Abstract
Fusarium fujikuroi, causing bakanae disease, is one of the most important seedborne pathogens of rice, the detection of which is paramount for seed certification and for preventing field infections. Molecular tests—qPCR and loop-mediated isothermal amplification (LAMP)—are replacing the blotter test in seed health procedures, due to higher sensitivity, specificity, fast turnaround results delivery, on-site application and the possibility of quantifying endophytic seed infections. A LAMP test, which had been previously developed with primers designed to target the elongation factor 1-α sequence of F. fujikuroi, was validated according to the international validation standard (EPPO, PM7/98) on thirty-four rice seed lines of different levels of susceptibility to the disease, thus comparing it to the blotter test and with two different DNA extraction procedures. The use of crude extracted DNA provided more sensitive results than the DNA extracted with the commercial kit Omega E.Z.N.A® Plant DNA kit. The results showed that the endophytic infection of F. fujikuroi is essential for the development of the disease in the field and that the minimum amount of the pathogen necessary for the development of the disease corresponds to 4.17 × 104 cells/µL. This study confirms the applicability of the LAMP technique on-site on rice seeds with fast and quantitative detection of the pathogen.
1. Introduction
Rice (Oryza sativa L.) is a staple food consumed worldwide, with a cultivated area of 162 million ha and a production of 755 million tons. The world’s largest producer is China, followed by India and Indonesia, while in Europe the largest producers are Italy and Spain [1]. In Italy, rice production is currently 1,498,133 tons, an area of 220,027 ha, concentrated in the northern regions [2] that apply technologically advanced rice cultivation systems [3].
Bakanae, caused by F. fujikuroi, a member of the Gibberella fujikuroi species complex (GFSC), is one of the most studied diseases of rice. First observed in Japan in 1828 [4] today it is widely spread throughout Asia [5] and in recent decades—California and Italy [6,7]. Yield losses worldwide can vary from 10% up to 90%, depending on the cultivar and the geographical region [8]. The most frequent symptoms of the disease are yellowing and excessive elongation of the affected seedlings [9,10] caused by the production of gibberellic acid by the pathogen [11], which has led to the Japanese name bakanae, meaning “foolish seedling”. Not all infected seeds cause symptoms in seedlings [8,12]. If the level of seed borne inoculum is high, the probability of causing infection in the field increases, but several factors can influence the infection cycle, such as weather conditions, cropping practices, resistance or susceptibility of the cultivar, the virulence of the strain of the pathogen and the efficiency of the inoculum [13]. In fact, the inoculum level on the seed detected with traditional or molecular methods, and the inoculum threshold necessary to develop the disease in the field do not always correlate [13]. F. fujikuroi is morphologically characterized by a white mycelium that turns purple gray with time and it produces macroconidia and microconidia [14]. The fungus can colonize seeds both internally and externally [15]. In recent years, the disease became more relevant to rice seed companies, increasing the need of specific diagnostic tools, as well as the development of effective management strategies [16].
Bakanae is a monocyclic disease, characterized by a high production of conidia on sick or dead culms in the field during the phenological stages of flowering and mature grain when the conidia can colonize the seeds [17,18]. Conidia are easily spread by wind and water [19]. Severely infected grains show red coloring due to the presence of mycelium and conidia. The fungus can also be isolated from asymptomatic seeds if they come from a highly affected field. The fungus overwinters in infected seeds or rice straw, where it can survive for at least three years in dry conditions. The pathogen can temporarily survive in soil, but it loses its pathogenicity after 180 days [17]. Low temperatures and relative humidity (30%–35%) influence the infection process and the survival of the pathogen in the soil. High levels of nitrogen in the soil increases fungal growth and host susceptibility [20].
The disease is present in all areas of rice cultivation. Bakanae causes both qualitative and quantitative losses under favorable conditions [17,21,22]. In Italy, an incidence ranging from 5% to 15% has been observed, depending on the season and the cultivar [23]. In 2008, 2009 and 2010, 146 strains of Fusarium spp. were isolated from infected rice plants and seeds from different cultivation areas in Piedmont [18]. Since the species belonging to the GFSC are morphologically indistinguishable, the strains have been identified at the molecular level by amplification and sequencing of the translation elongation factor gene (TEF) [24]. F. fujikuroi represents the most frequent Fusarium species present on rice causing bakanae symptoms [25,26]. Other species belonging to the GFSC, including F. verticillioides and F. proliferatum, are present in low percentages and are mainly isolated from seed [11]. Other species not belonging to the GFSC were isolated from rice, such as F. graminearum [27], F. equiseti [28] and F. chlamydosporum [29]. Of the 146 isolated strains, 121 were tested for pathogenicity and virulence in vivo on a susceptible cultivar, showing that only F. fujikuroi causes the symptoms of bakanae [18].
Different molecular techniques were used to characterize and identify the different populations of GFSC. Primer species-specifics used in PCR assays were obtained from sequences of different origin, including the elongation factor 1α. The multiple alignment of the elongation factor gene sequences (TEF) of different Fusarium spp. showed a deletion of six nucleotides in the F. fujikuroi sequence and a nucleotide polymorphism of two bases in the same region of F. proliferatum. These elements of variability were used to develop conventional and real-time PCR protocols [18]. The specificity of primers was confirmed by DNA analysis of the most representative species of GFSC and 298 strains of Fusarium spp. isolated from Italian rice plants and seeds [18]. Specific primers were used to detect fungal presence directly from infected tissues and rice seeds, providing a tool for early detection of contamination by pathogens. Based on the TEF-1α gene, 35 strains of F. fujikuroi and 8 strains of other species belonging to the genus Fusarium spp. were used to validate a real-time PCR with Taqman probe as a diagnostic method on rice seeds [30]. Specific primers and a Taqman probe were designed for the reaction. The combined action of primers and probe was tested in a quantitative PCR (qPCR), showing the amplifications of DNA fragments repeatedly and reliably from different strains of F. fujikuroi, but not from the other eight Fusarium species. The method was applied in vivo for the diagnosis of infected tissues and for the detection and quantification of the pathogen in batches of naturally contaminated rice seeds of different rice cultivars. Loop-mediated isothermal amplification (LAMP) represents a rapid, specific, sensitive and efficient molecular diagnostic technique that amplifies DNA sequences at a single temperature [31]. A LAMP protocol was validated to detect F. fujikuroi on rice [32]. Six primers, including two external (F3 and B3), two internal (FIP and BIP) and two loop primers (F-loop and B-loop), were designed for tef-1α target gene amplification, and the reaction was performed for 45 min at 65 °C. The fluorescence emitted was recorded during cooling by a portable instrument, GenieII (OptiGene Ltd., Horsham, UK). This method has the potential to be used directly in the field, as a screening tool for seed batches.
The aims of this work were (1) to validate an on-site rapid molecular assay (LAMP) to detect F. fujikuroi on rice seed, (2) to verify the correlation between the incidence of the disease in the field and the incidence of F. fujikuroi detected by the LAMP method on seed, and (3) to determine the minimum threshold of F. fujikuroi cells on seed necessary for the development of the disease in the field.
2. Materials and Methods
2.1. Rice Seed Samples
The 34 rice seed lines (Oryza sativa subsp. japonica) used in this study (Table 1) were produced during the summer of 2019, between March and September, in Piedmont, Lombardy and Sardinia (Italy), and provided by the company SA.PI.SE. (Vercelli, Italy). The 34 lines with different degree of tolerance to the disease—from resistant (cv. Selenio) to susceptible (cv. Dorella)—were tested by the seed company and data are not shown.

Table 1.
Rice seed lines (Oryza sativa subsp. japonica) used in the study.
2.2. Fungal Isolates
Strain I1.3 of F. fujikuroi, isolated from a diseased rice plant collected in Piedmont, Italy, in 2006 and stored in the collection at AGROINNOVA, Turin, Italy, at −80 °C in 20% glycerol was used in the study.
Five isolates of different species of Fusarium were used in the specificity test: F. graminearum, isolated from maize seed in 2019; F. oxysporum f. sp. lactucae, from lettuce in 2002; F. proliferatum, from crown tissues of sorghum in 2020; F. solani from roots of sorghum in 2020; F. verticillioides from crown tissues of maize in 2020.
Conidial suspensions of isolate I1.3 to be used to inoculate single seed were obtained growing it in potato dextrose broth (PDB, Sigma-Aldrich, Darmstadt, Germany) on a rotary shaker (120 rpm) for 7 days at room temperature, after that the mycelium was filtrated and the suspension of conidia was centrifuged at 6000 rpm for 20 min. The supernatant was removed and the pellet was resuspended in 3 mL of sterile Ringer solution. The suspension was used for spore counts on a haemocytometer.
2.3. Seed Health Evaluation
Seed health was evaluated with the blotter method [33]. Four-hundred seeds (40 replicates of 10 seeds) from each batch were placed, without previous disinfection, in 12 × 12 cm plastic boxes over three layers of sterile filter paper soaked with a 0.05% NaClO water solution. The boxes were placed in a growth chamber at 21 ± 2 °C, under a 12 h near-ultraviolet light (NUV), 12 h dark cycle, for 5 days. At the end of the incubation time, the rice seeds were examined for the presence of F. fujikuroi under a stereomicroscope and, when necessary, additional observations were made with the compound microscope. Seed transmission of F. fujikuroi was evaluated in the greenhouse with a growing out assay where ninety seeds of each batch were sown in plastic trays filled with sterile peaty substrate and grown at 21 ± 2 °C and irrigated twice a day. The test lasted 4 weeks, during which inspections were carried out at 7, 14, 21 and 28 days after sowing recording the percentage of seed germination and the appearance of symptoms of F. fujikuroi. An evaluation was carried out in the field in the experiment farm of SA.PI.SE. (Borgo Vercelli, VC) where 1200 seeds of each batch were sown in 4 m2 plots. Percentages of germination and of sick plants were evaluated at the phenological stages of tillering, stem elongation and heading [34].
2.4. Rice Seed DNA Extraction Methods
Two sub-samples of 50 dry seeds from each batch were ground separately in liquid nitrogen with mortar and pestle to obtain a powder. One hundred mg of the powder was transferred to a 2 mL microcentrifuge tube and used for the extraction of the DNA with the Omega E.Z.N.A.® Plant DNA kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturers’ instructions, after a 30-min cycle, at 25 Hz, in Tissuelyser (Qiagen®), that improves the yield of DNA extraction. To quantify the pathogen present on the surface of the seeds, two sub-samples of 50 seeds from each batch were washed twice, dipping the seeds in PEG 4600 (50 g/L) + KOH (20 mM) solution for 40 min. Seeds were then removed, and the solution was centrifuged at 6000 rpm for 20 min. The supernatant was removed, and the pellets resuspended in 2 mL of sterile Ringer solution. The sample was transferred in a 2 mL microcentrifuge tube and centrifuged at the maximum speed (25,000 rpm) for 5 min. At the end, the supernatant was removed, and the pellet was used for the DNA extraction with Omega E.Z.N.A.® Fungal DNA mini kit (Omega Bio-Tek) according to the manufacturers’ instructions, after a 15-min cycle, at 25 Hz, in Tissuelyser (QIAGEN®). The extracted DNA was quantified with the spectrophotometer Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
Two sub-samples of 25 seeds from each line were subjected to a DNA crude extraction where the seeds and 2 mL of a PEG 4600 (50 g/L) + KOH (20 mM), an alkaline buffer (pH 13.5), were put in a 5 mL tube and then vigorously shaken for 3 min [35]. The use of this alkaline buffer allows the testing in molecular assays of a wide variety of biological samples, such as rice seeds and their fungal pathogens, directly without previously neutralization or DNA isolation steps [35].
2.5. Real-Time PCR
Real-time PCR assays were carried out with specific primers TqF2/TqR and probe FfujiPq [29] and GoTaq® Probe qPCR Master Mix mix (Promega, Madison, WI, USA) to quantify F. fujikuroi. All real-time PCR tests were performed, including two biological replications for each seed batch and three technical replications for each biological replication. Each analysis included three technical replications of a positive and a negative control. The real-time PCR amplification was carried out using a StepOne Plus™ Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) thermal cycler, under the following conditions: 1 µL of genomic DNA as template, 5 µL of GoTaq® Probe qPCR Master Mix, 0.5 µL of each primer (5 µM), 0.25 µL of probe (0.25 µM) in a final volume of 10 µL. The reaction was carried out with an initial incubation at 50 °C for 2 min and at 95 °C for 10 min and followed by 40 cycles at 95 °C for 15 sec and at 62 °C for 1 min [30]. The results were displayed through the StepOne software, connected to the thermal cycler. A standard curve was obtained by the correlation of the Ct and the concentrations of ten-fold serial dilutions of the DNA of one strain of F. fujikuroi, ranging from 10 ng/µL to 100 fg/µL.
2.6. LAMP
The LAMP reaction was set up using six primers, two external (F3 and B3), two internal (FIP and BIP) and two loop primers (F-loop and B-loop), designed based on the EF-1α sequence [32]. The assays were carried out on a GenieII instrument (OptiGene Ltd., Horsham, UK) in a final volume of 10 µL containing 1 µL of genomic DNA as template, 0.5 µL of each external primer (10 µM), 0.5 µL of each internal primer (100 µM), 0.25 of each loop primer (100 µM) and 6 µL of Isothermal Mastermix ISO-004 (1×) (OptiGene Ltd.). The amplification protocol was carried out at 65 °C for 45 min, followed by an annealing step where the fluorescence was measured while cooling from 95 °C to 70 °C at 0.05 °C/s. The same protocol can be adopted on a StepOne Plus™ Real-Time PCR System thermal cycler. The protocol was verified by the evaluation of sensitivity, specificity, repeatability and reproducibility according to the EPPO PM7/98 standard (2019) [36].
The limit of analytical sensitivity was obtained with a standard curve by the correlation of time to positive (Tp) and the concentrations of ten-fold serial dilutions of the DNA extracted from mycelium of F. fujikuroi isolate I1.3. The limit was also tested with single rice seeds (cv. Dorella) artificially inoculated with serial dilutions of isolate I1.3 (0.48 and 4.8 ng/mL).
To verify the analytical specificity of the protocol to detect F. fujikuroi, an assay was set up with DNA samples extracted from F. proliferatum and F. verticillioides (GFSC) F. graminearum, F. oxysporum and F. solani.
DNA extracted from rice seed (cv. Dorella) artificially inoculated with a conidial suspension of F. fujikuroi isolate I1.3 (0.48 and 4.8 ng/mL) was used to verify repeatability and reproducibility. The repeatability of the method was tested through the degree of concordance between three independent tests carried out under unchanged analysis and instrument conditions, in the same laboratory and by the same operator, considering the results of biological and technical replication. The reproducibility of the method was tested by different operators on different days.
The validated protocol was adopted to test the 34 rice lines to compare two DNA extraction methods: extraction with the commercial kit Omega E.Z.N.A.® Plant DNA kit and crude extraction.
2.7. Relationship between Seed Contamination and Disease Development
Four samples of 150 seeds of line SPS.FUS.14, selected for the low percentage of contamination found both in the field and because of molecular analyses, were used to determine the minimum concentration of cells of F. fujikuroi necessary to observe the development of the disease in the seedlings. The seeds were artificially inoculated with serial dilutions of a conidial suspension of F. fujikuroi (102, 103, 104 and 105 conidia/mL), while a sample of 150 seeds was used as a healthy control. Sixty seeds of each group were used for the DNA extraction from the whole seed with Omega E.Z.N.A.® Plant DNA kit with a preliminary lysis with liquid nitrogen, while other 60 seeds of each group were used for the DNA extraction from the surface of the seeds with serial washes in PEG 4600 (50 g/L) + KOH (20 mM), followed by using the commercial kit Omega E.Z.N.A.® Fungal DNA mini kit. In addition, 30 seeds of each group were sown in plastic trays filled with sterile soil and incubated in the greenhouse at 21 ± 2 °C. The test lasted 4 weeks, during which inspections were carried out at 7, 14, 21 and 28 days after sowing to record symptoms of F. fujikuroi. Real-time PCR was carried out to quantify the pathogen [30]. The results obtained were used to calculate the number of F. fujikuroi cells per sample, using the parameter of interest “Quantity mean”, which is the average amount of DNA of F. fujikuroi of the three technical replications of the same sample analyzed. From this parameter, we calculated the approximate number of fungal cells in each sample by dividing the DNA quantity by the weight of the genome of the pathogen (0.000048 ng) [37].
2.8. Data Analyses
Baseline range, threshold cycle (CT) values and real-time PCR standard curves were automatically generated using StepOne software, while time to positive (Tp) and temperature of annealing of the LAMP products were visualized using Genie explorer software. Statistical analyses of the data were performed with the Statistical Package for Social Science (SPSS, IBM, Chicago, IL, USA) version 27.0.
3. Results
3.1. Seed Health Evaluation
The incubation tests with blotter method showed a percentage of seeds infected with F. fujikuroi always lower than 2.5%. These results, which are not statistically significant, may be due to a low sensitivity of the detection method or a low incidence of the pathogen in the seeds. The growing out assays carried out in the greenhouse showed a high variability among the different seed lines as percentage of plants with bakanae symptoms. Statistical analyses showed the highest percentage of plants showing symptoms of F. fujikuroi in lines SPS.FUS.01 and SPS.FUS.20, with values of up to 89% (Figure 1). The evaluation of the percentage of plants infected in the field did not show significant differences among the lines, with a percentage of symptomatic plants always lower than 14% (Table 2).
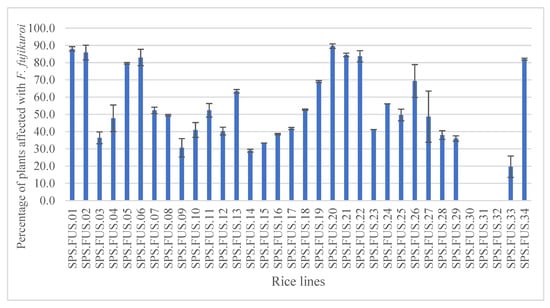
Figure 1.
Percentage of plants grown in the greenhouse, affected with F. fujikuroi. The values are the means ± standard deviations (ANOVA, Tukey test, p ≤ 0.05).

Table 2.
Percentage of seeds and plants infected with F. fujikuroi observed with blotter method (A), in the greenhouse (B) and in the field (C), in the 34 seed lines used in this study. Quantitation (pg/g) of F. fujikuroi in the seeds (D) and on their surface (E) with RealTime PCR with TaqMan probe and amplification with LAMP assay (Tp and T° annealing) of F. fujikuroi extracted by the seeds with commercial kit (F) and with crude extraction (G). * ND = not detected.
3.2. Real-Time PCR
Analytical sensitivity was assessed setting up a standard curve with ten-fold serial dilutions of DNA of F. fujikuroi. The cycle threshold (Ct) was interpolated with the initial concentration of the pathogen in the standard curve and the regression line was obtained with the determination coefficient (R2) value of 0.9992. The minimum Ct recorded corresponds to a concentration of F. fujikuroi of 10 ng/µL while the highest Ct to a concentration of the pathogen was 100 fg/µL. The average reaction efficiency, calculated from the slope of the line, was 97% (Figure 2A,B).
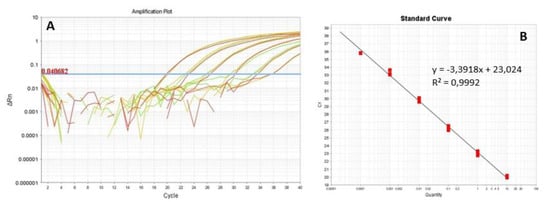
Figure 2.
(A) Amplification with real-time PCR assay of serial dilutions of DNA of F. fujikuroi I1.3. The threshold cycle values (Ct) of the fungal DNA ten-fold serial dilutions are represented in red and green. (B) Regression line obtained. The equation of the line and the coefficient of determination are shown in the graph.
Tests carried out on the rice samples showed the sensitivity of the method in quantifying the DNA of F. fujikuroi both when extracted from the whole seed and from the seed surface only. A high quantity of F. fujikuroi was detected in sample SPS.FUS.20, with an average concentration of 8.66 ng/g in the whole seed (Figure 3) and an average concentration of 3.8 pg/g on the seed surface that did not show statistically significant differences from the other rice lines. Ten out of 34 rice seed lots tested produced a negative result, indicating absence or a low concentration not detectable by the probe of F. fujikuroi (Table 2).
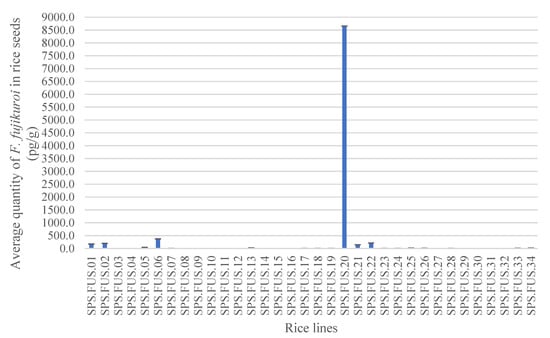
Figure 3.
Average quantity of F. fujikuroi in the whole seed obtained by the detection of 34 rice lines with real-time PCR with TaqMan probe in triplicate. The values are the means ± standard deviations (ANOVA, Tukey test, p ≤ 0.05).
3.3. LAMP
Analytical sensitivity of the LAMP reaction was determined with a standard curve obtained by correlating Tp and ten-fold serial DNA concentrations extracted from fresh mycelium of F. fujikuroi (I1.3). The curve set up by testing seven serial DNA dilutions of I1.3 showed amplification for the first six points (from 10 ng/µL to 100 fg/µL), determining the amount of 100 fg/μL as the sensitivity limit of the reaction (Figure 4). The analytical sensitivity limit was also tested on artificially inoculated single rice seeds (cv. Dorella) with serial dilutions (0.48 and 4.8 ng/mL) of the pathogen F. fujikuroi. The results of the amplifications obtained are shown in Table 3, confirming the sensitivity of the method to detect the pathogen in seeds.
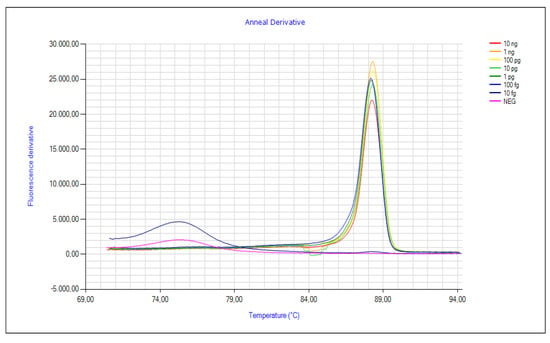
Figure 4.
Analytical sensitivity of the LAMP assay obtained by the amplification of ten-fold serial dilutions of DNA of F. fujikuroi (I1.3) from a concentration of 10 ng/µL to 100 fg/µL.

Table 3.
Results of the analytical sensitivity limit of the LAMP assay for F. fujikuroi on artificially inoculated rice seeds cv. Dorella. The table shows Time to positive (Tp) and average annealing temperature of the three technical replicates tested.
The LAMP assay was set up testing F. graminearum, F. solani and F. oxysporum f. sp. lactucae and three isolates belonging to the GFSC, F. fujikuroi, F. verticillioides and F. proliferatum to evaluate the analytical specificity of the reaction, that was confirmed by the amplification of F. fujikuroi only (Figure 5). The repeatability and reproducibility of LAMP assays for F. fujikuroi were confirmed by producing 100% reliable amplifications among replications, both biological and technical, when tested under unchanged analysis conditions or with different operators.
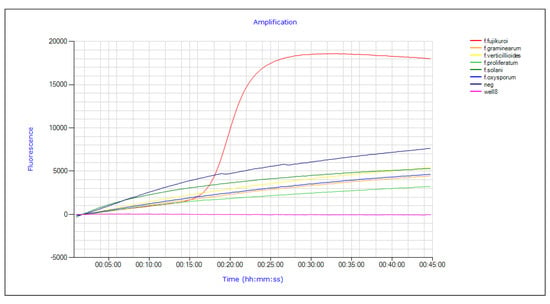

Figure 5.
Validation of the analytical specificity of the LAMP reaction obtained with amplification of F. fujikuroi DNA (I1.3) compared to five other isolates of Fusarium belonging to the GFSC, F. proliferatum and F. verticillioides, and F. graminearum, F. oxysporum and F. solani.
The results of the analyses carried out on the 34 rice lines tested are listed in Table 2. Positive amplifications were obtained with LAMP assays both with DNA extracted with the commercial kit E.Z.N.A. and with DNA obtained with crude extraction. Higher analytical sensitivity in the detection of F. fujikuroi was observed in six seed lines over a total of 34 tested with the use of DNA from crude extraction (Figure 6).
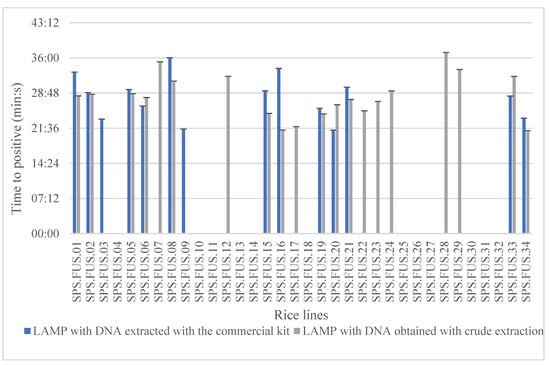
Figure 6.
Results obtained with LAMP in which 34 rice lines were used and the DNA was extracted using a kit extraction method and a crude extraction method in triplicate. The graph shows the time to positive (Tp) obtained using both types of DNA extraction method among the different rice lines. The values are the means ± standard deviations (ANOVA, Tukey test, p ≤ 0.05).
3.4. Relationship between Seed Contamination and Disease Development
The DNA extracted from whole seeds and from seed surface from artificially inoculated rice samples with ten-fold serial cell suspensions of F. fujikuroi was tested with a real-time PCR assay to quantify the pathogen and the results were used to quantify the number of F. fujikuroi cells per sample. From the quantity mean of each sample, the number of cells/µL was calculated. At the same time, artificially inoculated seeds were sown in the greenhouse and the percentage of symptomatic plants for each concentration of inoculum was recorded. Seed lines obtained from seeds inoculated with a conidial suspension of 102 conidia/mL did not show symptoms of the disease, while symptomatic seedlings were observed when seed was inoculated at 103, 104 and 105 conidia/mL. The minimum concentration of the fungus necessary to observe the development of the disease corresponds to a concentration of 4.17 × 104 cells/µL of F. fujikuroi (Table 4).

Table 4.
Number of cells/µL of F. fujikuroi detected in seed samples artificially inoculated with ten-fold serial dilution of the pathogen.
4. Discussion
The aim of the study was to optimize and validate a rapid, sensitive and efficient molecular diagnostic technique to replace the blotter test in seed health procedures, due to its higher sensitivity, specificity, fast turnaround results delivery, on-site application and the possibility of quantifying endophytic seed infections. Such a tool could represent an advantage for seed companies that may detect F. fujikuroi in rice seeds before sowing and decide to adopt seed dressing or other seed treatments.
The evaluation of seed health shows different percentages of infection of each seed lot when detected with different methods as a function of the sensitivity of the method and of the external conditions that can influence the development of the disease such as temperature, humidity and fertilization [20]. Blotter tests did not provide statistically significant results for seed health assessment in this study. Significant results were obtained in the greenhouse growing out tests that were carried out under controlled conditions of temperature and relative humidity. These parameters, like temperatures from 27 °C to 30 °C [18], create favorable conditions for the development of the disease and the appearance of the symptoms on plants affected by F. fujikuroi.
In the field, percentages of symptomatic plants were lower than in the experiments carried out in the greenhouse. These data could be influenced by environmental and crop management conditions. In fact, the rice field where the trial was carried out was often dry, influencing and the expression of symptomatic plants.
Moreover, the different degrees of virulence among different strains of F. fujikuroi present on or in rice seeds and plants in the same field and in the same cultivar can influence the level of infection in the field [18].
When the level of infection on or in whole rice seeds was assessed with real-time PCR it was observed that the endophytic infection was related to greenhouse expression of symptoms, although not statistically significant.
The quantitation of F. fujikuroi with real-time PCR showed different values among the different seed lines, as a function of the level of susceptibility of the cultivar, their origin and the environment and the soil conditions during their production. The survival of the fungus in the grains was strictly correlated to the soil temperature that is optimal at 35 °C [17] and the seeds infection can increase at high temperature during the flowering stage [38]. The disease incidence is also related to the genetic characteristics of the cultivar. Several studies have shown that aromatic cultivars were more sensitive that the non-aromatic ones [39], coarse varieties were more resistant [40] and the susceptibility may change throughout the crop stages [15]. The study highlighted that the microbiological component present on the seed surface did not affect the incidence of the disease, while the endophytic component of F. fujikuroi was relevant. Sunani et al. [41] hypothesized that the fungus can infect seeds and reach the embryo in three ways: by systemic infection of the vascular system, by stylar canal, or through the growth of hyphae from the husk to the ovary. Furthermore, the microconidia of F. verticillioides can represent a source of infection of the embryo [42] and it is possible that the conidia of F. fujikuroi also follow this inoculation mechanism and systemically infect the seeds [41]. This information is important because it supports the choice of the most effective chemical treatment for disease control [5].
A LAMP assay, based on the protocol described by Franco-Ortega et al. [32], was validated verifying the efficiency of the reaction by setting up a curve of standards obtained from ten-fold serial dilutions of DNA of F. fujikuroi. The original protocol was optimized reducing the final volume of the reaction to 10 µL. The method was validated following the EPPO (PM7/98) guidelines to determine the analytical sensitivity, specificity, repeatability and reproducibility. The analytical sensitivity of the LAMP assay was determined by testing ten-fold serial dilutions of F. fujikuroi and corresponds to a concentration of 100 fg/µL. The specificity was confirmed by testing the DNA of Fusarium species belonging or not to the same species complex and highlighting the amplification of only F. fujikuroi. Repeatability and reproducibility were confirmed by replication of LAMP tests under unchanged and modified test conditions. The validated protocol was used to quantify F. fujikuroi in the commercial rice seed lines testing both the DNA extracted with the commercial kit “E.Z.N.A. Plant DNA kit” of Omega Bio-Tek and crude extraction. The use of crude extraction produces more sensitive results in the detection of the pathogen, and it simplifies the diagnostics assays, reducing time and enabling the processing of many samples with lower costs [35].
Results obtained with artificial single seed inoculation with serial DNA dilutions and detection with real-time PCR showed that the minimum amount necessary for the development of the disease in the greenhouse corresponds to a concentration of 4.17 × 104 cells/µL of F. fujikuroi in the seeds. Therefore, this result can be considered as a warning threshold for seed phytosanitary quality assessment. A similar evaluation was made on the quantity of F. fujikuroi detected with real-time PCR carried out with DNA extracted from artificially inoculated single rice seeds by Franco-Ortega et al. [32].
5. Conclusions
This study confirms the on-site applicability of the LAMP method as a fast, effective and sensitive diagnostic technique, such as published previously by Rong et al. [43], who developed a similar technique based on the IGS region. The advantage of this diagnostic tool is represented by the easy application of the protocol by field operators without prior molecular experience or the support of a laboratory structure [44]. In addition, the use of the crude extraction allows obtaining a sensitive screening of the level of infection with F. fujikuroi in rice seed batches. Finally, the evaluation of the minimum concentration of the pathogen necessary for the development of the disease represents a way to select rice batches and exclude those with high levels of contamination, which would lead to consequent yield and economic losses for the seed company.
Author Contributions
Conceptualization, M.S., M.M. and D.S.; methodology, M.S. and M.M.; validation, M.S.; formal analysis, M.S. and M.M.; investigation, M.S. and M.M.; writing—original draft preparation, M.S. and M.M.; review and editing, D.S. and M.L.G.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research project has been carried out thanks to the grant “Bando Talenti della Società Civile” 2019 edition promoted by Fondazione CRT with Fondazione Giovanni Goria and SAPISE Soc. Coop. (Vercelli, Italy).
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Carlo Minoia and Diego Greppi from SAPISE Soc. Coop. (Vercelli, Italy) for their help in providing the rice lines and in performing the field trials.
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation or writing of the study.
References
- FAOSTAT Food & Agriculture Organization of the United Nations Statistic Division. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 17 April 2021).
- ISTAT. Coltivazioni: Cereali, Legumi, Radici Bulbi e Tuberi. Available online: https://www.istat.it/ (accessed on 17 April 2021).
- Blengini, G.A.; Busto, M. The life cycle of rice: LCA of alternative agri-food chain management systems in Vercelli (Italy). J. Environ. Manag. 2009, 90, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kimura, J. Studies on the bakanae disease of the rice plant. Rep. Hokkaido Agric. Exp. Stn. 1931, 27, 1–95. [Google Scholar]
- Gupta, A.K.; Solanki, I.S.; Bashyal, B.M.; Singh, Y.; Srivastava, K. Bakanae of rice—An emerging disease in Asia. J. Anim. Plant Sci. 2015, 25, 1499–1514. [Google Scholar]
- Carter, L.L.A.; Leslie, J.F.; Webster, R.K. Population structure of Fusarium fujikuroi from California rice and water grass. Phytopathology 2008, 98, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldacci, E. The presence in Italy of Fusarium moniliforme on rice and its phytopathological significance. Int. Bull. Pl. Prot. Int. Agr. Rome 1946, 20, 1–2. [Google Scholar]
- CABI. Gibberella Fujikuroi. In Crop Protection Compendium; CAB International: Wallingford, UK, 2021; Available online: www.cabi.org/cpc (accessed on 17 April 2021).
- Hsieh, H.W.; Smith, S.N.; Snyder, W.C. Mating groups in Fusarium moniliforme. Phytopathology 1977, 67, 1041–1043. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.K.; Gunnel, P.S. Compendium of Rice Disease; APS Press: St. Paul, MN, USA, 1992; Volume 86. [Google Scholar]
- Siciliano, I.; Amaral Carneiro, A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Jasmonic acid, abscisic acid and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agr. Food Chem. 2015, 63, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Wulff, E.G.; Sorensen, J.L.; Lubeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef]
- Mew, T.W.; Gonzales, P. A Handbook of Rice Seedborne Fungi; IRRI: Montpellier, France, 2002; pp. 6–34. [Google Scholar]
- Choi, H.W.; Hong, S.K.; Lee, Y.K.; Kim, W.G.; Chun, S. Taxonomy of Fusarium fujikuroi species complex associated with bakanae on rice in Korea. Australas. Plant Pathol. 2018, 47, 23–34. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, P.; Laha, G.S. Present status of bakanae of rice caused by Fusarium fujikuroi Nirenberg. Indian Phytopathol. 2019, 72, 587–597. [Google Scholar] [CrossRef]
- Matic, S.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol. Control. 2014, 73, 59–67. [Google Scholar] [CrossRef]
- Ou, S.H. Rice Diseases, 2nd ed.; Commonwealth Agricultural Bureaux International: Wallingford, UK, 1985; p. 380. [Google Scholar]
- Matic, S.; Gullino, M.L.; Spadaro, D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front. Biosci. 2017, 9, 333–344. [Google Scholar]
- Gupta, A.K.; Singh, Y.; Jain, A.K.; Singh, D. Prevalence and incidence of bakanae disease of rice in northern India. J. Agrisearch. 2014, 1, 233–237. [Google Scholar]
- Singh, R.; Sunder, S. Foot rot and bakanae of rice: An overview. Rev. Plant Pathol. 2012, 5, 565–604. [Google Scholar]
- Iqbal, M.; Javed, N.; Sahi, S.T.; Cheema, N.M. Genetic management of bakanae disease of rice and evaluation of various fungicides against Fusarium moniliforme in vitro. Pakistan J. Phytopathol. 2011, 23, 103–107. [Google Scholar]
- Fiyaz, R.A.; Krishnan, S.G.; Rajashekara, H.; Yadav, A.K.; Bashyal, B.M.; Bhowmick, P.K.; Singh, N.K.; Prabhu, K.V.; Singh, A.K. Development of high throughput screening protocol and identification of novel sources of resistance against bakanae disease in rice (Oryza sativa L.). Indian Soc. Genet. Plant Breed. 2014, 74, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, S. Fusarium spp., Analisi e Metodi di Controllo. Available online: http://www.enterisi.it (accessed on 17 April 2021).
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Nur Ain Izzati, M.Z.; Salleh, B. Variability of Fusarium species associated with bakanae disease of rice based on virulence, vegetative and biological compatibility. Sydowia 2010, 62, 89–104. [Google Scholar]
- Bashyal, B.M. Etiology of an emerging disease: Bakanae of rice. Indian Phytopathol. 2018, 71, 485–494. [Google Scholar] [CrossRef]
- Gomes, L.B.; Ward, T.J.; Badiale-Furlong, E.; Del Ponte, E.M. Species composition, toxigenic potential, and pathogenicity of Fusarium graminearum species complex isolates from southern Brazilian rice. Plant Pathol. 2015, 69, 980–987. [Google Scholar] [CrossRef]
- Avila, C.F.; Moreira, G.M.; Nicolli, C.P.; Gomes, L.B.; Abreu, L.M.; Pfenning, L.H.; Haidukowski, M.; Moretti, A.; Logrieco, A.; Del Ponte, E.M. Fusarium incarnatum-equiseti species complex associated with Brazilian rice: Phylogeny, morphology, and toxigenic potential. Int. J. Food Microbiol. 2019, 306, 108267. [Google Scholar] [CrossRef]
- Islam, N.F.; Borthakur, S.K. Screening of mycota associated with Aijung rice seed and their effects on seed germination and seedling vigour. Plant Pathol. Quar. 2012, 2, 75–85. [Google Scholar] [CrossRef]
- Amaral Carneiro, G.; Matic, S.; Ortu, G.; Garibaldi, A.; Spadaro, D.; Gullino, M.L. Development and validation of a TaqMan Real-Time PCR assay for the specific detection and quantification of Fusarium fujikuroi in rice plants and seeds. Phytopathology 2017, 107, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco Ortega, S.; Tomlinson, J.; Hodgetts, J.; Spadaro, D.; Gullino, M.L.; Boonham, N. Development of Loop-Mediated Isothermal Amplification assays for the detection of seedborne fungal pathogens Fusarium fujikuroi and Magnaporthe oryzae in rice seed. Plant Dis. 2018, 102, 1549–1558. [Google Scholar] [CrossRef] [Green Version]
- ISTA. Seed Health Method 2020; International Seed Testing Association: Bassersdorf, Switzerland, 2020; Available online: https://www.seedtest.org/en/seed-health-methods-_content---1--1452.html (accessed on 17 April 2021).
- IRRI. Standard Evaluation System for Rice (SES); International Rice Research Institute: Los Baños, Philippines, 2002; p. 56. [Google Scholar]
- Chomczynski, P.; Rymaszewski, M. Alkaline polyethilene glycol based method for direct PCR from bacteria, eukaryotic tissue samples, and whole blood. Biotechniques 2006, 40, 454–458. [Google Scholar] [CrossRef] [PubMed]
- European and Mediterranean Plant Protection Organization (EPPO). PM 7/98 (4) Specific requirements for laboratories preparing accreditation for a plant pest diagnostic activity. OEPP/EPPO Bull. 2019, 49, 530–563. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Lee, S.; Choi, J.; Lee, T.; Yun, S. Draft genome sequence of Fusarium fujikuroi B14, the causal agent of the bakanae disease of rice. Genome Announc. 2013, 1, e00035-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, S. Climatic effect on seed infection of rice plant with bakanae disease and disinfection with organic mercury compounds. Proc. Kansai Plant Prot. Soc. 1972, 14, 14–19. [Google Scholar]
- Singh, R.; Sunder, S.; Kumar, P.; Ram, M.; Sekhar, C. Study of bakanae disease of rice in Haryana. Plant Dis. Res. 2018, 3, 15–22. [Google Scholar]
- Ghazanfar, M.U.; Javed, N.; Wakil, W.; Iqbal, M. Screening of some fine and coarse rice varieties against bakanae disease. J. Agric. Res. 2013, 51, 41–49. [Google Scholar]
- Sunani, S.K.; Bashyal, B.M.; Kharayat, B.S.; Prakash, G.; Krishnan, S.G.; Aggarwal, R. Identification of rice seed infection routes of Fusarium fujikuroi inciting bakanae disease of rice. J. Plant Pathol. 2020, 102, 113–121. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A. Fusarium species of the Liseola section associated with stalk and ear rot of maize in southern Italy, and their ability to produce moniliformin. Trans. Br. Mycol. Soc. 1988, 90, 215–219. [Google Scholar] [CrossRef]
- Rong, Z.; Yuan, Y.; Ye, W.; Wang, X.; Zheng, X. Rapid diagnosis of rice bakanae caused by Fusarium fujikuroi and F. proliferatum using loop-mediated isothermal amplification assays. J. Phytopathol. 2018, 166, 283–290. [Google Scholar] [CrossRef]
- Lee, P.L. DNA amplification in the field: Move over PCR, here comes LAMP. Mol. Ecol. Resour. 2017, 17, 138–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).