Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Growth Parameters
2.3. Photosynthetic Pigments
2.4. Anatomical Features of Leaves
2.5. Stomatal Traits
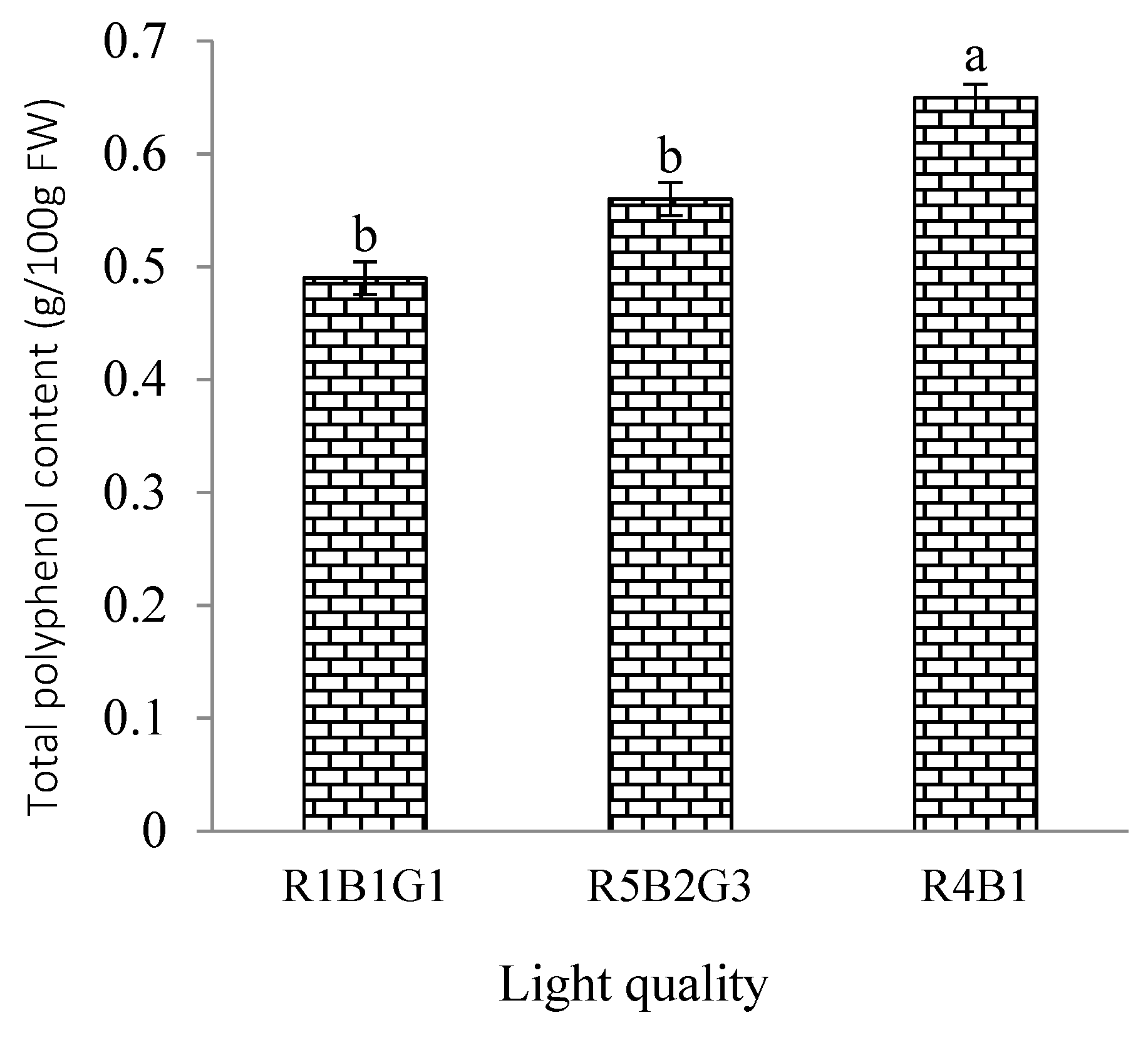
2.6. Quality Parameters
2.7. Statistical Analysis
3. Results
3.1. Effect of Light Quality on Growth Parameters of Spinach Plant
3.2. Effect of Light Quality on Photosynthetic Pigments of Spinach Plant
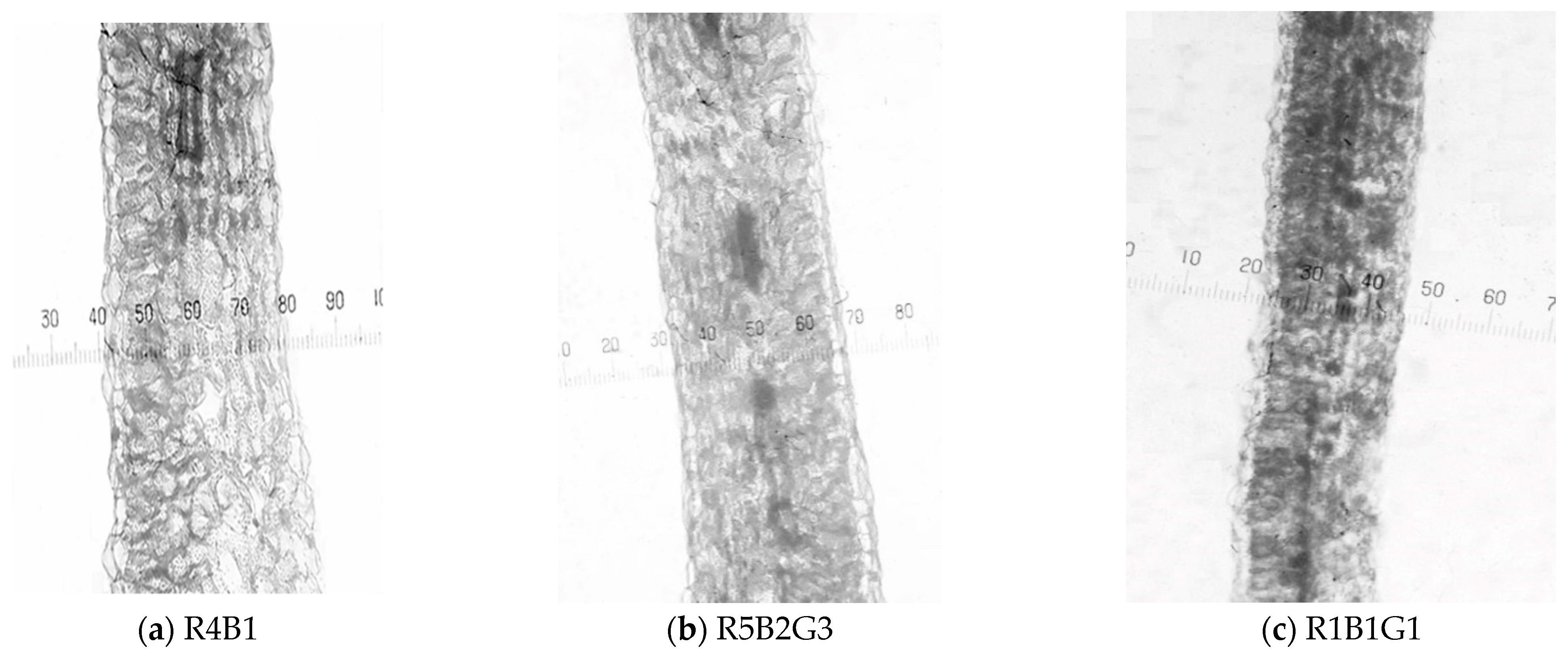
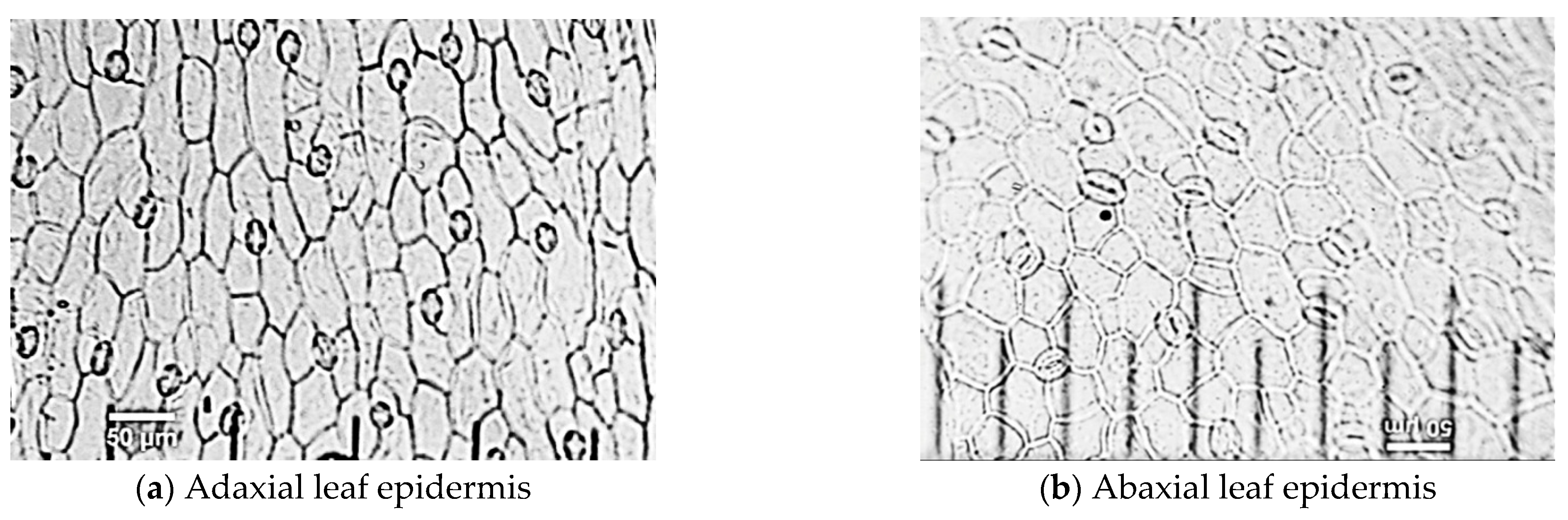
3.3. Effect of Light Quality on Leaf Structure Characteristics of Spinach Plant
3.3.1. Anatomy of the Leaf Cross-Sections
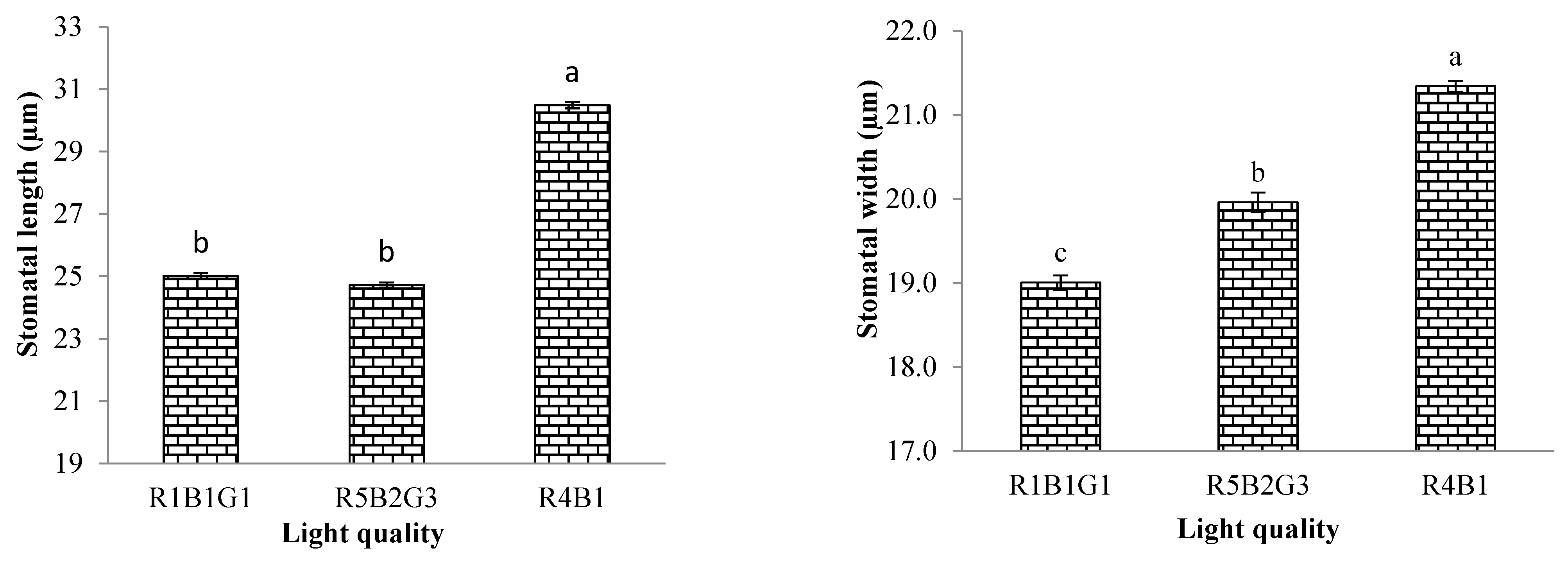
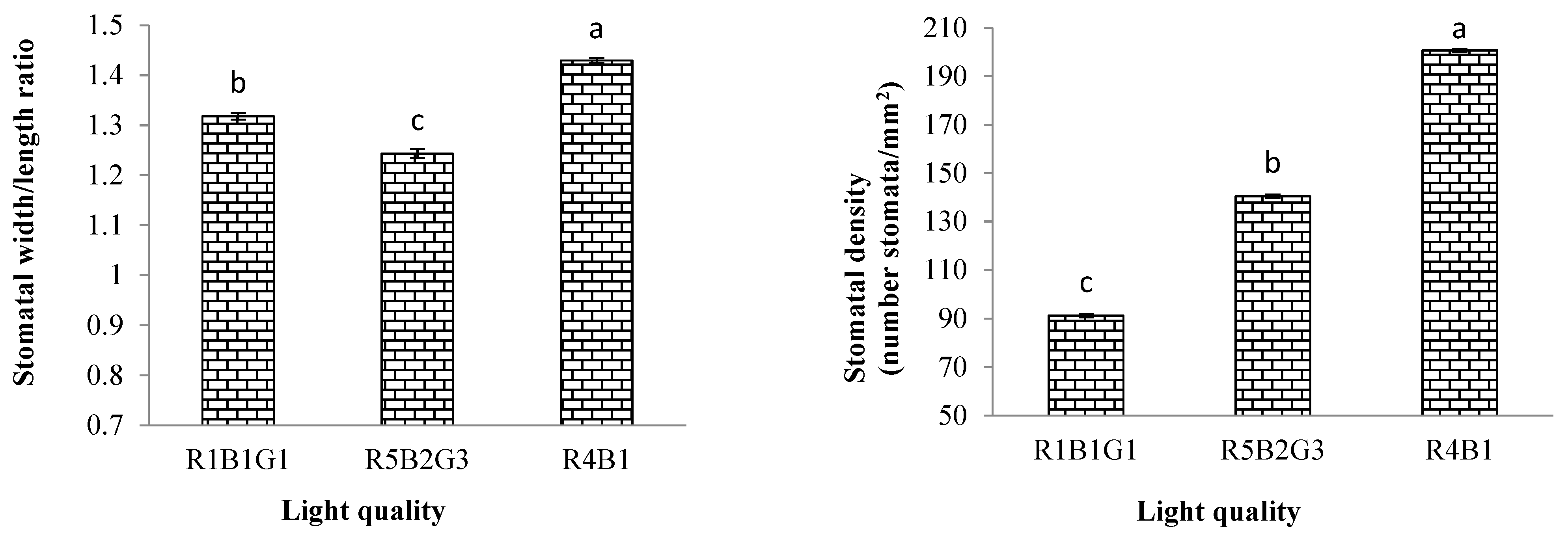
3.3.2. Characteristics of Stomata Cells
3.4. Effect of Light Quality on Nutrition Content and Quality of Spinach Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Qi, L.D.; Liu, S.Q.; Xu, L. Effects of light qualities on accumulation of oxalate: Tannin and nitrate in spinach. Trans. Chin. Soc. Agric. Eng. 2007, 4, 201–205. [Google Scholar]
- Saebo, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Rapid suppression of growth by blue light. Plant Physiol. 1981, 67, 584–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senger, H. The effect of blue light on plants and microorganisms. Phytochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Chen, X.L.; Yang, Q.C.; Song, W.P.; Wang, L.C.; Guo, W.Z.; Xue, X.Z. Growth and nutritional properties of lettuce affected by different alternating intervals of red and blue LED irradiation. Sci. Hortic. 2017, 223, 44–52. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo-Lewandowski, M.M.; Brown, C.S. Life cycle experiments with Arabidopsis under red light-emitting diodes (LEDs). Life Support Biosph. Sci. 1998, 5, 143–149. [Google Scholar] [PubMed]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kagawa, T.; Sato, Y. Chloroplast movement. Annu. Rev. Plant Biol. 2003, 54, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Loreto, F.; Tsonev, T.; Centritto, M. The impact of blue light on leaf mesophyll conductance. J. Exp. Bot. 2009, 60, 2283–2290. [Google Scholar] [CrossRef]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Viršilė, A.; Olle, M.; Duchovskis, P. LED Lighting in Horticulture. In Light Emitting Diodes for Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 113–147. [Google Scholar]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hortic. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Kaneko, K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Cui, J.; Ma, Z.; Xu, Z.; Zhang, H.; Chang, T.; Liu, H. Effects of supplemental lighting with different light qualities on growth and physiological characteristics of cucumber, pepper and tomato seedlings. Acta Hortic. Sin. 2009, 36, 663–670. [Google Scholar]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef] [Green Version]
- De Keyser, E.; Dhooghe, E.; Christiaens, A.; Van Labeke, M.C.; Van Huylenbroeck, J. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Kim, H.H.; Wheeler, R.; Sager, J.; Norikane, J. Photosynthesis of lettuce exposed to different short-term light qualities. Environ. Control Biol. 2005, 43, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandenberger, L.; Cavins, T.; Payton, M.; Wells, L.; Johnson, T. Yield and quality of spinach cultivars for greenhouse production in Oklahoma. HortTechnology 2007, 17, 269–272. [Google Scholar] [CrossRef] [Green Version]
- Lisiewska, Z.; Kmiecik, W.; Gębczyński, P.; Sobczyńska, L. Amino acid profile of raw and as-eaten products of spinach (Spinacia oleracea L.). Food Chem. 2011, 126, 460–465. [Google Scholar] [CrossRef]
- Lisiewska, Z.; Gębczyński, P.; Bernaś, E.; Kmiecik, W. Retention of mineral constituents in frozen leaf vegetables prepared for consumption. J. Food Compos. Anal. 2009, 22, 218–223. [Google Scholar] [CrossRef]
- Nguyen, T.P.D.; Tran, T.T.H.; Dong, C.J.; Kim, I.S.; Nguyen, Q.T. Effects of supplemental green LEDs to red and blue light on the growth, yield and quality of hydroponic cultivated spinach (Spinacia oleracea L.) in plant factory. Prot. Hortic. Plant Fact. 2020, 29, 171–180. [Google Scholar] [CrossRef]
- Nguyen, T.P.D.; Tran, T.T.H.; Nguyen, Q.T. Effects of light intensity on the growth, photosynthesis and leaf microstructure of hydroponic cultivated spinach (Spinacia oleracea L.) under a combination of red and blue LEDs in house. Int. J. Agric. Technol. 2019, 15, 75–90. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Expt. Stat. Circ. 1950, 347, 1–32. [Google Scholar]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, P. Growth analysis formulae-their use and abuse. Crop. Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different slovents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Clark, G. Staining Procedures, 4th ed.; Williams and Wilkins: London, UK, 1981; pp. 325–326. [Google Scholar]
- Yao, X.Y.; Liu, X.Y.; Xu, Z.G.; Jiao, X.L. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Tuan, T.A.; Valya, V.; Petar, P.; Petrova, P.L. Cadmium-induced structural disturbances in Pisum sativum leaves are alleviated by nitric oxide. Turk. J. Bot. 2013, 37, 698–707. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1980. [Google Scholar]
- Antial, B.S.; Akpanz, E.J.; Okonl, P.A.; Umorenl, I.U. Nutritive and anti-Nutritive evaluation of sweet potatoes. Pak. J. Nutr 2006, 5, 166–168. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitrocultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Stryjewski, E.; Goins, G.; Kelly, C. Quantitative morphological analysis of spinach leaves grown under light-emitting diodes or sulfur-microwave lamps. In Proceedings of the 31st International Conference on Environmental Systems, Orlando, FL, USA, 9–12 July 2001; SAE International: Warrendale, PA, USA, 2001. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Sakalauskienė, S.; Jankauskienė, J.; Duchovskis, P.; Novičkovas, A. The impact of supplementary short-term red led lighting on the antioxidant properties of microgreens. Acta Hortic. 2012, 956, 649–655. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2019, 301, 125799. [Google Scholar] [CrossRef]

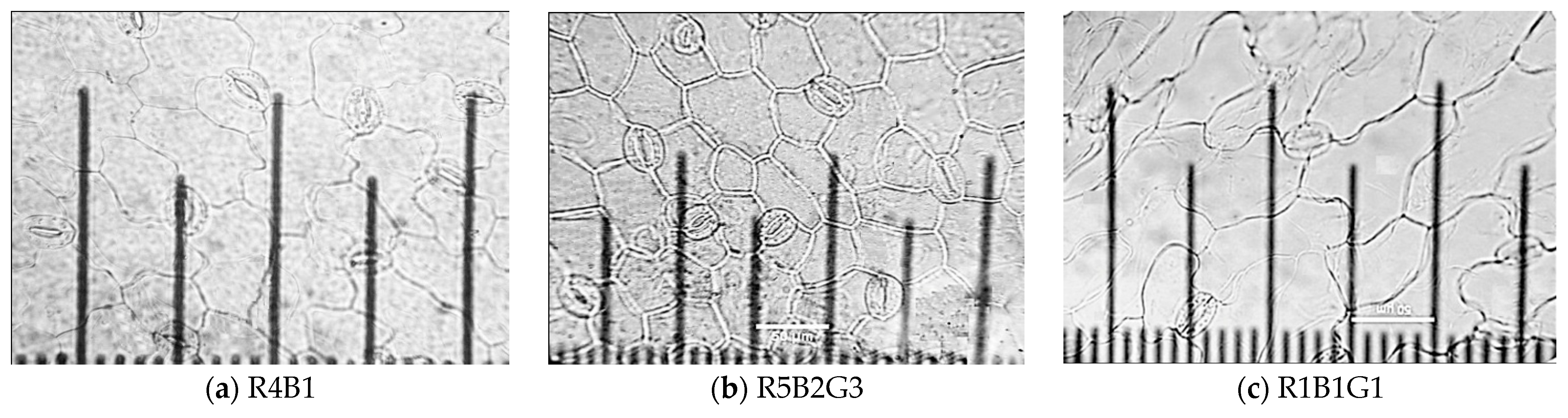
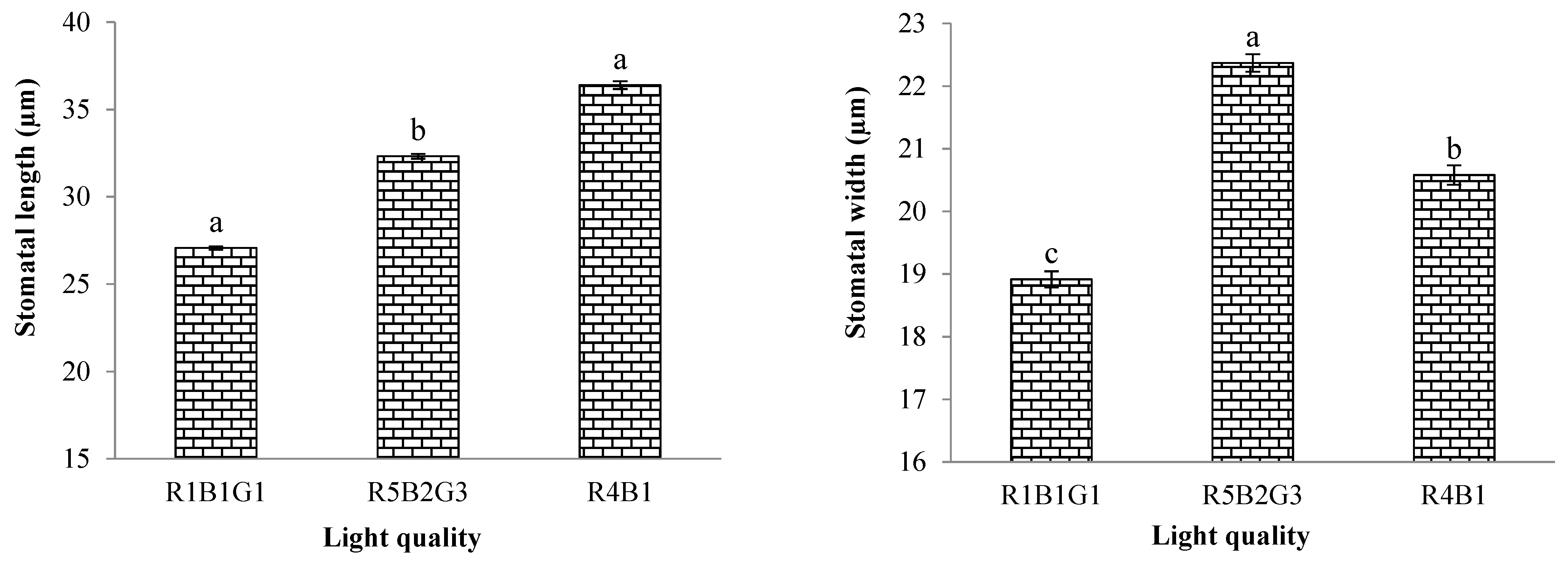
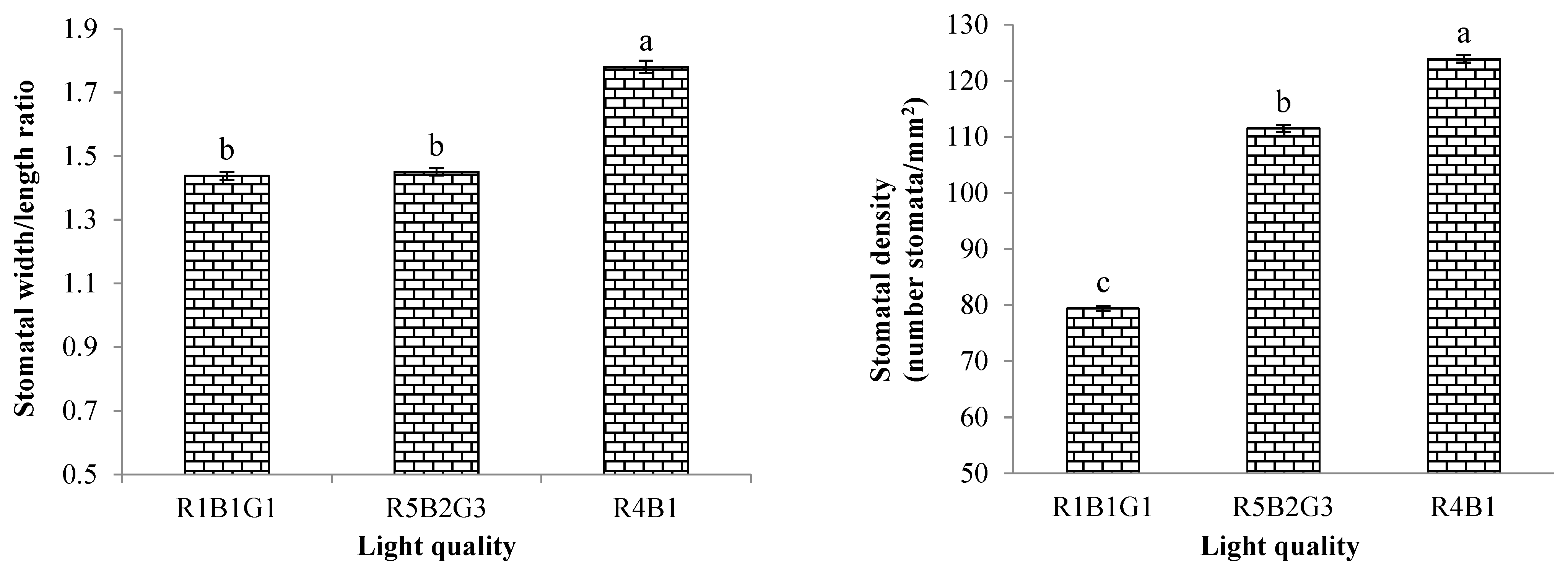
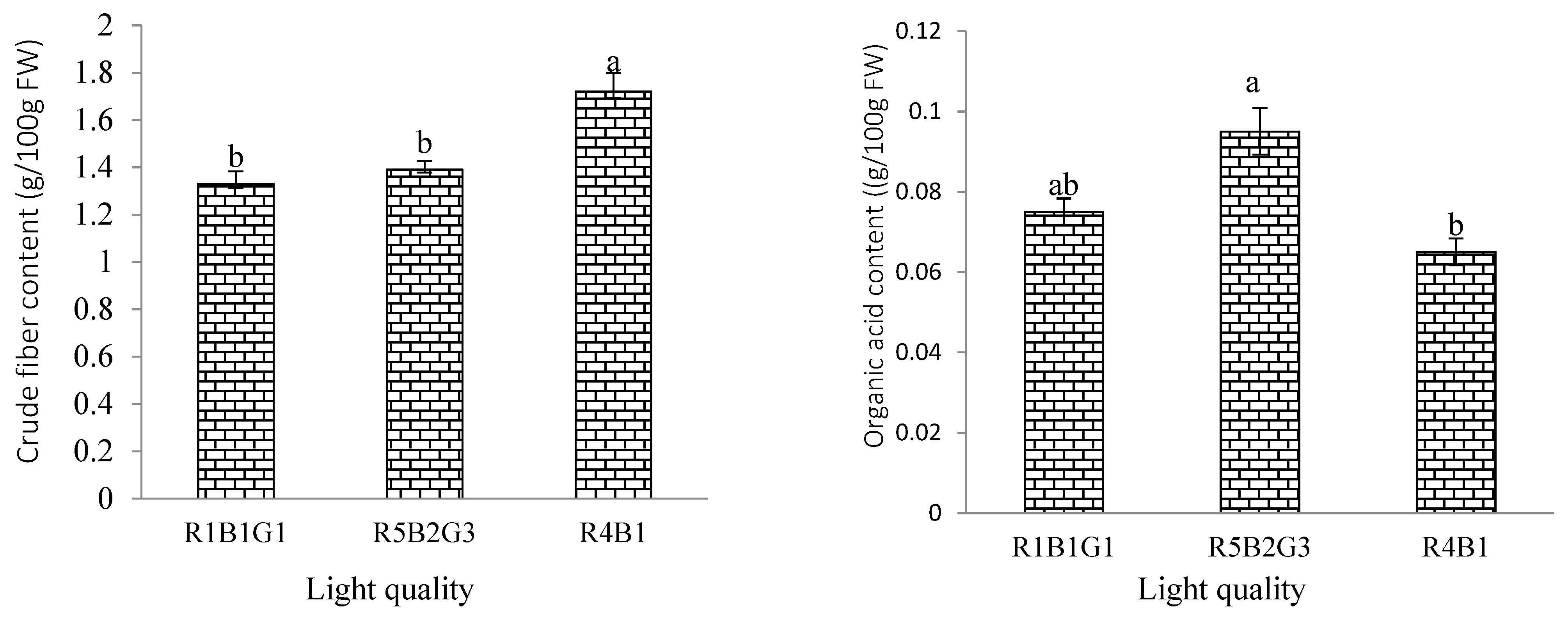
| Treatment | SLA (cm2/g) | RGR (g/day) | NAR (g/m2/day) | FW of Whole Plant (g/Plant) | DW of Whole Plant (g/Plant) |
|---|---|---|---|---|---|
| R4B1 | 2.99 c | 0.140 a | 5.56 a | 74.69 a | 4.20 a |
| R5B2G3 | 4.08 a | 0.132 b | 5.35 b | 53.57 b | 2.45 b |
| R1B1G1 | 3.57 b | 0.121 c | 5.22 c | 36.31 c | 2.19 b |
| CV% | 4.40 | 2.71 | 1.14 | 2.76 | 6.90 |
| LSD0.05 | 0.31 | 0.007 | 0.12 | 3.02 | 0.36 |
| Treatment | Chla (mg/g) | Chlb (mg/g) | Chl(a + b) (mg/g) | Chla/Chlb | Carotenoids (mg/g) |
|---|---|---|---|---|---|
| R4B1 | 0.290 a | 0.522 a | 0.812 a | 0.555 a | 0.183 a |
| R5B2G3 | 0.274 b | 0.515 a | 0.789 a | 0.532 ab | 0.181 a |
| R1B1G1 | 0.268 b | 0.498 b | 0.766 b | 0.538 b | 0.169 b |
| CV% | 1.68 | 1.51 | 1.38 | 1.60 | 2.34 |
| LSD0.05 | 0.009 | 0.015 | 0.022 | 0.017 | 0.008 |
| Treatment | Palisade Tissue Length (µm) | Spongy Tissue Length (µm) | Leaf Thickness (µm) | PT/ST | Leaf Compactness |
|---|---|---|---|---|---|
| R4B1 | 78.31 a | 253.00 a | 382.43 a | 0.310 a | 0.205 a |
| R5B2G3 | 65.00 b | 218.59 b | 320.92 b | 0.297 b | 0.203 a |
| R1B1G1 | 46.91 c | 163.48 c | 251.88 c | 0.287 c | 0.186 b |
| CV% | 2.34 | 0.71 | 0.59 | 2.51 | 2.15 |
| LSD0.05 | 1.36 | 1.38 | 1.74 | 0.007 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-P.-D.; Jang, D.-C.; Tran, T.-T.-H.; Nguyen, Q.-T.; Kim, I.-S.; Hoang, T.-L.-H.; Vu, N.-T. Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. https://doi.org/10.3390/agronomy11091724
Nguyen T-P-D, Jang D-C, Tran T-T-H, Nguyen Q-T, Kim I-S, Hoang T-L-H, Vu N-T. Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy. 2021; 11(9):1724. https://doi.org/10.3390/agronomy11091724
Chicago/Turabian StyleNguyen, Thi-Phuong-Dung, Dong-Cheol Jang, Thi-Thanh-Huyen Tran, Quang-Thach Nguyen, Il-Seop Kim, Thi-Lan-Huong Hoang, and Ngoc-Thang Vu. 2021. "Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.)" Agronomy 11, no. 9: 1724. https://doi.org/10.3390/agronomy11091724
APA StyleNguyen, T.-P.-D., Jang, D.-C., Tran, T.-T.-H., Nguyen, Q.-T., Kim, I.-S., Hoang, T.-L.-H., & Vu, N.-T. (2021). Influence of Green Light Added with Red and Blue LEDs on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy, 11(9), 1724. https://doi.org/10.3390/agronomy11091724







