Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sampling Methods and Data Analysis
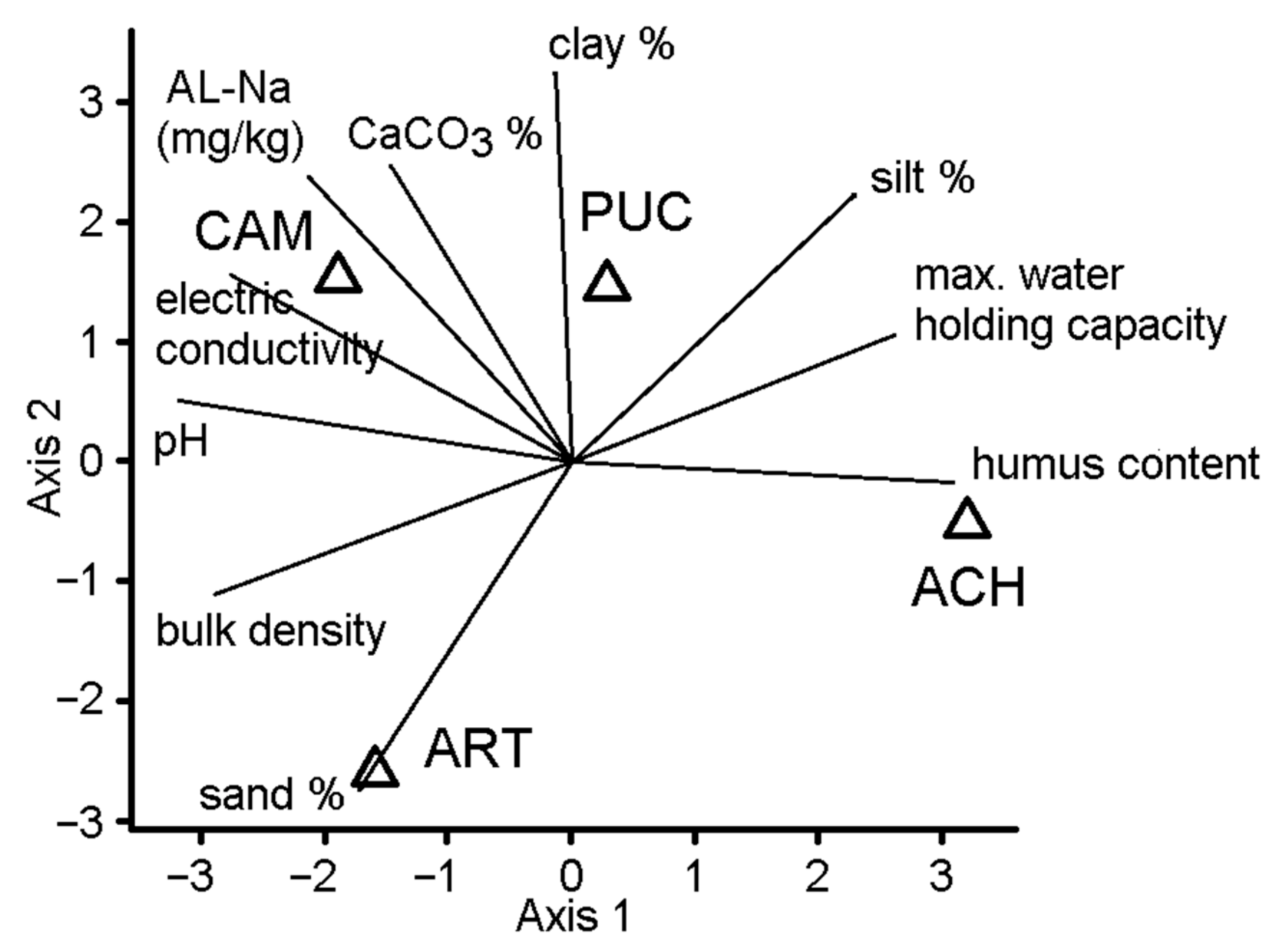
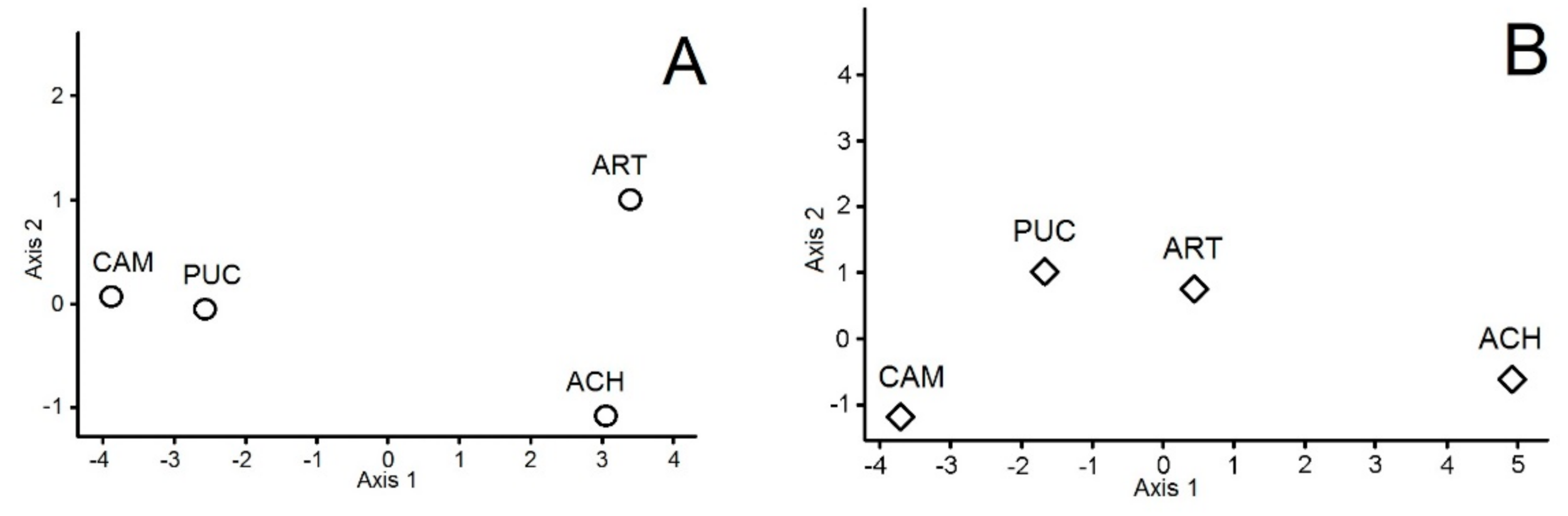
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CAM | PUC | ART | ACH | |||||
|---|---|---|---|---|---|---|---|---|
| Carbon Sources | Jun. | Sep. | Jun. | Sep. | Jun. | Sep. | Jun. | Sep. |
| D-galactose | 0.1257 | 0.1124 | 0.4986 | 0.2061 | 1.3822 | 0.4314 | 1.7890 | 0.9274 |
| L-(+)-Arabinose | 0.1289 | 0.1177 | 0.4453 | 0.2109 | 1.8403 | 0.4919 | 1.8730 | 1.0139 |
| Trehalose | 0.1293 | 0.1127 | 0.6485 | 0.2173 | 1.6057 | 0.4111 | 1.5199 | 0.8489 |
| D-(−)-Fructose | 0.1459 | 0.1272 | 0.7867 | 0.2322 | 2.7781 | 0.5926 | 2.7487 | 1.3151 |
| D-Glucose | 0.1467 | 0.1208 | 0.9347 | 0.2377 | 3.3780 | 0.7120 | 3.1372 | 1.4760 |
| DL-Malic acid | 0.5774 | 0.1708 | 0.7527 | 0.3958 | 3.9764 | 0.5171 | 3.0146 | 1.3326 |
| Citric acid monohydrate | 0.4407 | 0.1678 | 0.4073 | 0.4666 | 1.8965 | 0.4185 | 1.4027 | 0.7385 |
| L-Alanine | 0.2324 | 0.2099 | 0.2952 | 0.2828 | 0.8097 | 0.3591 | 0.9323 | 0.6061 |
| Succinic acid | 0.1302 | 0.1182 | 0.6086 | 0.2865 | 1.6200 | 0.4556 | 1.5116 | 0.8497 |
| L-Glutamine | 0.1986 | 0.1863 | 0.3473 | 0.2919 | 1.2973 | 0.4514 | 1.1825 | 0.7143 |
| L-lysine-monohydrochloride | 0.1682 | 0.1668 | 0.2062 | 0.2686 | 0.5203 | 0.3056 | 0.4748 | 0.3756 |
| L-Arginine | 0.0964 | 0.1273 | 0.1054 | 0.2031 | 0.2651 | 0.2899 | 0.3328 | 0.4129 |
| L-Leucine | 0.1437 | 0.1471 | 0.1956 | 0.2047 | 0.4626 | 0.2784 | 0.5684 | 0.3908 |
| L-Glutamic acid | 0.1652 | 0.1594 | 0.4768 | 0.2795 | 1.1811 | 0.5562 | 1.2450 | 0.8223 |
| 3.4-Dihydroxi-benzoic acid | 0.1200 | 0.1106 | 0.3484 | 0.5163 | 2.1173 | 0.5310 | 0.6941 | 0.3596 |
| Species Name | CAM | PUC | ART | ACH |
|---|---|---|---|---|
| Achillea setacea W. et K. | 0 | 0 | 0 | 12.2 |
| Agrimonia eupatoria L. | 0 | 0 | 0 | 0.02 |
| Arenaria serpyllifolia L. | 0 | 0 | 0 | 0.22 |
| Artemisia santonicum L. | 0 | 0 | 21 | 0 |
| Bromus commutatus Schrad. | 0 | 0 | 0 | 1.8 |
| Bromus hordeaceus L. | 0 | 0 | 0 | 1.6 |
| Calamagrostis epigeios (L.) Roth | 0 | 0 | 0 | 0.02 |
| Camphorosma annua Pall. | 8.8 | 0.02 | 0 | 0 |
| Carduus nutans L. | 0 | 0 | 0 | 1.44 |
| Centaurea pannonica (Heuff.) Simk. | 0 | 0 | 0 | 0.02 |
| Cerastium pumilum Curt. | 0 | 0 | 0 | 5.02 |
| Convolvulus arvensis L. | 0 | 0 | 0 | 1.8 |
| Cruciata pedemontana (Bell.) Ehrend. | 0 | 0 | 0 | 0.46 |
| Dactylis glomerata L. | 0 | 0 | 0 | 1.42 |
| Daucus carota L. | 0 | 0 | 0 | 0.08 |
| Elymus repens (L.) Gould | 0 | 0 | 0.2 | 2.22 |
| Eryngium campestre L. | 0 | 0 | 0 | 0.84 |
| Euphorbia cyparissias L. | 0 | 0 | 0 | 0.2 |
| Falcaria vulgaris Bernh. | 0 | 0 | 0 | 0.02 |
| Festuca pseudovina Hack. ex Wiesb. | 0 | 0 | 7.6 | 37 |
| Galium verum L. | 0 | 0 | 0 | 0.82 |
| Hieracium caespitosum Dum. | 0 | 0 | 0 | 1.24 |
| Hordeum hystrix Roth | 0 | 0 | 0 | 0.04 |
| Koeleria cristata (L.) Pers. | 0 | 0 | 0 | 4.8 |
| Leontodon hispidus L. | 0 | 0 | 0 | 2.02 |
| Lepidium crassifolium W. et K. | 6.2 | 0.22 | 0 | 0 |
| Lepidium draba L. | 0 | 0 | 0 | 0.06 |
| Limonium gmelinii (Willd.) O. Kuntze | 0 | 0 | 0 | 0.02 |
| Linum austriacum L. | 0 | 0 | 0 | 0.2 |
| Lolium perenne L. | 0 | 0 | 0 | 0.02 |
| Lotus corniculatus L. | 0 | 0 | 0 | 5.66 |
| Medicago falcata L. | 0 | 0 | 0 | 1.2 |
| Medicago lupulina L. | 0 | 0 | 0 | 1.2 |
| Medicago minima (L.) Grufbg. | 0 | 0 | 0 | 0.6 |
| Myosotis ramosissima Rochel | 0 | 0 | 0 | 0.06 |
| Ononis spinosa L. | 0 | 0 | 0 | 0.2 |
| Phragmites australis (Cav.) Trin. | 0.1 | 0.4 | 0 | 0 |
| Plantago lanceolata L. | 0 | 0 | 0 | 10 |
| Plantago maritima L. | 0.04 | 0.02 | 15 | 0 |
| Poa angustifolia L. | 0 | 0 | 0 | 0.04 |
| Poa bulbosa L. | 0 | 0 | 0.02 | 0 |
| Podospermum canum C.A. Mey. | 0 | 0 | 0.06 | 0.04 |
| Potentilla argentea L. | 0 | 0 | 0 | 3.02 |
| Puccinellia limosa (Schur) Holmbg. | 1.44 | 68 | 1.26 | 0 |
| Thymus pannonicus All. | 0 | 0 | 0 | 4.02 |
| Tragopogon dubius Scop. | 0 | 0 | 0 | 0.02 |
| Trifolium campestre Schreb. | 0 | 0 | 0 | 1.62 |
| Trifolium repens L. | 0 | 0 | 0 | 3 |
| Veronica arvensis L. | 0 | 0 | 0 | 0.28 |
| Veronica prostrata L. | 0 | 0 | 0 | 0.02 |
| Vicia angustifolia L. | 0 | 0 | 0 | 0.24 |
| indet. dicotyledonous seedling | 0 | 0.02 | 0 | 0 |
| Number of species | 5 | 6 | 7 | 44 |
References
- Juhász-Nagy, P. Lack, Need and Tasks of An Oprative Ecology; Akadémiai Kiadó: Budapest, Hungary, 1986; pp. 1–251. (In Hungarian) [Google Scholar]
- Piernik, A. Inland halophilious vegetation as indicator of soil salinity. Basic Appl. Ecol. 2003, 4, 525–536. [Google Scholar] [CrossRef]
- Clements, F.E. Nature and Structure of the Climax. J. Ecol. 1936, 24, 252–284. Available online: https://www.jstor.org/stable/2256278?seq=1#metadata_info_tab_contents (accessed on 18 August 2021). [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. II. A gradient analysis of the south slope. Ecology 1965, 46, 429–452. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, production, and diversity along the elevation gradient. Ecology 1975, 56, 771–790. [Google Scholar] [CrossRef]
- Gentry, A.H. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Ann. Mo. Bot. Gard. 1988, 75, 1–34. [Google Scholar] [CrossRef]
- Pennings, S.C.; Grant, M.B.; Bertness, M.D. Plant zonation in low-latitude salt marshes: Disentangling the roles of flooding, salinity and competition. J. Ecol. 2005, 93, 159–167. [Google Scholar] [CrossRef]
- Hemp, A. Continuum or zonation? Altitudinal gradients in the forest vegetation of Mt. Kilimanjaro. Plant Ecol. 2006, 184, 27–42. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Rice, W.A.; Clayton, G.W. Soil microbial diversity and community structure under wheat as influenced by tillage and crop rotation. Soil Biol. Biochem. 1998, 30, 1733–1741. [Google Scholar] [CrossRef]
- Elhottova, D.; Szili-Kovács, T.; Triska, J. Soil microbial community of abandoned sand fields. Folia Microbiol. 2002, 47, 435–440. [Google Scholar] [CrossRef]
- Merilä, P.; Malmivaara-Lämsä, M.; Spetz, P.; Stark, S.; Vierikko, K.; Derome, J.; Fritze, H. Soil organic matter quality as a link between microbial community structure and vegetation composition along a successional gradient in a boreal forest. Appl. Soil Ecol. 2010, 46, 259–267. [Google Scholar] [CrossRef]
- Felföldi, T. Microbial communities of soda lakes and pans in the Carpathian Basin: A review. Biol. Futur. 2020, 71, 393–404. [Google Scholar] [CrossRef]
- Niering, W.A.; Warren, R.S. Vegetation patterns and processes in New England salt marshes. BioScience 1980, 30, 301–307. [Google Scholar] [CrossRef]
- Corwin, D.L.; Kaffka, S.R.; Hopmans, J.W.; Mori, Y.; van Groenigen, J.W.; van Kessel, C.; Lesch, S.M.; Oster, J.D. Assessment and field-scale mapping of soil quality properties of a saline-sodic soil. Geoderma 2003, 114, 231–259. [Google Scholar] [CrossRef]
- Semple, W.S.; Koen, T.B.; Eldridge, D.J.; Düttmer, K.M.; Parker, B. Variation in soil properties on two partially revegetated saline scalds in south-eastern Australia. Aust. J. Exp. Agr. 2006, 46, 1279–1289. [Google Scholar] [CrossRef]
- Bodrogközy, G.; Györffy, G. Ecology of the halophilic vegetation of the Pannonicum. VII. Zonation study along the Bega-backwatersin the Viovodina (Yugoslavia). Acta Biol. Szeged 1970, 16, 25–41. Available online: http://acta.bibl.u-szeged.hu/21732/1/biologica_016_fasc_003_004_025-041.pdf (accessed on 18 August 2021).
- Tóth, T.; Rajkai, K. Soil and plant correlations in a solonetzic grassland. Soil Sci. 1994, 157, 253–262. [Google Scholar] [CrossRef]
- Piernik, A. Vegetation-environment relations on inland saline habitats in Central Poland. Phytocoenologia 2005, 35, 19–37. [Google Scholar] [CrossRef]
- Piernik, A.; Hulisz, P.; Rokicka, A. Micropattern of halophytic vegetation on technogenic soils affected by the soda industry. Soil Sci. Plant. Nutr. 2013, 61 (Suppl. S1), 98–112. [Google Scholar] [CrossRef]
- Ping, Y.; Cui, L.; Pan, X.; Li, W.; Li, Y.; Kang, X.; Song, T.; He, P. Decomposition Processes in Coastal Wetlands: The Importance of Suaeda salsa Community for Soil Cellulose Decomposition. Pol. J. Ecol. 2018, 66, 217–226. [Google Scholar] [CrossRef]
- Sardinha, M.; Muller, T.; Schmeisky, H.; Joergensen, R.G. Microbial performance in soils along a salinity gradient under acidic conditions. Appl. Soil Ecol. 2003, 23, 237–244. [Google Scholar] [CrossRef]
- Borsodi, A.K.; Bárány, Á.; Krett, G.; Márialigeti, K.; Szili-Kovács, T. Diversity and ecological tolerance of bacteria isolated from the rhizosphere of halophyton plants living nearby Kiskunság soda ponds, Hungary. Acta Microbiol. Immunol. Hung. 2015, 62, 183–197. [Google Scholar] [CrossRef]
- Canfora, L.; Lo Papa, G.; Antisari, L.V.; Bazan, G.; Dazzi, C.; Benedetti, A. Spatial microbial community structure and biodiversity analysis in "extreme" hypersaline soils of a semiarid Mediterranean area. Appl. Soil Ecol. 2015, 93, 120–129. [Google Scholar] [CrossRef]
- Molnár, Z.; Borhidi, A. Hungarian alkali vegetation: Origins, landscape history, syntaxonomy, conservation. Phytocoenologia 2003, 33, 377–408. [Google Scholar] [CrossRef]
- Tóth, T. Salt-affected soils and their native vegetation in Hungary. In Sabkha Ecosystems: Volume III: Africa and Southern Europe; Ozturk, M., Böer, B., Barth, H.-J., Clüsener-Godt, M., Ajmal Khan, M., Breckle, S.-W., Eds.; Springer Science & Business Media B.V.: Dordrecht, The Netherlands, 2011; pp. 113–132. [Google Scholar] [CrossRef]
- Borhidi, A.; Kevey, B.; Lendvai, G. Plant. Communities of Hungary; Akadémiai Kiadó: Budapest, Hungary, 2012; p. 544. [Google Scholar]
- Buzás, I. Manual for Soil and Agrochemical Analyses 2: Physico-Chemical and Chemical Analysis of Soils; Mezőgazdasági Kiadó: Budapest, Hungary, 1988; pp. 1–242. (In Hungarian) [Google Scholar]
- Buzás, I. Manual for Soil and Agrochemical Analyses 1: Physical, Water Management and Mineral Analysis of Soils; Inda Kiadó: Budapest, Hungary, 1993; pp. 1–357. (In Hungarian) [Google Scholar]
- MSZ 2006. Hungarian Standard MSz 21470-50/2006. Environmental Testing of Soils. Determination of Total and Soluble Toxic Element, Heavy Metal and Chromium(VI) Content; Hungarian Standards Institution: Budapest, Hungary, 2006; p. 33. (In Hungarian) [Google Scholar]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Szili-Kovács, T.; Bárány, Á.; Füzy, A.; Takács, T.; Krett, G.; Kovács, R.; Borsodi, A. Analysis of the microbial metabolic activity patterns and mycorrhizal fungal colonisation in the rhizosphere of three soils neighbouring sodic lakes. Agrokémia és Talajt. 2017, 66, 149–164. [Google Scholar] [CrossRef][Green Version]
- Csontos, P.; Mjazovszky, Á.; Ragályi, P.; Szili Kovács, T. Studies on the Minimal Area of Salt Affected Plant Communities. In Abstracts of the "XVIII. Apáczai-napok" Scientific Conference, Győr, Hungary, 21–22 October 2014; University of Western Hungary, Apáczai Csere János Faculty: Győr, Budapest, 2014; p. 58. (In Hungarian) [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley & Sons: New York, NY, USA, 1974; pp. 1–547. [Google Scholar]
- Mucsi, M.; Csontos, P.; Borsodi, A.; Krett, G.; Gazdag, O.; Szili-Kovács, T. Use of the microrespiration method to analyse the metabolic activity patterns in the soil of four characteristic sodic plant associations. Agrokémiai és Talajt. 2017, 66, 165–179, (In Hungarian, English summary). [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Jackson, D.A. How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia 2001, 129, 169–178. [Google Scholar] [CrossRef]
- Podani, J. SYN-TAX Version 5.0, User’s Guide; Scientia Publishing: Budapest, Hungary, 1993; p. 104. [Google Scholar]
- Podani, J. SYN-TAX 2000—Computer Programs for Data Analysis in Ecology and Syste–Matics. User’s Manual; Scientia Publishing: Budapest, Hungary, 2001; p. 53. [Google Scholar]
- Zalatnai, M.; Körmöczi, L.; Tóth, T. Community boundaries and edaphic factors in saline-sodic grassland communities along an elevation gradient. Tiscia 2007, 36, 7–15. Available online: https://www.researchgate.net/publication/237325142_Community_boundaries_and_edaphic_factors_in_saline-sodic_grassland_communities_along_an_elevation_gradient (accessed on 18 August 2021).
- Deák, B.; Valkó, O.; Alexander, C.; Mücke, W.; Kania, A.; Tamás, J.; Heilmeier, H. Fine-scale vertical position as an indicator of vegetation in alkali grasslands—Case study based on remotely sensed data. Flora 2014, 209, 693–697. [Google Scholar] [CrossRef]
- Borsodi, A.K.; Mucsi, M.; Krett, G.; Szabó, A.; Felföldi, T.; Szili-Kovács, T. Variation in sodic soil bacterial communities associated with different alkali vegetation types. Microorganisms 2021, 9, 1673. [Google Scholar] [CrossRef]
- Gönczöl, J.; Csontos, P.; Révay, Á. Catchment scale patterns of aquatic hyphomycetes. The role of physicochemical variables and substrate composition in structuring conidial communities. Arch. Hydrobiol. 2003, 157, 249–266. [Google Scholar] [CrossRef]
- Singh, B.K.; Munro, S.; Reid, E.; Ord, B.; Potts, J.M.; Paterson, E.; Millard, P. Investigating microbial community structure in soils by physiological, biochemical and molecular fingerprinting methods. Eur. J. Soil Sci. 2006, 57, 72–82. [Google Scholar] [CrossRef]
- Dirilgen, T.; Arroyo, J.; Dimmers, W.J.; Faber, J.; Stone, D.; da Silva, P.M.; Carvalho, F.; Schmelz, R.; Griffiths, B.S.; Francisco, R.; et al. Mite community composition across a European transect and its relationships to variation in other components of soil biodiversity. Appl. Soil Ecol. 2016, 97, 86–97. [Google Scholar] [CrossRef]
- Luo, G.; Li, B.; Lo, L.G.; Zhang, T.; Angelidaki, I. Antibiotic resistance genes and correlations with microbial community and metal resistance genes in full-scale biogas reactors as revealed by metagenomic analysis. Environ. Sci. Technol. 2017, 51, 4069–4080. [Google Scholar] [CrossRef] [PubMed]



| Carbon Source | Abbreviation | Concentration (g/1000·cm−3) | |
|---|---|---|---|
| 1. | D-galactose | GAL | 80 |
| 2. | L-(+)-Arabinose | ARA | 80 |
| 3. | Trehalose | TRE | 80 |
| 4. | D-(-)-Fructose | FRU | 80 |
| 5. | D-Glucose | GLC | 80 |
| 6. | DL-Malic acid | MAL | 40 |
| 7. | Citric acid monohydrate | CIT | 40 |
| 8. | L-Alanine | ALA | 40 |
| 9. | Succinic acid | SUC | 40 |
| 10. | L-Glutamine | GLN | 20 |
| 11. | L-lysine-monohydrochloride | LYS | 40 |
| 12. | L-Arginine | ARG | 12 |
| 13. | L-Leucine | LEU | 12 |
| 14. | L-Glutamic acid | GLU | 12 |
| 15. | 3,4-Dihydroxi-benzoic acid | DHB | 12 |
| Soil Properties | CAM | PUC | ART | ACH |
|---|---|---|---|---|
| Max. water-holding capacity, m/m% (pF0) | 34.61 | 32.44 | 27.6 | 41.882 |
| Bulk density, g/cm3 | 1.466 | 1.3049 | 1.492 | 1.2825 |
| Sand % (2–0.05 mm) | 23.87 | 21.127 | 41.64 | 21.554 |
| Silt % (0.05–0.002 mm) | 48.44 | 51.49 | 37.23 | 54.298 |
| Clay % (0.002 > mm) | 27.69 | 27.383 | 21.13 | 24.148 |
| pH (H2O) | 10.36 | 9.4825 | 9.928 | 8.035 |
| Electric Conductivity, µS/cm | 1993 | 1148.3 | 1048 | 282 |
| Humus content, m/m% | 0.495 | 1.9559 | 1.051 | 2.6681 |
| CaCO3, m/m% | 20.18 | 23.018 | 14.69 | 12.35 |
| AL-Na, mg/kg | 4779 | 3041 | 1127 | 74 |
| Soil Variables | Soil Microbes in June | Soil Microbes in September | Vascular Vegetation | |
|---|---|---|---|---|
| Soil Variables | 0 | 0.402 | 0.363 | 0.210 |
| Soil Microbes in June | 0 | 0.292 | 0.613 | |
| Soil Microbes in September | 0 | 0.191 | ||
| Vascular Vegetation | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csontos, P.; Mucsi, M.; Ragályi, P.; Tamás, J.; Kalapos, T.; Pápay, G.; Mjazovszky, Á.; Penksza, K.; Szili-Kovács, T. Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures. Agronomy 2021, 11, 1652. https://doi.org/10.3390/agronomy11081652
Csontos P, Mucsi M, Ragályi P, Tamás J, Kalapos T, Pápay G, Mjazovszky Á, Penksza K, Szili-Kovács T. Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures. Agronomy. 2021; 11(8):1652. https://doi.org/10.3390/agronomy11081652
Chicago/Turabian StyleCsontos, Péter, Márton Mucsi, Péter Ragályi, Júlia Tamás, Tibor Kalapos, Gergely Pápay, Ákos Mjazovszky, Károly Penksza, and Tibor Szili-Kovács. 2021. "Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures" Agronomy 11, no. 8: 1652. https://doi.org/10.3390/agronomy11081652
APA StyleCsontos, P., Mucsi, M., Ragályi, P., Tamás, J., Kalapos, T., Pápay, G., Mjazovszky, Á., Penksza, K., & Szili-Kovács, T. (2021). Standing Vegetation Exceeds Soil Microbial Communities in Soil Type Indication: A Procrustes Test of Four Salt-Affected Pastures. Agronomy, 11(8), 1652. https://doi.org/10.3390/agronomy11081652







