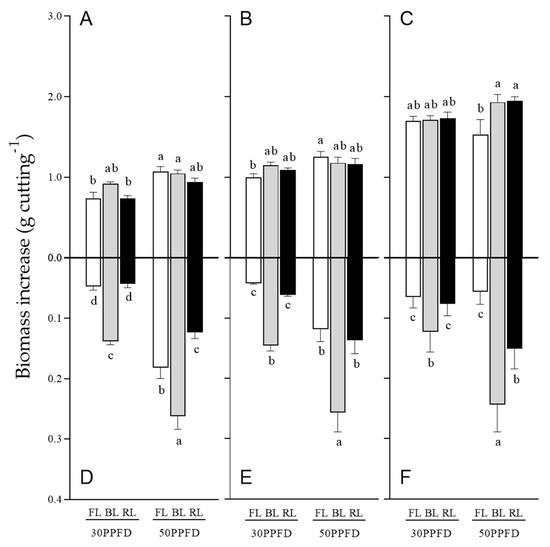
Figure 5.
(A–C) Shoot and (D–F) root growth of (A,D) apical-, (B,E) middle-, and (C,F) basal-position rosemary cuttings irradiated with different photosynthetic photon flux densities (PPFDs) of fluorescent lamp light (FL), blue light (BL), and red light (RL) for 4 weeks. Error bars indicate the mean ± SE from three replicates (n = 8). Different letters indicate statistical differences determined by Tukey’s HSD test at p < 0.05.
Root growth increased when the higher light intensity was applied to all cutting positions, except for apical cuttings under FL, which showed no significant difference between 30 and 50 PPFD. BL at 50 PPFD induced the highest root growth (approximately 0.26 g per cutting), and no significant difference was observed among cutting positions. No significant difference was observed for root growth under 30 PPFD BL among cutting positions; however, growth was approximately two-fold lower than that under 50 PPFD.
Our results clearly showed that the root growth rates of apical and middle cuttings were more sensitive than basal cuttings to light intensity, and the root growth rates increased when the light intensity was increased. Cuttings generally obtain nutrients from starch in their leaves to form adventitious roots. The photosynthetic rate of cuttings increases as starch degrades to maintain the stored starch level [43]. Under low-intensity light, cuttings cannot survive because of the nutritional shortage resulting from the lack of light energy needed to promote photosynthesis [44]. Therefore, an adequate light intensity is an essential factor to ensure better and faster establishment of rosemary cuttings. However, excessive BL intensity should be avoided owing to possible leaf damage, as we previously reported in other plant cuttings [11].
4. Conclusions
The rooting abilities of rosemary stem cuttings varied depending on their position of origin on the mother stem. Irregular rooting limits propagation efficiency, which restricts the production of healthy cuttings. Application of BL significantly increased the expression levels of IAA synthesis-related genes. From this perspective, BL irradiation at an adequate intensity guarantees a successful production of uniform cuttings, regardless of cutting maturity and promotes cutting establishment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11091725/s1, Table S1: Arabidopsis auxin pathway related genes and Pfam annotation of studied genes, Table S2: List of primer for qRT-PCR, Table S3: Transcriptome sequence of in R. officinalis auxin pathway related genes.
Author Contributions
Conceptualization, S.-H.E.; methodology, C.-S.G., O.-J.L., H.-Y.J., C.L. and S.-H.E.; software, C.-S.G. and H.-Y.J.; validation, S.-H.E.; formal analysis, C.-S.G. and H.-Y.J.; investigation, C.-S.G. and O.-J.L.; data curation, C.-S.G., S.-J.K., O.-J.L. and C.L.; writing—original draft preparation, C.-S.G. and S.-J.K.; writing—review and editing, S.-H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Radiation Technology R&D program (NRF-2017M2A2A6A05018538) through the National Research Foundation of Korea funded by the Ministry of Science and ICT. This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NO. NRF-2019R1A2C1009623).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMI1 | AMIDASE 1 |
| AO1 | ALDEHYDE OXIDASE 1 |
| CYP79B2 | CYTOCHROME P450 79B2 |
| IAAId | Indole-3-acetaldehyde |
| IAM | Indole-3-acetamide |
| IAN | Indole-3-acetonitrile |
| IAOx | Indole-3-acetaldoxime |
| IG | Indole glucosinolate |
| IPyA | Indole-3-pyruvic acid |
| NIT1 | NITRILASE 1 |
| SUR1 | SUPERROOT 1 |
| TAA1 | TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 |
| TAM | Tryptamine |
| TGG | β-THIOGLUCOSIDE GLUCOHYDROLASE |
| Trp | L-Tryptophan |
| UGT74B1 | UDP-GLUCOSYL TRANSFERASE |
| VAS1 | REVERSAL OF SAV3 PHENOTYPE 1 |
| YUCs | YUCCAs |
References
- Naiel, M.A.E.; Ismael, N.E.M.; Negm, S.S.; Ayyat, M.S.; Al-Sagheer, A.A. Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 526, 735370. [Google Scholar] [CrossRef]
- Still, S.M. Manual of Herbaceous Ornamental Plants, 4th ed.; Stipes Publishing Champaign: Champaign, IL, USA, 1994; pp. 570–571. [Google Scholar]
- Pence, V.C. Evaluating costs for the in vitro propagation and preservation of endangered plants. In Vitro Cell. Dev. Biol. 2011, 47, 176–187. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, T.D.; Geneve, T.L., Jr. Plant Propagation: Principles and Practices, 6th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1997; p. 288. [Google Scholar]
- Hansen, J.; Kristensen, K. Axillary bud growth in relation to adventitious root formation in cuttings. Physiol. Plant. 1990, 79, 39–44. [Google Scholar] [CrossRef]
- Bredmose, N.; Kristiansen, K.; Nielsen, B. Propagation temperature, PPFD, auxin treatment, cutting size and cutting position affect root formation, axillary bud growth and shoot development in miniature rose (Rosa hybrida L.) plants and alter homogeneity. J. Hortic. Sci. Biotechnol. 2004, 79, 458–465. [Google Scholar] [CrossRef]
- Gil, C.S.; Jung, H.Y.; Lee, C.; Eom, S.H. Blue light and NAA treatment significantly improve rooting on single leaf-bud cutting of Chrysanthemum via upregulated rooting-related genes. Sci. Hort. 2020, 274, 109650. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Ersoy, N.; Kalyoncu, I.; Özer, N. Rooting of apical softwood cuttings of Cotoneaster horizontalis Dcne. with application of IBA and air humidity. Selcuk J. Agric. Food Sci. 2017, 30, 67–73. [Google Scholar]
- De Almeida, M.R.; Aumond, M.; Da Costa, C.T.; Schwambach, J.; Ruedell, C.M.; Correa, L.R.; Fett-Neto, A.G. Environmental control of adventitious rooting in Eucalyptus and Populus cuttings. Trees 2017, 31, 1377–1390. [Google Scholar] [CrossRef]
- Lim, Y.J.; Eom, S.H. Effects of different light types on root formation of Ocimum basilicum L. cuttings. Sci. Hort. 2013, 164, 552–555. [Google Scholar] [CrossRef]
- Sager, J.C.; Wheeler, R.M. Application of sunlight and lamps for plant irradiation in space bases. Adv. Space Res. 1992, 12, 133–140. [Google Scholar] [CrossRef]
- Thomas, B.; Dickinson, H.G. Evidence for two photoreceptors controlling growth in de-etiolated seedlings. Planta 1979, 146, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, O.H.; Blaauw-Jansen, G. The phototropic responses of Avena coleoptiles. Acta Bot. Neerlandica 1970, 19, 755–763. [Google Scholar] [CrossRef]
- Schwartz, A.; Zeiger, E. Metabolic energy for stomatal opening. Roles of photophosphorylation and oxidative phosphorylation. Planta 1984, 161, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Brown, C.S. Root-shoot interaction in the greening of wheat seedlings grown under red light. Plant Physiol. 1995, 107, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Hoenecke, M.E.; Bula, R.J.; Tibbitts, T.W. Importance of ‘Blue’ photon levels for lettuce seedlings grown under red-light-emitting Diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Di, D.W.; Zhang, C.; Luo, P.; An, C.W.; Guo, G.Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant growth regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Ullah, H.; Chen, J.G.; Temple, B.; Boyes, D.C.; Alonso, J.M.; Davis, K.R.; Ecker, J.R.; Jones, A.M. The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 2003, 15, 393–409. [Google Scholar] [CrossRef] [Green Version]
- Tivendale, N.D.; Ross, J.J.; Cohen, J.D. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014, 19, 44–51. [Google Scholar] [CrossRef]
- Shirke, P.A. Leaf photosynthesis, dark respiration and fluorescence as influenced by leaf age in an evergreen tree, Prosopis juliflora. Photosynthetica 2001, 39, 305–311. [Google Scholar] [CrossRef]
- Christie, J.M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goins, G.D.; Yorio, N.C.; Sanwo-Lewandowski, M.M.; Brown, C.S. Life cycle experiments with Arabidopsis grown under red light-emitting diodes (LEDs). Life Support Biosphere Sci. 1998, 5, 143–149. [Google Scholar]
- Johnson, D.M.; Smith, W.K.; Vogelmann, T.C.; Brodersen, C.R. Leaf architecture and direction of incident light influence mesophyll fluorescence profiles. Am. J. Bot. 2005, 92, 1425–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis. Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Amri, E.; Lyaruu, H.V.M.; Nyomora, A.S.; Kanyeka, Z.L. Vegetative propagation of African Blackwood (Dalbergia melanoxylon Guill. & Perr.): Effects of age of donor plant, IBA treatment and cutting position on rooting ability of stem cuttings. New For. 2010, 39, 183–194. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Hall, R.B.; Bauer, E.O.; Riemenschneider, D.E. Shoot position affects root initiation and growth of dormant unrooted cuttings of Populus. Silvae Genet. 2003, 52, 273–279. [Google Scholar]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Alallaq, S.; Ranjan, A.; Brunoni, F.; Novák, O.; Lakehal, A.; Bellini, C. Red light controls adventitious root regeneration by modulating hormone homeostasis in Picea abies seedlings. Front. Plant Sci. 2020, 11, 1397. [Google Scholar] [CrossRef]
- Sæbø, A.; Skjeseth, G.; Appelgren, M. Light quality of the in vitro stage affects the subsequent rooting and field performance of Betula pendula (Roth). Scand. J. For. Res. 1995, 10, 155–160. [Google Scholar] [CrossRef]
- Quittenden, L.J.; Davies, N.W.; Smith, J.A.; Molesworth, P.P.; Tivendale, N.D.; Ross, J.J. Auxin biosynthesis in pea: Characterization of the tryptamine pathway. Plant Physiol. 2009, 151, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Normanly, J.; Slovin, J.P.; Cohen, J.D. Auxin biosynthesis and metabolism. In Plant Horm; Davies, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 36–62. [Google Scholar]
- Li, R.; Jiang, J.; Jia, S.; Zhu, X.; Su, H.; Li, J. Overexpressing broccoli tryptophan biosynthetic genes BoTSB1 and BoTSB2 promotes biosynthesis of IAA and indole glucosinolates. Physiol. Plant. 2020, 168, 174–187. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Voß, U. Auxin metabolism controls developmental decisions in land plants. Trends Plant Sci. 2019, 24, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Normanly, J.; Grisafi, P.; Fink, G.R.; Bartel, B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell 1997, 9, 1781–1790. [Google Scholar] [PubMed]
- Seo, M.; Akaba, S.; Oritani, T.; Delarue, M.; Bellini, C.; Caboche, M.; Koshiba, T. Higher activity of an aldehyde oxidase in the auxin-overproducing superroot1 mutant of Arabidopsis thaliana. Plant Physiol. 1998, 116, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, T.; Zhou, M.; Li, M.; Zhao, Y.; Xu, J.; Hu, F.; Li, H. Caenorhabditis elegans extracts stimulate IAA biosynthesis in Arthrobacter pascens ZZ21 via the indole-3-pyruvic acid pathway. Microorganisms 2021, 9, 970. [Google Scholar] [CrossRef]
- Singh, K.K.; Tomar, Y.K. Effect of planting time and indole butyric acid levels on rooting of woody cuttings of phalsa (Grewia asiatica L.). HortFlora Res. Spect. 2015, 4, 39–43. [Google Scholar]
- Singh, K.K.; Chauhan, J.S.; Rawat, J.M.S.; Rana, D.K. Effect of different growing conditions and various concentrations of IBA on the rooting and shooting of hardwood cutting of phalsa (Grewia asetica L.) under valley condition of Garhwal Himalayas. Plant Arch. 2015, 15, 131–136. [Google Scholar]
- Braha, S.; Rama, P. The effects of indol butyric acid and naphthalene acetic acid of adventitious root formation to green cuttings in blueberry cv. Vaccinium corymbosum L. Int. J. Sci. Res. 2016, 5, 876–879. [Google Scholar]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, L.; Brunes, L. Light effects on root formation in aspen and willow cuttings. Physiol. Plantarum 1980, 48, 261–265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).