Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana
Abstract
1. Introduction
2. Materials and Methods
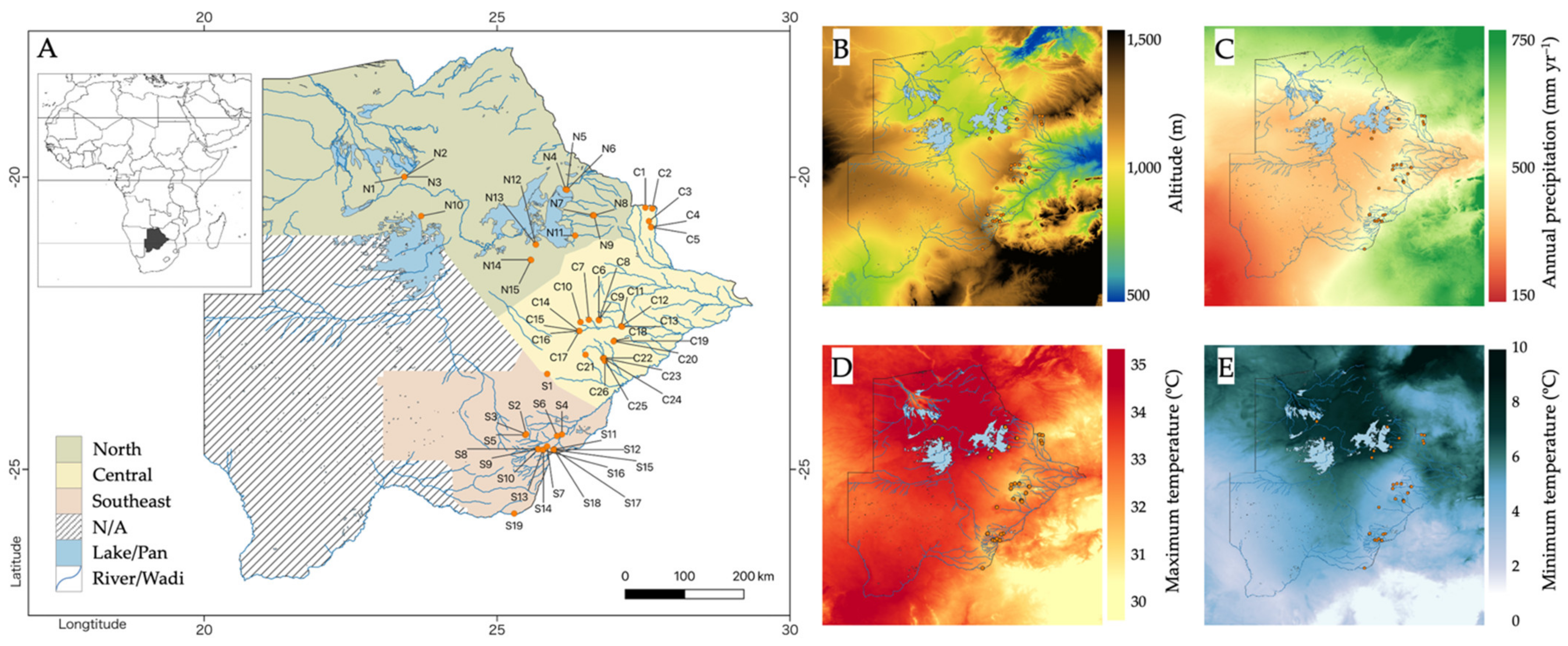
2.1. Climate and Geography of Botswanan Jatropha Collection Sites
2.2. Plant Materials
2.3. Lipid Extraction
2.4. Fatty Acid Analysis
2.5. Phorbol Ester Extraction
2.6. PE Analysis
2.7. Meta-Analysis of Jatropha Seed Chemical Composition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Geographical and Environmental Features of the Botswanan Jatropha Accession Collection Sites
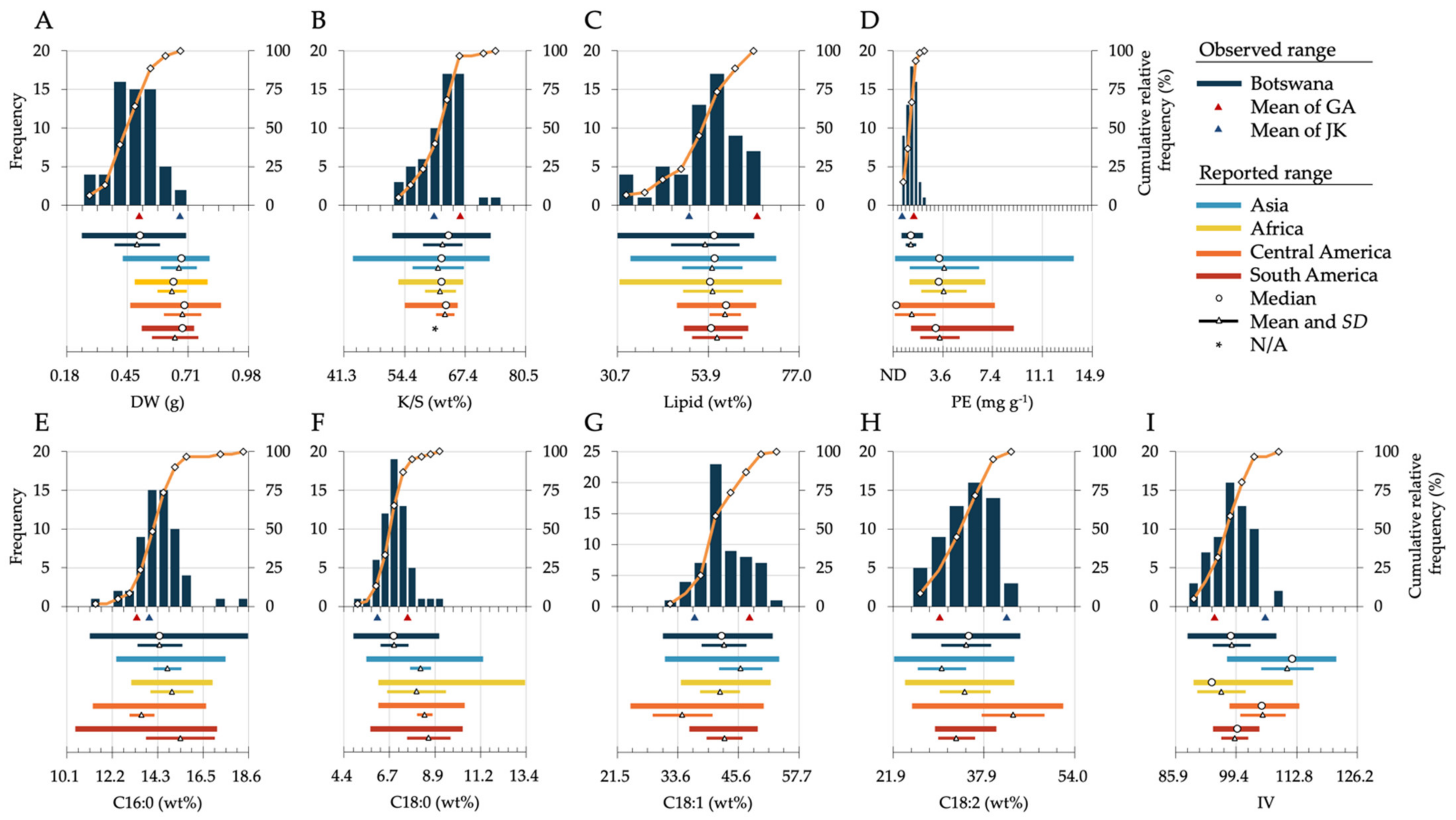
3.2. Frequency Distribution of Seed Chemical Traits among Botswanan Accessions
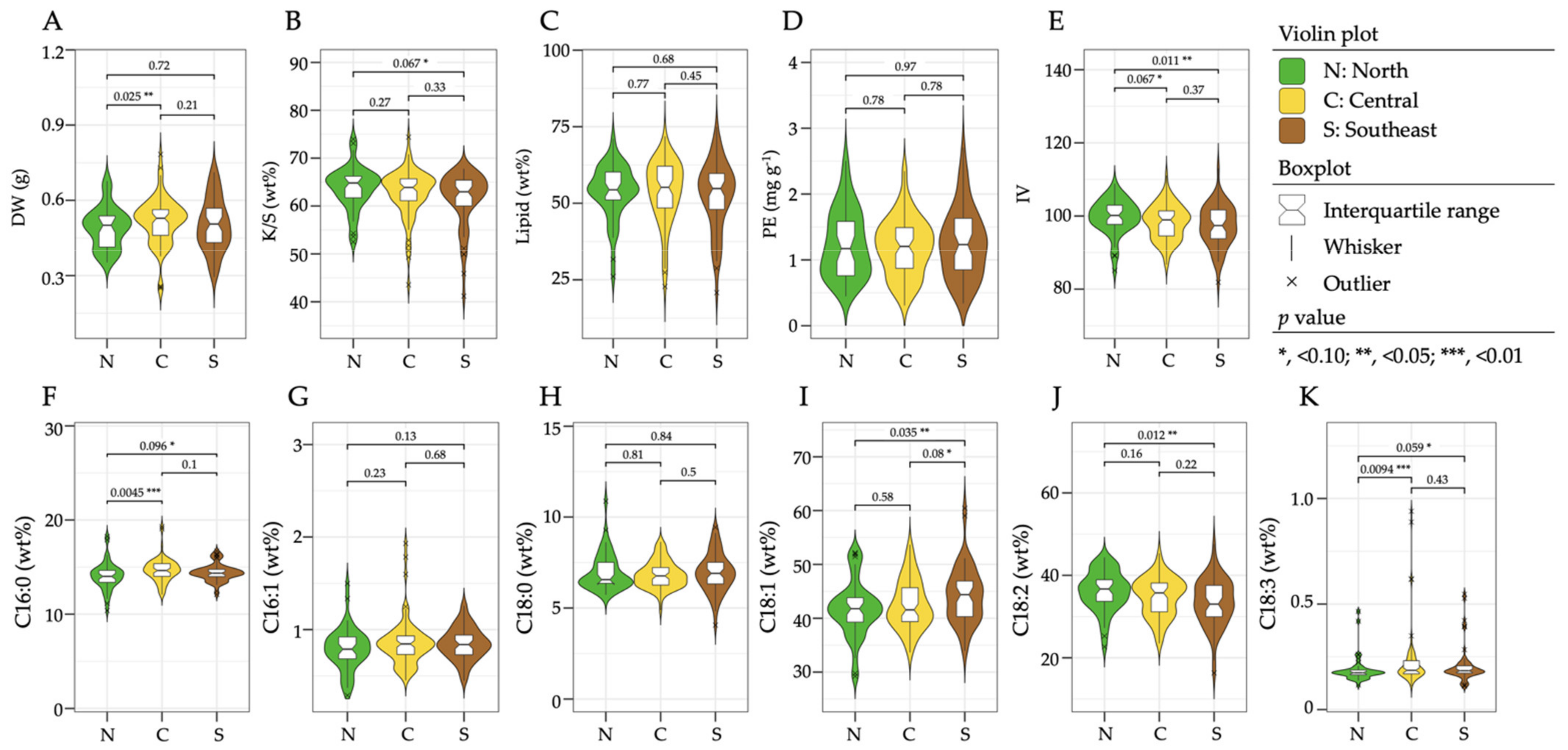
3.3. Regional Differences in the Seed Chemical Compositions of Botswanan Accessions
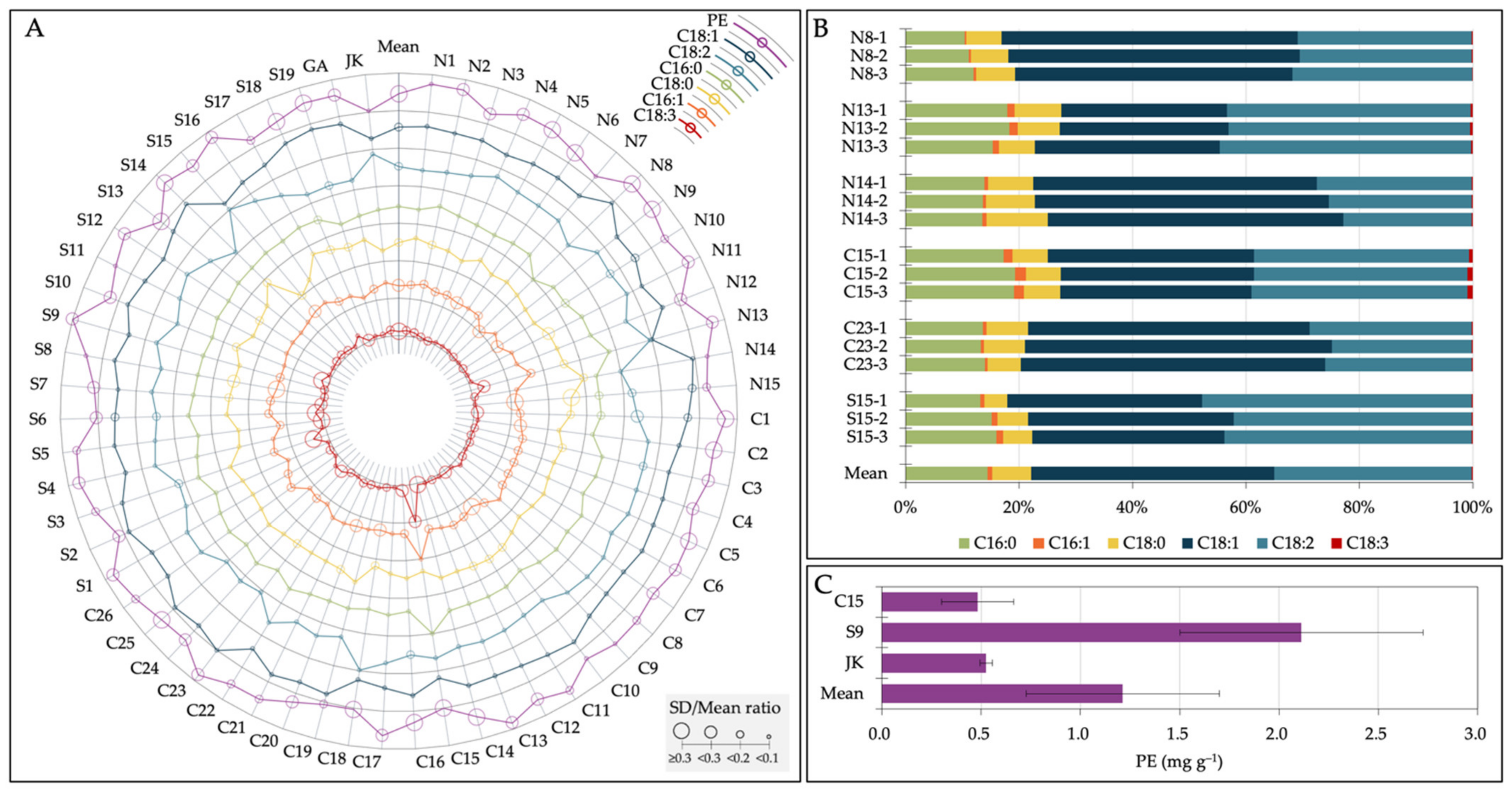
3.4. Accessions with Unique Seed Chemical Compositions
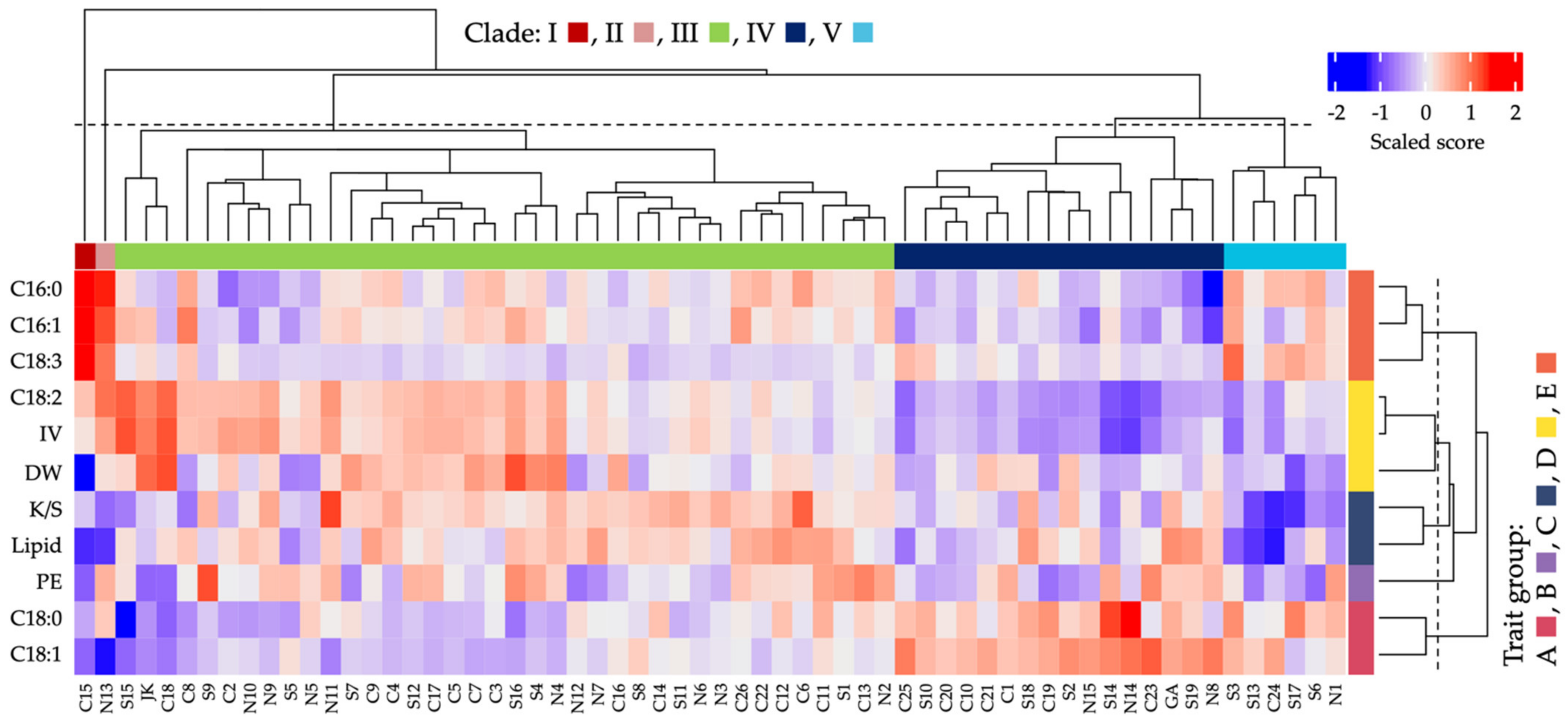
3.5. Categorization of Botswanan Accessions Based on Their Seed Chemical Traits
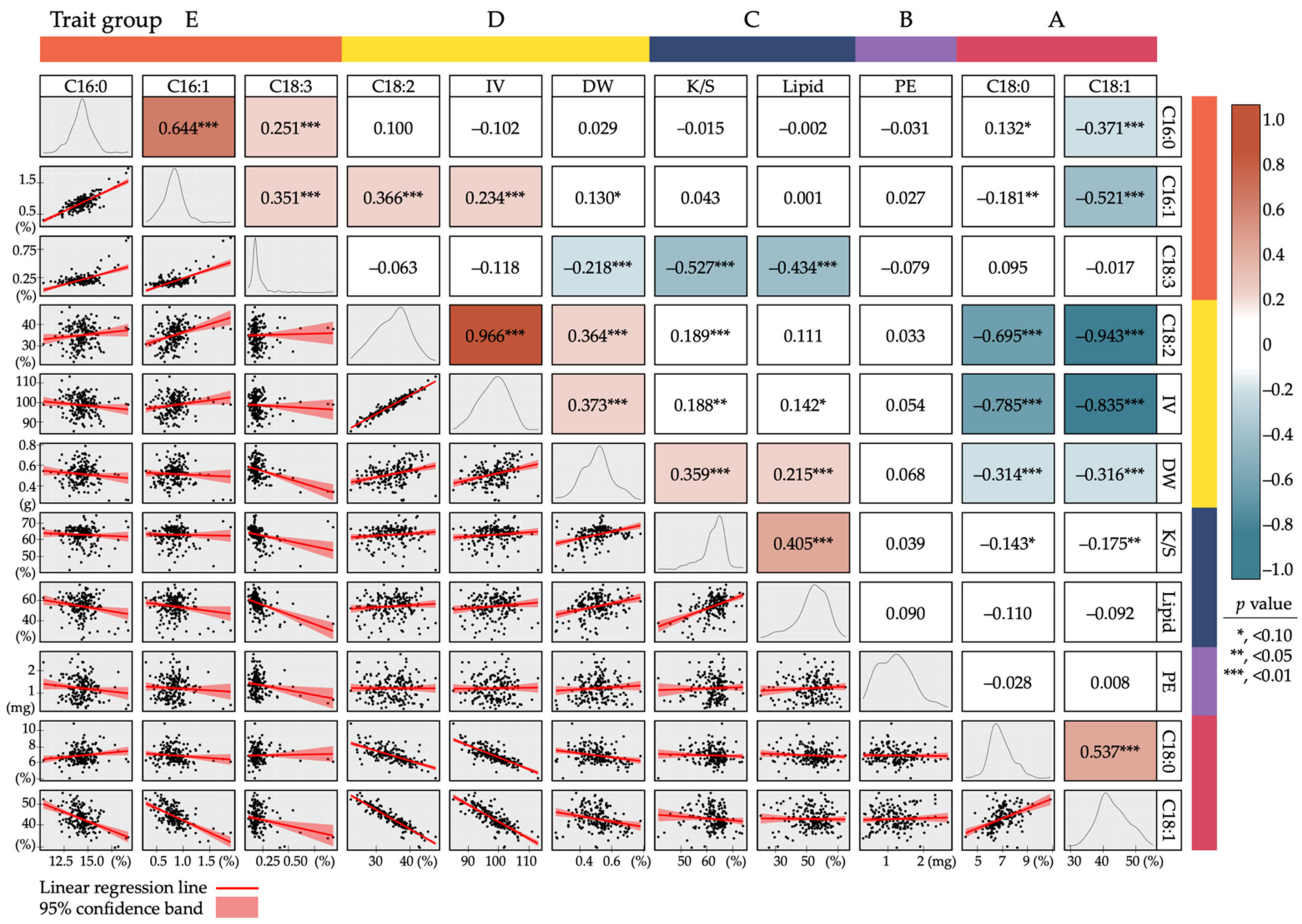
3.6. Correlations between Seed Chemical Traits
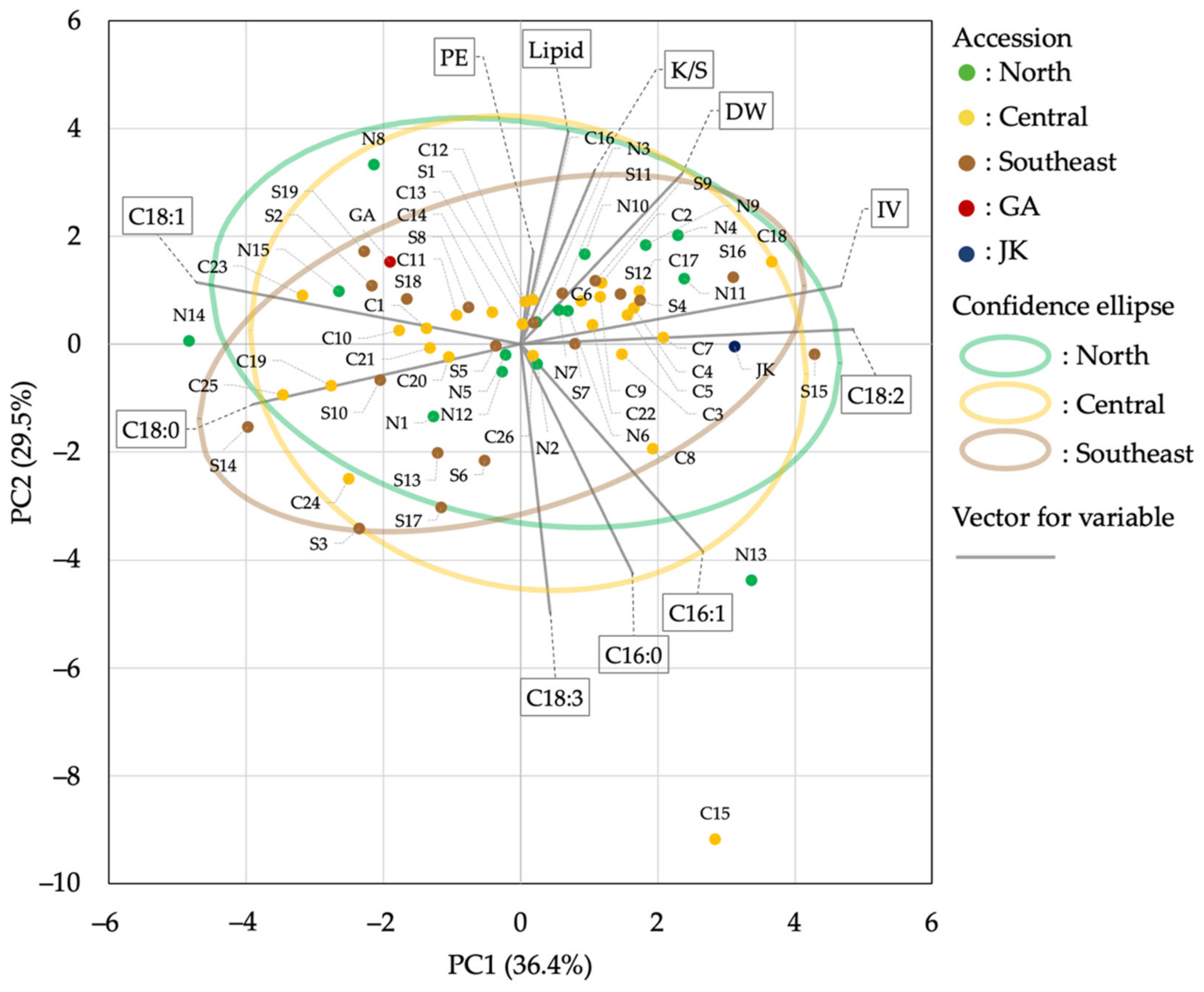
3.7. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achten, W.M.J.; Verchot, L.; Franken, Y.J.; Mathijs, E.; Singh, V.P.; Aerts, R.; Muys, B. Jatropha bio-diesel production and use. Biomass Bioenergy 2008, 32, 1063–1084. [Google Scholar] [CrossRef]
- Montes, J.M.; Melchinger, A.E. Domestication and breeding of Jatropha curcas L. Trends Plant Sci. 2016, 21, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Kheira, A.A.A.; Atta, N.M.M. Response of Jatropha curcas L. to water deficits: Yield, water use efficiency, and oilseed characteristics. Biomass Bioenergy 2009, 33, 1343–1350. [Google Scholar] [CrossRef]
- Gasparatos, A.; von Maltitz, G.P.; Johnson, F.X.; Lee, L.; Mathai, M.; de Oliveira, J.A.P.; Willis, K.J. Biofuels in Sub-Sahara Africa: Drivers, impacts and priority policy areas. Renew. Sustain. Energy Rev. 2015, 45, 879–901. [Google Scholar] [CrossRef]
- Quinn, L.D.; Straker, K.C.; Guo, J.; Kim, S.; Thapa, S.; Kling, G.; Lee, D.K.; Voigt, T.B. Stress-tolerant feedstocks for sustainable bioenergy production on marginal land. BioEnergy Res. 2015, 8, 1081–1100. [Google Scholar] [CrossRef]
- Gonzáles, N.F.C. International experiences with the cultivation of Jatropha curcas for biodiesel production. Energy 2016, 112, 1245–1258. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha toxicity—A review. J. Toxicol. Environ. Health Part B 2010, 13, 476–507. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef]
- Martin, M.; Bohlinger, B.; Senger, E.; Dongmeza, E.; Andrianirina, Z.T.; Montes, J.M. Genetic improvement of edible and non-edible Jatropha for marginal environments in Sub-Saharan Africa. In Jatropha, Challenges for a New Energy Crop: Volume 3: A Sustainable Multipurpose Crop; Mulpuri, S., Carels, N., Bahadur, B., Eds.; Springer: Singapore, 2019; pp. 3–27. [Google Scholar] [CrossRef]
- Environment, Computer, and Geophysical Application. The Feasibility Study for the Production and Use of Biofuels in Botswana; Ministry of Minerals, Energy and Water Affairs: Gaborone, Botswana, 2007. [Google Scholar]
- Kgathi, D.L.; Mmopelwa, G.; Chanda, R.; Kashe, K.; Murray-Hudson, M. A review of the sustainability of Jatropha cultivation projects for biodiesel production in Southern Africa: Implications for energy policy in Botswana. Agric. Ecosyst. Environ. 2017, 246, 314–324. [Google Scholar] [CrossRef]
- Inafuku-Teramoto, S.; Mazereku, C.; Coetzee, T.; Gwafila, C.; Lekgari, L.A.; Ketumile, D.; Fukuzawa, Y.; Yabuta, S.; Masukujane, M.; George, D.G.M.; et al. Production approaches to establish effective cultivation methods for Jatropha (Jatropha curcas L.) under cold and Semi-arid climate conditions. Int. J. Agron. Plant Prod. 2013, 4, 3804–3815. [Google Scholar]
- Ishimoto, Y.; Kgokong, S.; Yabuta, S.; Tominaga, J.; Coetzee, T.; Konaka, T.; Mazereku, C.; Kawamitsu, Y.; Akashi, K. Flowering pattern of biodiesel plant Jatropha in frost- and drought-prone regions of Botswana. Int. J. Green Energy 2017, 14, 908–915. [Google Scholar] [CrossRef]
- Masukujane, M.; Coetzee, T.; Ngwanathebe, R.B.; Ishimoto, Y.; Akashi, K. Diversity and seasonal variation of insect pests of Jatropha in Gaborone, Botswana. Int. J. Trop. Insect Sci. 2018, 38, 294–298. [Google Scholar] [CrossRef]
- Moseki, O.; Murray-Hudson, M.; Kashe, K. Crop water and irrigation requirements of Jatropha curcas L. in Semi-arid conditions of Botswana: Applying the CROPWAT model. Agric. Water Manag. 2019, 225, 105754. [Google Scholar] [CrossRef]
- Tominaga, J.; Inafuku, S.; Coetzee, T.; Kawamitsu, Y. Diurnal regulation of photosynthesis in Jatropha curcas under drought during summer in a Semi-arid region. Biomass Bioenergy 2014, 67, 279–287. [Google Scholar] [CrossRef]
- Vurayai, R.; Nkoane, B.; Moseki, B.; Chaturvedi, P. Phytoremediation potential of Jatropha curcas and Pennisetum clandestinum grew in polluted soil with and without coal fly ash: A case of BCL Cu/Ni mine, Selibe-Phikwe, Botswana. J. Biodivers. Environ. Sci. 2017, 10, 193–206. [Google Scholar]
- Konaka, T.; Ishimoto, Y.; Yamada, M.; Moseki, B.; Tsujimoto, H.; Mazereku, C.; Akashi, K. Tolerance evaluation of Jatropha curcas and Acacia burkei to acidic and copper/nickel-contaminated soil. J. Lucknow 2019, 40, 1109–1114. [Google Scholar] [CrossRef]
- Ogura, T.; Date, Y.; Masukujane, M.; Coetzee, T.; Akashi, K.; Kikuchi, J. Improvement of physical, chemical, and biological properties of Aridisol from Botswana by the incorporation of torrefied biomass. Sci. Rep. 2016, 6, 28011. [Google Scholar] [CrossRef]
- Kethobile, E.; Ketlogetswe, C.; Gandure, J. Characterisation of the non-oil Jatropha biomass material for use as a source of solid fuel. Biomass Convers. Biorefinery 2020, 10, 1251–1267. [Google Scholar] [CrossRef]
- Jonas, M.; Ketlogetswe, C.; Gandure, J. Variation of Jatropha curcas seed oil content and fatty acid composition with fruit maturity stage. Heliyon 2020, 6, e03285. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Yabuta, S.; Kgokong, S.; Motsepe, M.; Tominaga, J.; Teramoto, S.; Konaka, T.; Mmopelwa, G.; Kawamitsu, Y.; Akashi, K.; et al. Environmental evaluation with greenhouse gas emissions and absorption based on life cycle assessment for a Jatropha cultivation system in frost- and drought-prone regions of Botswana. Biomass Bioenergy 2018, 110, 33–40. [Google Scholar] [CrossRef]
- Kashe, K.; Kgathi, D.L.; Murray-Hudson, M.; Mfundisi, K.B. Assessment of benefits and risks of growing Jatropha (Jatropha curcas) as a biofuel crop in sub-Saharan Africa: A contribution to agronomic and socio-economic policies. J. For. Res. 2018, 29, 1–12. [Google Scholar] [CrossRef]
- Gwafila, C.; Batlang, U.; Ngwako, S. Morphological and molecular characterization of Jatropha curcas L. germplasm in Botswana. Afr. J. Biotechnol. 2019, 18, 726–734. [Google Scholar] [CrossRef]
- GADM Home Page, Version 3.6. Available online: https://gadm.org (accessed on 1 May 2021).
- DIVA-GIS Home Page, Version 7.5. Available online: https://www.diva-gis.org (accessed on 1 May 2021).
- Danko, D.M. The digital chart of the world project. Photogramm. Eng. Remote Sens. 1992, 58, 1125–1128. [Google Scholar]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The shuttle radar topography mission. Rev. Geophys. 2007, 45, 25–36. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- WorldClim Home Page, Version 2.1. Available online: https://www.worldclim.org (accessed on 1 May 2021).
- QGIS Home Page, Version 3.12. Available online: https://qgis.org (accessed on 1 May 2021).
- Osorio, L.R.M.; Salvador, A.F.T.; Jongschaap, R.E.E.; Perez, C.A.A.; Sandoval, J.E.B.; Trindade, L.M.; Visser, R.G.F.; Van Loo, E.N. High level of molecular and phenotypic biodiversity in jatropha curcas from central America compared to Africa, Asia, and South America. BMC Plant Biol. 2014, 14, 77. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. Recommended practice Cd 1c-85. In Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Champaign, IL, USA, 1998. [Google Scholar]
- He, W.; King, A.J.; Khan, M.A.; Cuevas, J.A.; Ramiaramanana, D.; Graham, I.A. Analysis of seed phorbol-ester and curcumin content together with genetic diversity in multiple provenances of jatropha curcas L. from Madagascar and Mexico. Plant Physiol. Biochem. 2011, 49, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Baldini, M.; Ferfuia, C.; Bortolomeazzi, R.; Verardo, G.; Pascali, J.; Piasentier, E.; Franceschi, L. Determination of phorbol esters in seeds and leaves of Jatropha curcas and animal tissue by high-performance liquid chromatography-tandem mass spectrometry. Ind. Crop. Prod. 2014, 59, 268–276. [Google Scholar] [CrossRef]
- Faria-Machado, A.F.; Licurgo, F.M.S.; Pires, J.M.F.; Campos, R.d.S.; Wilhelm, A.E.; De Souza, M.d.L.M.; Antoniassi, R. Method validation for analysis of phorbol esters from jatropha curcas. Ind. Crop. Prod. 2019, 140, 111627. [Google Scholar] [CrossRef]
- Vogg, G.; Achatz, S.; Kettrup, A.; Sandermann, H. Fast, sensitive and selective liquid chromatographic-tandem mass spectrometric determination of tumor-promoting diterpene esters. J. Chromatogr. A 1999, 855, 563–573. [Google Scholar] [CrossRef]
- Google Scholar Home Page. Available online: https://scholar.google.com (accessed on 1 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Freedman, D.; Diaconis, P. On the histogram as a density estimator: L2 theory. Z. Für Wahrscheinlichkeitstheorie Verwandte Geb. 1981, 57, 453–476. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Version 3.3.2; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofmann, H.; et al. GGally: Extension to Ggplot2, Version 1.5.0. Available online: https://github.com/ggobi/ggally (accessed on 1 May 2021).
- Horn, J.L. A rationale and test for the number of factors in factor analysis. Psychometrika 1965, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hayton, J.C.; Allen, D.G.; Scarpello, V. Factor retention decisions in exploratory factor analysis: A tutorial on parallel analysis. Organ. Res. Methods 2004, 7, 191–205. [Google Scholar] [CrossRef]
- Revelle, W. psych: Procedures for Personality and Psychological Research, Version 2.1.3. Available online: https://CRAN.R-project.org/package=psych (accessed on 1 May 2021).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, Third Edition. Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 1 May 2021).
- Brittaine, R.; Lutaladio, N. Jatropha: A Smallholder Bioenergy Crop: The Potential for Pro-Poor Development; FAO: Rome, Italy, 2010; Volume 8, ISBN 978-92-5-106438-2. [Google Scholar]
- Garg, K.K.; Karlberg, L.; Wani, S.P.; Berndes, G. Jatropha production on wastelands in India: Opportunities and trade-offs for soil and water management at the watershed scale. Biofuels Bioprod. Biorefining 2011, 5, 410–430. [Google Scholar] [CrossRef]
- Maes, W.H.; Trabucco, A.; Achten, W.M.J.; Muys, B. Climatic growing conditions of Jatropha curcas L. Biomass Bioenergy 2009, 33, 1481–1485. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K.; Sporer, F.; Wink, M. Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J. Agric. Food Chem. 1997, 45, 3152–3157. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Aderibigbe, A.O.; Becker, K. Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability, and toxic factors. Food Chem. 1998, 62, 207–215. [Google Scholar] [CrossRef]
- Basha, S.D.; Francis, G.; Makkar, H.P.S.; Becker, K.; Sujatha, M. A comparative study of biochemical traits and molecular markers for assessment of genetic relationships between Jatropha curcas L. germplasm from different countries. Plant Sci. 2009, 176, 812–823. [Google Scholar] [CrossRef]
- Herrera, J.M.; Ayala, A.L.M.; Makkar, H.; Francis, G.; Becker, K. Agroclimatic conditions, chemical and nutritional characterization of different provenances of Jatropha curcas L. from Mexico. Eur. J. Sci. Res. 2010, 39, 396–407. [Google Scholar]
- Mulpuri, S.; Muddanuru, T.; Francis, G. Start codon targeted (SCoT) polymorphism in toxic and non-toxic accessions of Jatropha curcas L. and development of a codominant SCAR marker. Plant Sci. Int. J. Exp. Plant Biol. 2013, 207, 117–127. [Google Scholar] [CrossRef]
- Marínez, A.M.; Brito, R.F.; Ayala, A.M.; Herrera, J.M.; Vargas, G.P.; Dávila, J.G. Chemical and physical characterizaton of Jatropha curcas L. seed from the Northern Sierra of Puebla, México. J. Plant Sci. 2018, 6, 25. [Google Scholar] [CrossRef]
- EN 14214:2008. Automotive Fuels. Fatty Acid Methyl Esters (FAME) for Diesel Engines. Requirements and Test Methods; European Standards: Pilsen, Czech Republic, 2019. [Google Scholar]
- Wilson, R.F. Seed composition. In Soybeans: Improvement, Production, and Uses; Shibles, R.M., Harper, J.E., Wilson, R.F., Shoemaker, R.C., Eds.; Wiley: Hoboken, NJ, USA, 2004; pp. 621–677. [Google Scholar] [CrossRef]
- Tsakraklides, V.; Kamineni, A.; Consiglio, A.L.; MacEwen, K.; Friedlander, J.; Blitzblau, H.G.; Hamilton, M.A.; Crabtree, D.V.; Su, A.; Afshar, J.; et al. High-oleate yeast oil without polyunsaturated fatty acids. Biotechnol. Biofuels 2018, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Diédhiou, I.; Sambou, Y.S.; Sagna, M.D.; Diédhiou, P.M. Genetic parameters for fruits, seeds and oil content traits of Jatropha curcas L. in a Semi-arid region of Senegal. J. Exp. Agric. Int. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Islam, A.K.M.A.; Sultana, N.; Chakraborty, P. Genetic diversity of Jatropha curcas L. genotypes: A potential biofuel crop in Bangladesh. Biofuels 2019. [Google Scholar] [CrossRef]
- McVetty, P.B.E.; Scarth, R. Breeding for improved oil quality in brassica oilseed species. J. Crop Prod. 2002, 5, 345–369. [Google Scholar] [CrossRef]
- Montes, J.M.; Technow, F.; Bohlinger, B.; Becker, K. Seed quality diversity, trait associations and grouping of accessions in Jatropha curcas L. Ind. Crops Prod. 2013, 51, 178–185. [Google Scholar] [CrossRef]
- Yeilaghi, H.; Arzani, A.; Ghaderian, M.; Fotovat, R.; Feizi, M.; Pourdad, S.S. Effect of salinity on seed oil content and fatty acid composition of safflower (Carthamus tinctorius L.) genotypes. Food Chem. 2012, 130, 618–625. [Google Scholar] [CrossRef]
- Browse, J.; Somerville, C. Glycerolipid synthesis: Biochemistry and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 467–506. [Google Scholar] [CrossRef]
- Flagella, Z.; Giuliani, M.M.; Rotunno, T.; Di Caterina, R.; De Caro, A. Effect of saline water on oil yield and quality of a high oleic Sunflower (Helianthus annuus L.) hybrid. Eur. J. Agron. 2004, 21, 267–272. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Li, W.; Lu, D.; Chen, F.; Li, J. Quantitative determination of phorbol ester derivatives in Chinese Jatropha curcas seeds by high-performance liquid chromatography/mass spectrometry. Ind. Crop. Prod. 2013, 47, 29–32. [Google Scholar] [CrossRef]
- King, A.J.; Montes, L.R.; Clarke, J.G.; Affleck, J.; Li, Y.; Witsenboer, H.; Van Der Vossen, E.; Linde, P.; Van Der Tripathi, Y.; Tavares, E.; et al. Linkage mapping in the oilseed crop Jatropha curcas L. Reveals a locus controlling the biosynthesis of phorbol esters which cause seed toxicity. Plant Biotechnol. J. 2013, 11, 986–996. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Brown, G.D.; Gilday, A.D.; Larson, T.R.; Graham, I.A. Production of bioactive diterpenoids in the Euphorbiaceae depends on evolutionarily conserved gene clusters. Plant Cell 2014, 26, 3286–3298. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Ohtani, M.; Polsri, W.; Usami, T.; Sambongi, K.; Demura, T. Characterization of the carbine synthase homolog from Jatropha (Jatropha Curcas L.). Plant Biotechnol. 2012, 29, 185–189. [Google Scholar] [CrossRef]
- Hirakawa, H.; Tsuchimoto, S.; Sakai, H.; Nakayama, S.; Fujishiro, T.; Kishida, Y.; Kohara, M.; Watanabe, A.; Yamada, M.; Aizu, T.; et al. Upgraded genomic information of Jatropha Curcas L. Plant Biotechnol. 2012, 29, 123–130. [Google Scholar] [CrossRef]
- Sato, S.; Hirakawa, H.; Isobe, S.; Fukai, E.; Watanabe, A.; Kato, M.; Kawashima, K.; Minami, C.; Muraki, A.; Nakazaki, N.; et al. Sequence analysis of the genome of an oil-bearing tree, Jatropha curcas L. DNA Res. 2011, 18, 65–76. [Google Scholar] [CrossRef]
- De Almeida, N.P.; Neto, D.F.M.; Carneiro, G.R.A.; de Farias, A.R.B.; Domont, G.B.; de Assis de Paiva Campos, F.; Nogueira, F.C.S. Monitoring carbene synthase in Jatropha curcas tissues using targeted proteomics. Plant Methods 2021, 17, 15. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadano, S.; Chiyapo, G.; Ishimoto, Y.; Konaka, T.; Mazereku, C.; Tsujimoto, H.; Akashi, K. Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana. Agronomy 2021, 11, 1570. https://doi.org/10.3390/agronomy11081570
Tadano S, Chiyapo G, Ishimoto Y, Konaka T, Mazereku C, Tsujimoto H, Akashi K. Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana. Agronomy. 2021; 11(8):1570. https://doi.org/10.3390/agronomy11081570
Chicago/Turabian StyleTadano, Shota, Gwafila Chiyapo, Yudai Ishimoto, Takafumi Konaka, Charles Mazereku, Hisashi Tsujimoto, and Kinya Akashi. 2021. "Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana" Agronomy 11, no. 8: 1570. https://doi.org/10.3390/agronomy11081570
APA StyleTadano, S., Chiyapo, G., Ishimoto, Y., Konaka, T., Mazereku, C., Tsujimoto, H., & Akashi, K. (2021). Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana. Agronomy, 11(8), 1570. https://doi.org/10.3390/agronomy11081570




