Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation
Abstract
1. Introduction
2. Materials and Methods
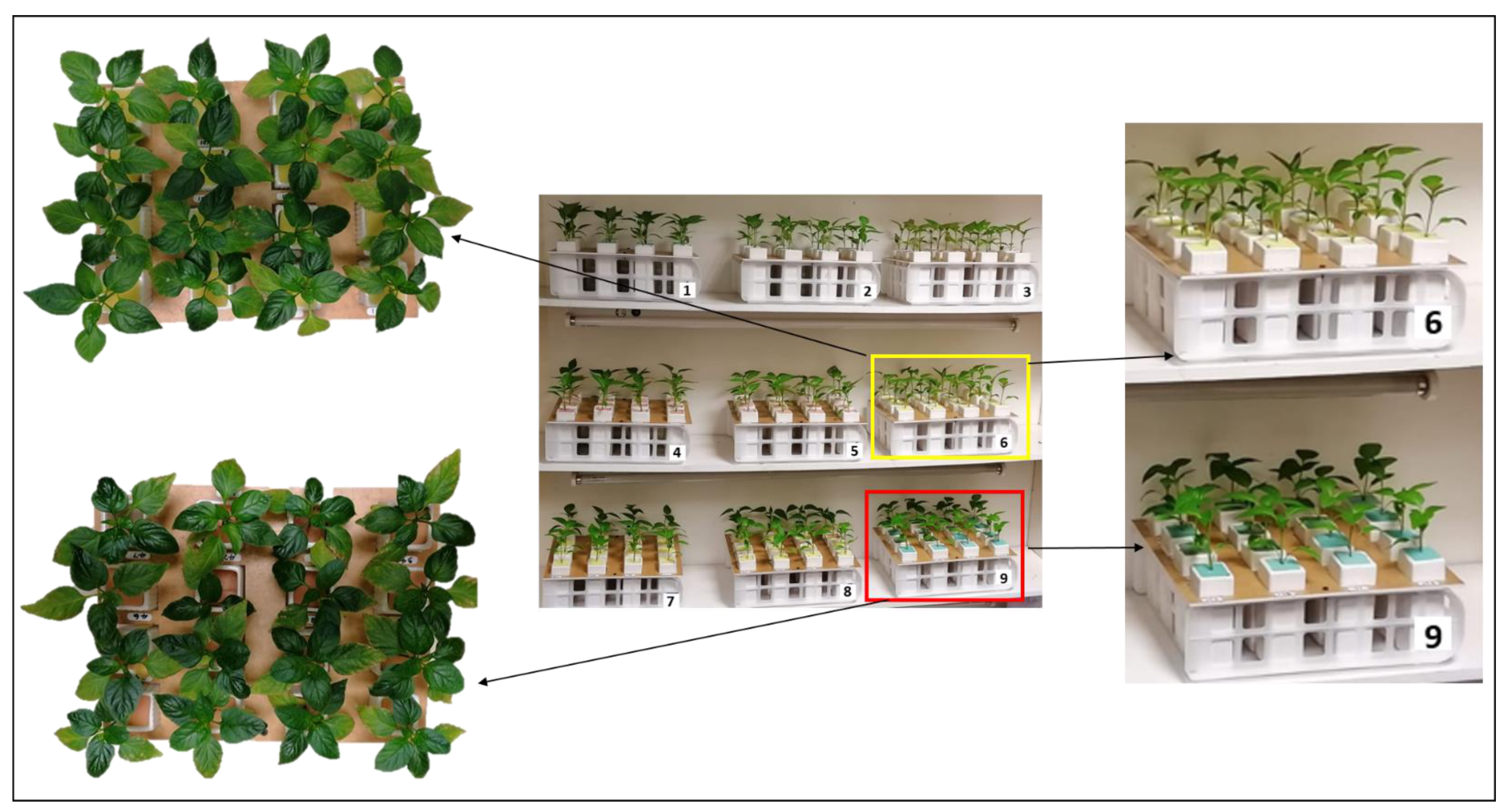
2.1. Plant Material and Culture Condition
2.2. Cyanobacteria Strains Cultivation and Growth Conditions
2.3. Preparation of Cyanobacteria Extracts
2.4. Cyanobacteria and Its Characterization
2.4.1. Morphological Characterization
2.4.2. DNA Extraction and Gene Sequencing
2.4.3. Phylogenetic Analysis
2.5. Experimental Design
2.6. Growth Parameter and Spad Index
2.7. Data Analysis
3. Results
3.1. Cyanobacteria Characterization, Pigment, and Molecular Identification
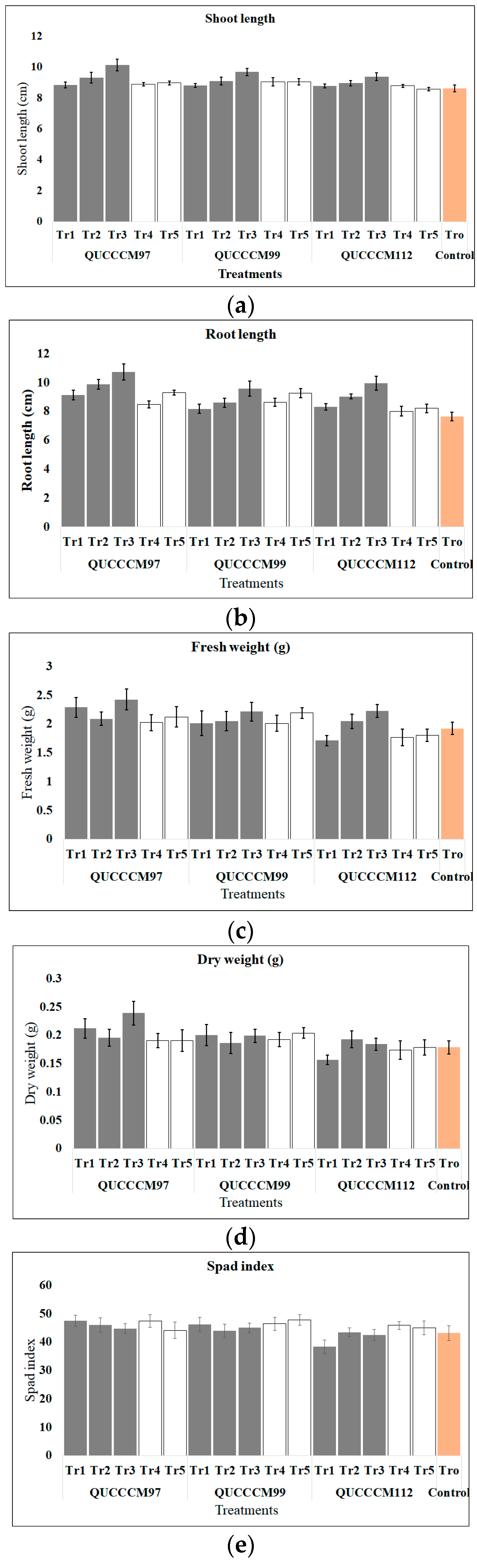
3.2. Growth Parameters of Seedlings and Spad Index at the End of the Experimental Period
3.2.1. Efficacy of Roholtiella sp. (QUCCCM97) Extracts and Biomass as a Growth Enhancer
3.2.2. Efficacy of Nostoc ellipsosporum (QUCCCM99) Extracts and Biomass as a Growth Enhancer
3.2.3. Efficacy of Desmonostoc danxiaense (QUCCCM112) Extracts and Biomass as a Growth Enhancer
3.2.4. Strain Nature, Pigment, Dose and Effect Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73–80. [Google Scholar] [CrossRef]
- Lee, K.-E.; Adhikari, A.; Kang, S.-M.; You, Y.-H.; Joo, G.-J.; Kim, J.-H.; Kim, S.-J.; Lee, I.-J. Isolation and characterization of the high silicate and phosphate solubilizing novel strain Enterobacter ludwigii GAK2 that promotes growth in rice plants. Agronomy 2019, 9, 144. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.; Nelson, P.; Bird, M. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Foppen, R.P.B.; Van Turnhout, C.A.M.; De Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, R.; Bhadouria, R.; Tripathi, S.; Singh, P.; Singh, H.; Raghubanshi, A.S. Organic amendment impact on SOC dynamics in dry tropics: A possible role of relative availability of inorganic-N pools. Agric. Ecosyst. Environ. 2016, 235, 38–50. [Google Scholar] [CrossRef]
- Van Der Sluijs, J.P.; Amaral-Rogers, V.; Belzunces, L.P.; Van Lexmond, M.F.I.J.B.; Bonmatin, J.-M.; Chagnon, M.; Downs, C.A.; Furlan, L.; Gibbons, D.W.; Giorio, C.; et al. Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ. Sci. Pollut. Res. 2015, 22, 148–154. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Darki, B.Z. Soil conservation in an abandoned agricultural rain-fed land through inoculation of cyanobacteria. Catena 2020, 187, 104341. [Google Scholar] [CrossRef]
- Saadaoui, I.; Sedky, R.; Rasheed, R.; Bounnit, T.; AlMahmoud, A.; Elshekh, A.; Dalgamouni, T.; Al Jmal, K.; Das, P.; Al Jabri, H. Assessment of the algae-based biofertilizer influence on date palm (Phoenix dactylifera L.) cultivation. J. Appl. Phycol. 2018, 31, 457–463. [Google Scholar] [CrossRef]
- Jaiswal, P.; Prasanna, R.; Kashyap, A.K. Modulation of carbonic anhydrase activity in two nitrogen fixing cyanobacteria, Nostoc calcicola and Anabaena sp. J. Plant Physiol. 2005, 162, 1087–1094. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Riahi, H.; Hashtroudi, M.S.; Ghassempour, A.; Aghashariatmadary, Z. Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci. Plant Nutr. 2013, 59, 535–547. [Google Scholar] [CrossRef]
- Dunker, S. Book Review on: The Cell Biology of Cyanobacteria; Flores, E., Herrero, A., Eds.; Caister Academic Press: Norfolk, UK, 2014; p. 308. ISBN 978-1-908230-38-6. [Google Scholar]
- Dorina, S.; Judith, S.; Björn, W.; Schwing, J.; Andrea, S.; Kai, M.; Roland, U. A new strategy for a combined isolation of EPS and pigments from cyanobacteria. J. Appl. Phycol. 2020, 32, 1729–1740. [Google Scholar] [CrossRef]
- Vaishampayan, A.; Sinha, R.P.; Hader, D.-P.; Dey, T.; Gupta, A.K.; Bhan, U.; Rao, A.L. Cyanobacterial biofertilizers in rice agriculture. Bot. Rev. 2001, 67, 453–516. [Google Scholar] [CrossRef]
- Mishra, U.; Pabbi, S. Cyanobacteria: A potential biofertilizer for rice. Resonance 2004, 9, 6–10. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Riahi, H.; Abdi, M.; Hashtroudi, M.S.; Ghassempour, A. Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. J. Appl. Phycol. 2015, 27, 2279–2287. [Google Scholar] [CrossRef]
- Garlapati, D.; Chandrasekaran, M.; Devanesan, A.; Mathimani, T.; Pugazhendhi, A. Role of cyanobacteria in agricultural and industrial sectors: An outlook on economically important byproducts. Appl. Microbiol. Biotechnol. 2019, 103, 4709–4721. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Mueller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Misra, S.; Kaushik, B. Growth promoting substances of cyanobacteria. I: Vitamins and their influence on rice plant. Proc. Indian Natl. Sci. Acad. Part B Biol. Sci. 1989, 55, 295–300. [Google Scholar]
- Misra, S.; Kaushik, B. Growth promoting substances of cyanobacteria II. Detection of amino acids, sugars and auxins. Proc. Indian Sci. Acad. B. 1989, 55, 499–504. [Google Scholar]
- Karthikeyan, N.; Prasanna, R.; Nain, L.; Kaushik, B.D. Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur. J. Soil Biol. 2007, 43, 23–30. [Google Scholar] [CrossRef]
- Obana, S.; Miyamoto, K.; Morita, S.; Ohmori, M.; Inubushi, K. Effect of Nostoc sp. on soil characteristics, plant growth and nutrient uptake. J. Appl. Phycol. 2007, 19, 641–646. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, D.P.; Prabha, R.; Sharma, A.K. Role of cyanobacteria in nutrient cycle and use efficiency in the soil. In Nutrient Use Efficiency: From Basics to Advances; Springer: Berlin/Heidelberg, Germany, 2015; pp. 163–171. [Google Scholar]
- Al Shrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Sci. Res. J. Eng. Technol. Sci. (ASRJETS) 2017, 27, 247–255. [Google Scholar]
- Al-Karaki, G.N.; Al-Hashimi, M. Green fodder production and water use efficiency of some forage crops under hydroponic conditions. ISRN Agron. 2012, 2012, 924672. [Google Scholar] [CrossRef]
- Maqubela, M.P.; Mnkeni, P.N.S.; Muchaonyerwa, P.; D’Acqui, L.P.; Pardo, M.T. Effects of cyanobacteria strains selected for their bioconditioning and biofertilization potential on maize dry matter and soil nitrogen status in a South African soil. Soil Sci. Plant Nutr. 2010, 56, 552–559. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Bosland, P.W.; Votava, E.J.; Votava, E.M. Peppers: Vegetable and Spice Capsicums; CABI: Wallingford, UK, 2012; Volume 22. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In Circular. California Agricultural Experiment Station; CABI: Wallingford, UK, 1950; Volume 347, p. 32. [Google Scholar]
- Saadaoui, I.; Al Ghazal, G.; Bounnit, T.; Al Khulaifi, F.; Al Jabri, H.; Potts, M. Evidence of thermo and halotolerant Nannochloris isolate suitable for biodiesel production in Qatar Culture Collection of Cyanobacteria and Microalgae. Algal Res. 2016, 14, 39–47. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef]
- Saadaoui, I.; Cherif, M.; Rasheed, R.; Bounnit, T.; Ben Hmadou, R.; Manning, S.; Al Jabri, H. Screening of Fresh water and Sea water Microalgae strains from Qatar for feed supplement production. In Qatar Foundation Annual Research Conference Proceedings; Hamad bin Khalifa University Press (HBKU Press): Ar-Rayyan, Qatar, 2018; Volume 2018. [Google Scholar]
- Pitcher, D.; Saunders, N.; Owen, R. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 1989, 8, 151–156. [Google Scholar] [CrossRef]
- Kiselev, K.; Dubrovina, A.; Tyunin, A. The methylation status of plant genomic DNA influences PCR efficiency. J. Plant Physiol. 2015, 175, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Photosynthesis: The light reactions. Plant Physiol. 2010, 5, 163–198. [Google Scholar]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, J.; Sommerfeld, M.R. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef]
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081. [Google Scholar] [CrossRef]
- Irisarri, P.; Gonnet, S.; Monza, J. Cyanobacteria in Uruguayan rice fields: Diversity, nitrogen fixing ability and tolerance to herbicides and combined nitrogen. J. Biotechnol. 2001, 91, 95–103. [Google Scholar] [CrossRef]
- Jha, M.N.; Prasad, A.N. Efficacy of new inexpensive cyanobacterial biofertilizer including its shelf-life. World J. Microbiol. Biotechnol. 2006, 22, 73–79. [Google Scholar] [CrossRef]
- Pereira, Í.; Ortega, R.; Barrientos, L.; Moya, M.; Reyes, G.; Kramm, V. Development of a biofertilizer based on filamentous nitrogen-fixing cyanobacteria for rice crops in Chile. J. Appl. Phycol. 2009, 21, 135–144. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Dias, J.S. Nutritional quality and health benefits of vegetables: A review. Food Nutr. Sci. 2012, 3, 1354–1374. [Google Scholar] [CrossRef]
- Riahi, H.; Shariatmadari, Z.; Khanjir, M.; Azizi, A. Effect of Anabaena vaginicola inoculum on growth of pot plants. In International Symposium on Growing Media, Composting and Substrate Analysis 1013; ISHS: Leuven, Belgium, 2011. [Google Scholar]
- Gantar, M.; Kerby, N.W.; Rowell, P.; Obreht, Z.; Scrimgeour, C. Colonization of wheat (Triticum vulgare L.) by N2− fixing cyanobacteria: IV. Dark nitrogenase activity and effects of cyanobacteria on natural 15N abundance in the plants. New Phytol. 1995, 129, 337–343. [Google Scholar] [CrossRef]
- Nilsson, M.; Bhattacharya, J.; Rai, A.N.; Bergman, B. Colonization of roots of rice (Oryza sativa) by symbiotic Nostoc strains. New Phytol. 2002, 156, 517–525. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of supercritical extracts of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016, 7, 1591. [Google Scholar] [CrossRef]
- Sobiechowska-Sasim, M.; Stoń-Egiert, J.; Kosakowska, A. Quantitative analysis of extracted phycobilin pigments in cyanobacteria—an assessment of spectrophotometric and spectrofluorometric methods. J. Appl. Phycol. 2014, 26, 2065–2074. [Google Scholar] [CrossRef]
- Hegazi, A.Z.; Mostafa, S.S.; Ahmed, H.M. Influence of different cyanobacterial application methods on growth and seed production of common bean under various levels of mineral nitrogen fertilization. Nat. Sci. 2010, 8, 183–194. [Google Scholar]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Aghofack-Nguemezi, J.; Schinzoumka, P.; Tatchago, V. Effects of extracts or powder of Jatropha curcas and Spirulina platensis on the growth and development of tomato plant. J. Appl. Biosci. 2015, 90, 8413–8420. [Google Scholar] [CrossRef][Green Version]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar]
- Herrera, R.M.H.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Mógor, Á.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Samoraj, M.; Witkowska, Z.; Chojnacka, K. Biofortification of maize with micronutrients by Spirulina. Open Chem. 2015, 13, 1119–1126. [Google Scholar] [CrossRef]
- Nanda, B.; Tripathy, S.K.; Padhi, S. Effect of algalization on seed germination of vegetable crops. World J. Microbiol. Biotechnol. 1991, 7, 622–623. [Google Scholar] [CrossRef]
- Shanab, S.M.; Saker, M.M.; Abdel-Rahman, M.H. Crude extracts of some fresh water cyanobacteria have auxin-like activity on potato tissue culture. Arab. J. Biotechnol. 2003, 6, 297–312. [Google Scholar]
- Gupta, A.; Gupta, K. The effect of Phormidium foveolarum extract on growth and development of pea seedlings. Labdev J. Sci. Technol. B 1970, 8, 151–154. [Google Scholar]
- Shukla, A.C.; Gupta, A.B. Influence of algal growth-promoting substances on growth, yield and protein contents of rice plants. Nature 1967, 213, 744. [Google Scholar] [CrossRef]
- Mutale-Joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef]

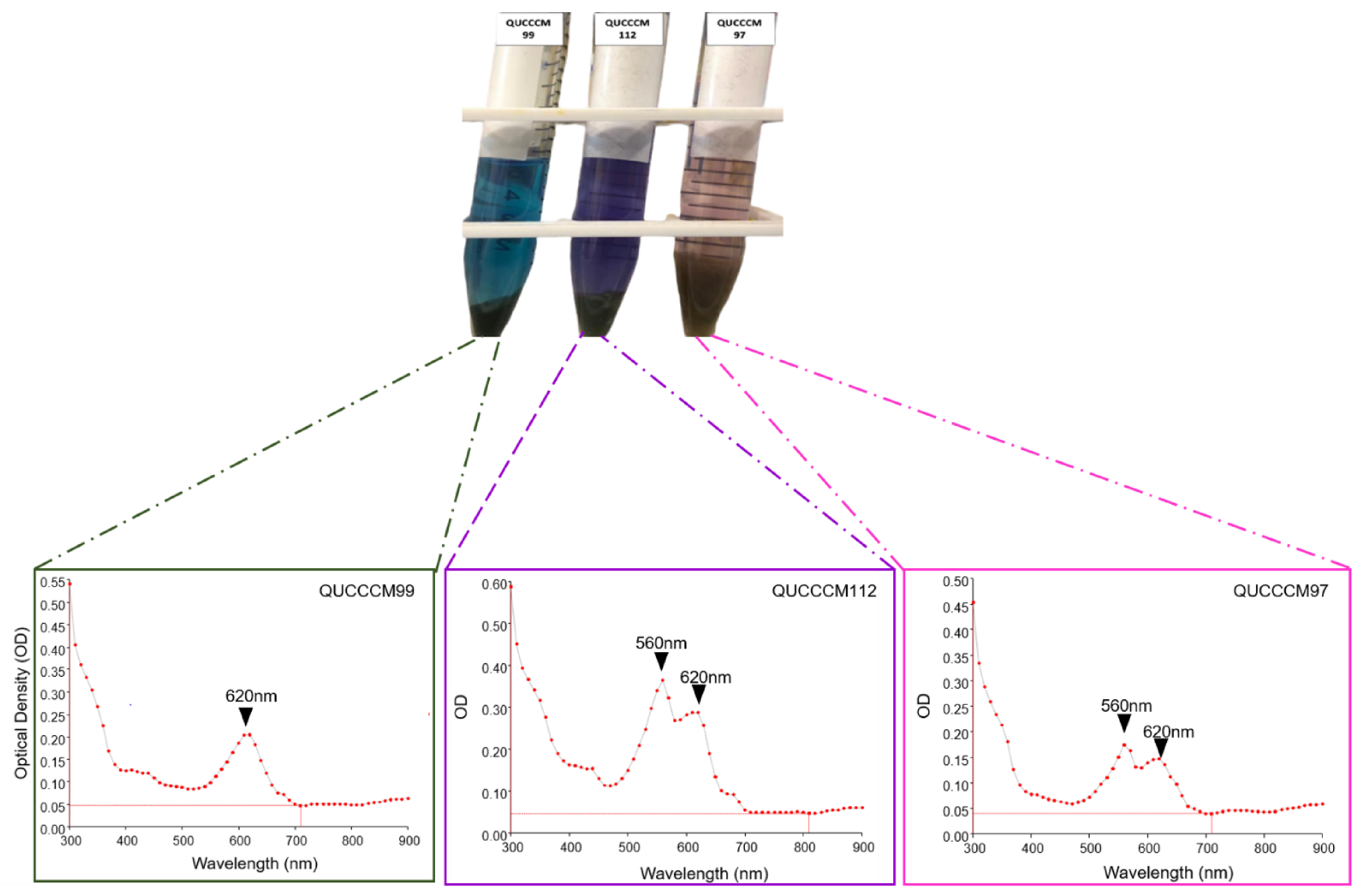
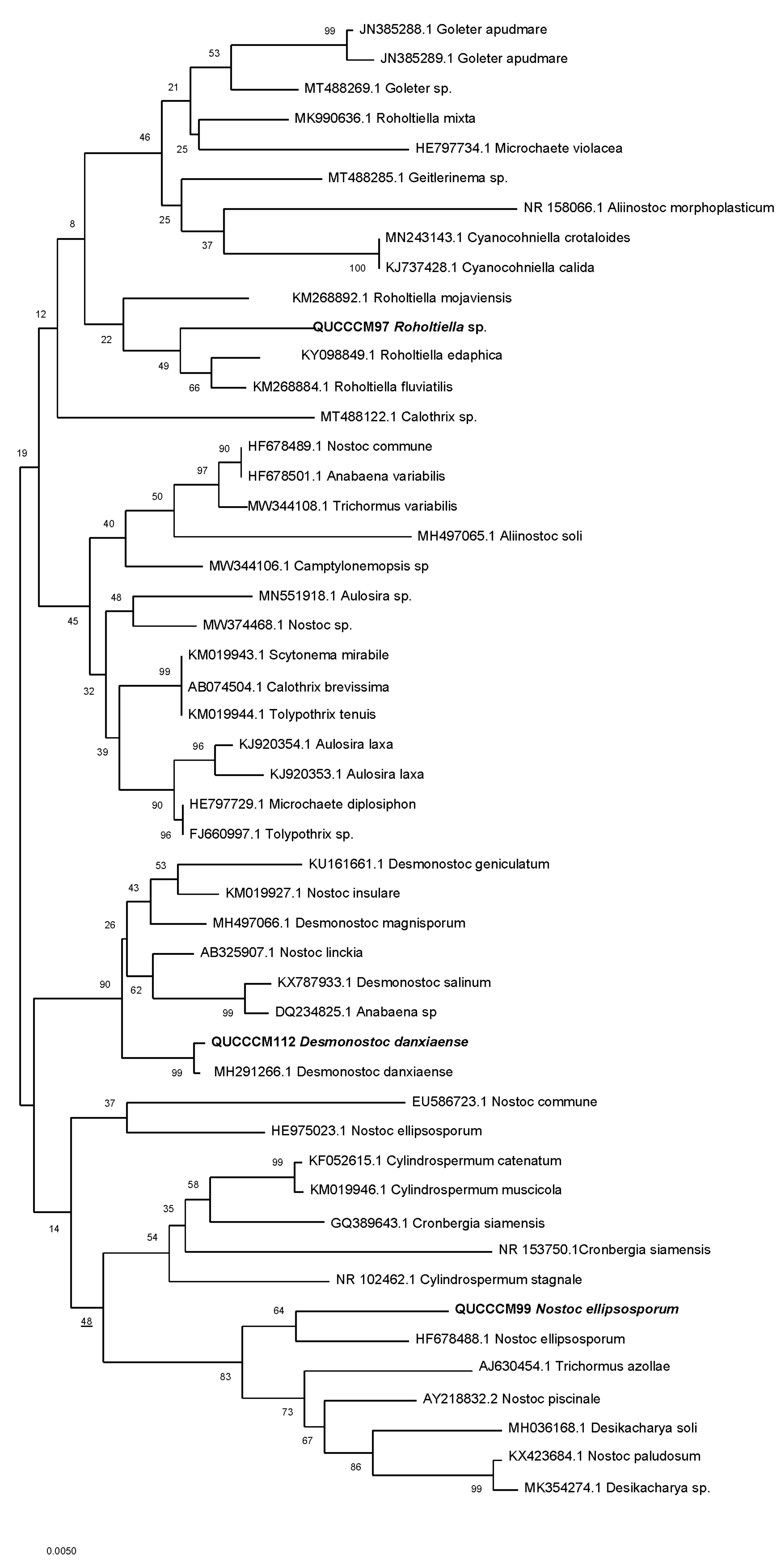
| Strain/Isolate | GenBank Accession Number | Molecular Classification |
|---|---|---|
| QUCCCM97 | MW791421 | Roholtiella sp. |
| QUCCCM99 | MW791422 | Nostoc ellipsosporum |
| QUCCCM112 | MW791423 | Desmonostoc danxiaense |
| Source of Variation | DF | Mean Square (MS) | ||||||
|---|---|---|---|---|---|---|---|---|
| SL | RL | FW | DW | CC | NL | GR | ||
| Block | 8 | 0.979 | 3.8925 | 1.1712 | 0.0065 | 242.3572 | 5.7153 | 0.9544 |
| Treatment | 15 | 1.485 ** | 6.1416 ** | 0.3328 ** | 0.0029 ns | 50.0803 ns | 1.7884 ** | 1.5226 ** |
| Error | 120 | 0.386 | 0.8399 | 0.1245 | 0.00173 | 30.22 | 0.7801 | 0.3815 |
| Total | 143 | |||||||
| Strains | Treatments | Con. (in Hoagland) mL L−1 | Growth Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | Spad Index | Number of Leaves/Plant | Growth Rate | |||
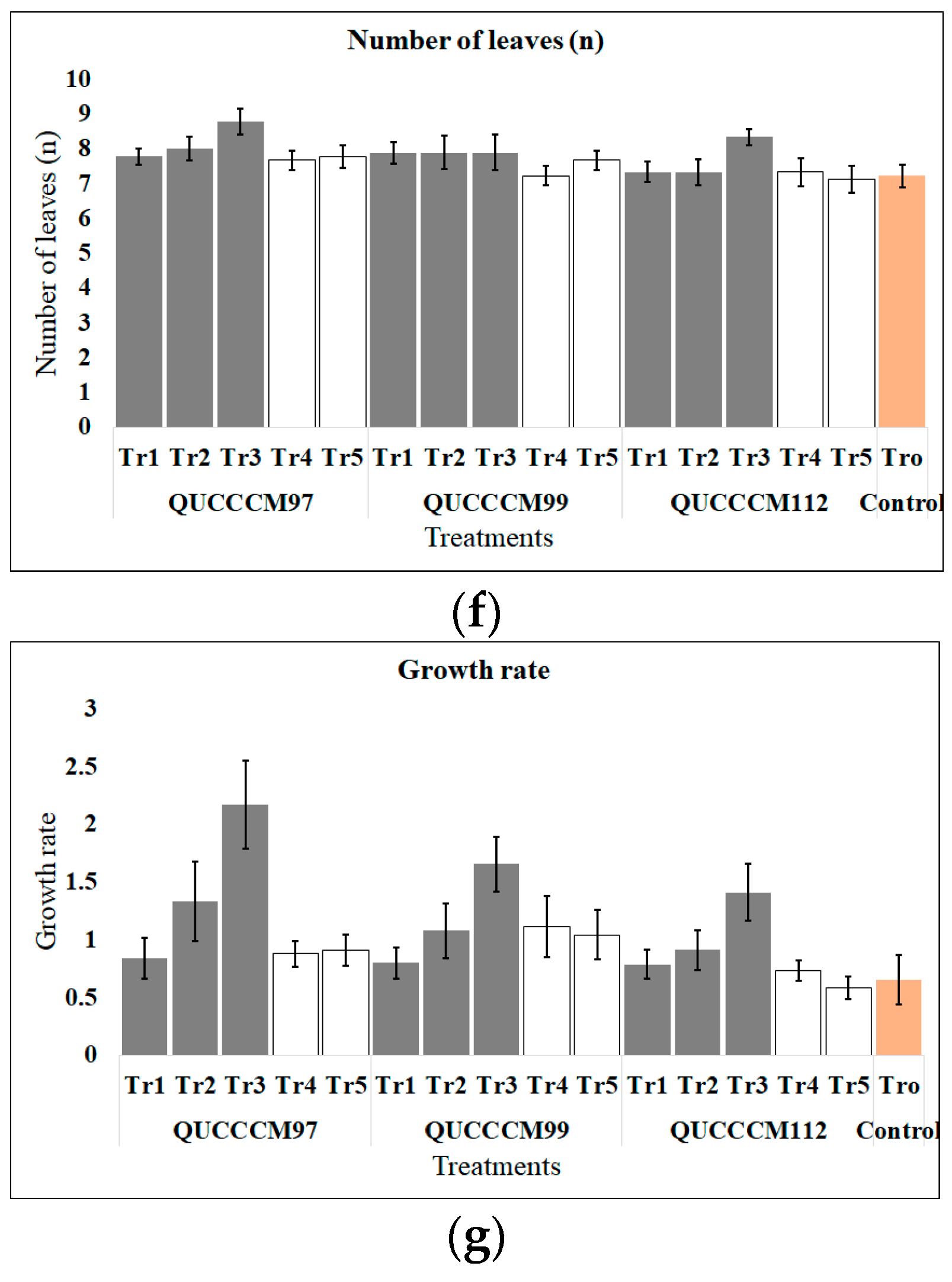
| Roholtiella sp. (QUCCCM97) | Tr1 | 1 (2 mL L−1) | 8.83 ± 0.19 (bc) | 9.13 ± 0.33 (bcde) | 2.29 ± 0.17 (ab) | 0.21 ± 0.02 (ab) | 47.48 ± 1.96 (a) | 7.78 ± 0.22 (ab) | 0.84 ± 0.18 (bc) |
| Tr2 | 2 (4 mL L−1) | 9.319 ± 0.34 (abc) | 9.88 ± 0.32 (ab) | 2.09 ± 0.12 (ab) | 0.20 ± 0.02 (ab) | 46.01 ± 2.50 (ab) | 8.00 ± 0.33) (b) | 1.33 ± 0.34 (abc) | |
| Tr3 | 3 (6 mL L−1) | 10.13 ± 0.38 (a) | 10.72 ± 0.55 (a) | 2.42 ± 0.18 (a) | 0.24 ± 0.02 (a) | 44.72 ± 1.65 (ab) | 8.78 ± 0.36 (a) | 2.17 ± 0.38 (a) | |
| Tr4 | 1 (2 mL L−1) | 8.88 ± 0.12 (bc) | 8.47 ± 0.24 (bcde) | 2.02 ± 0.14 (ab) | 0.19 ± 0.01 (ab) | 47.39 ± 2.31 (a) | 7.67 ± 0.29 (ab) | 0.88 ± 0.11 (bc) | |
| Tr5 | 2 (4 mL L−1) | 8.97 ± 0.14 (bc) | 9.29 ± 0.16 (abcd) | 2.12 ± 0.18 (ab) | 0.19 ± 0.02 (ab) | 44.06 ± 2.89 (ab) | 7.78 ± 0.32 (ab) | 0.91 ± 0.14 (bc) | |
| Nostoc ellipsosporum (QUCCCM99) | Tr1 | 1 (2 mL L−1) | 8.80 ± 0.13 (bc) | 8.18 ± 0.30 (cde) | 2.01 ± 0.21 (ab) | 0.20 ± 0.02 (ab) | 46.19 ± 2.54 (ab) | 7.89 ± 0.31 (ab) | 0.80 ± 0.13 (bc) |
| Tr2 | 2 (4 mL L−1) | 9.09 ± 0.24 (bc) | 8.59 ± 0.30 (bcde) | 2.04 ± 0.17 (ab) | 0.19 ± 0.02 (ab) | 43.98 ± 2.27 (ab) | 7.89 ± 0.484 (ab) | 1.08 ± 0.23 (bc) | |
| Tr3 | 3 (6 mL L−1) | 9.68 ± 0.23 (a) | 9.57 ± 0.52 (abc) | 2.21 ± 0.17 (ab) | 0.20 ± 0.01 (ab) | 45.01 ± 1.64 (ab) | 7.89 ± 0.51 (ab) | 1.66 ± 0.24 (ab) | |
| Tr4 | 1 (2 mL L−1) | 9.06 ± 0.27 (bc) | 8.62 ± 0.29 (bcde) | 2.01 ± 0.14 (ab) | 0.19 ± 0.01 (ab) | 46.38 ± 2.37 (ab) | 7.22 ± 0.28 (b) | 1.11 ± 0.27 (bc) | |
| Tr5 | 2 (4 mL L−1) | 9.03 ± 0.21 (bc) | 9.26 ± 0.30 (abcd) | 2.19 ± 0.10 (ab) | 0.20 ± 0.01 (ab) | 47.76 ± 1.94 (a) | 7.67 ± 0.29 (ab) | 1.04 ± 0.22 (bc) | |
| Desmonostoc danxiaense (QUCCCM112) | Tr1 | 1 (2 mL L−1) | 8.77 ± 0.13 (bc) | 8.31 ± 0.22 (cde) | 1.71 ± 0.09 (b) | 0.16 ± 0.01 (b) | 38.28 ± 2.44 (b) | 7.33 ± 0.29 (ab) | 0.79 ± 0.13 (bc) |
| Tr2 | 2 (4 mL L−1) | 8.96 ± 0.17 (bc) | 9.03 ± 0.16 (bcde) | 2.04 ± 0.13 (ab) | 0.19 ± 0.01 (ab) | 43.48 ± 1.56 (ab) | 7.33 ± 0.37 (ab) | 0.91 ± 0.17 (bc) | |
| Tr3 | 3 (6 mL L−1) | 9.37 ± 0.25 (a) | 9.94 ± 0.48 (ab) | 2.22 ± 0.11 (ab) | 0.18 ± 0.01 (ab) | 42.46 ± 2.03 (ab) | 8.33 ± 0.24 (ab) | 1.41 ± 0.25 (abc) | |
| Tr4 | 1 (2 mL L−1) | 8.78 ± 0.10 (c) | 8.01 ± 0.34 (de) | 1.77 ± 0.15 (b) | 0.17 ± 0.02 (ab) | 45.82 ± 1.42 (ab) | 7.33 ± 0.41 (ab) | 0.73 ± 0.10 (bc) | |
| Tr5 | 2 (4 mL L−1) | 8.56 ± 0.11 (c) | 8.20 ± 0.30 (cde) | 1.80 ± 0.11 (b) | 0.18 ± 0.01 (ab) | 44.92 ± 2.41 (ab) | 7.11 ± 0.39 (b) | 0.59 ± 0.10 (c) | |
| Control | TO | Hoagland | 8.62 ± 0.21 (c) | 7.64 ± 0.29 (e) | 1.92 ± 0.11(b) | 0.18 ± 0.01 (ab) | 43.11 ± 2.62 (ab) | 7.22 ± 0.32 (b) | 0.66 ± 0.21 (bc) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, A.S.; Saadaoui, I.; Ahmed, T.; Hamdi, H.; Cherif, M.; Dalgamouni, T.; Al Ghazal, G.; Ben-Hamadou, R. Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation. Agronomy 2021, 11, 1624. https://doi.org/10.3390/agronomy11081624
Bello AS, Saadaoui I, Ahmed T, Hamdi H, Cherif M, Dalgamouni T, Al Ghazal G, Ben-Hamadou R. Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation. Agronomy. 2021; 11(8):1624. https://doi.org/10.3390/agronomy11081624
Chicago/Turabian StyleBello, Adewale Suraj, Imen Saadaoui, Talaat Ahmed, Helmi Hamdi, Maroua Cherif, Tasneem Dalgamouni, Ghamza Al Ghazal, and Radhouane Ben-Hamadou. 2021. "Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation" Agronomy 11, no. 8: 1624. https://doi.org/10.3390/agronomy11081624
APA StyleBello, A. S., Saadaoui, I., Ahmed, T., Hamdi, H., Cherif, M., Dalgamouni, T., Al Ghazal, G., & Ben-Hamadou, R. (2021). Enhancement in Bell Pepper (Capsicum annuum L.) Plants with Application of Roholtiella sp. (Nostocales) under Soilless Cultivation. Agronomy, 11(8), 1624. https://doi.org/10.3390/agronomy11081624








