1. Introduction
The sugarcane (
Saccharum officinarum) industry is a major economic driver in Florida, with a total value of over
$4.5 billion annually [
1]. With approximately 1600 km
2 in South Florida, sugarcane is one of the major agricultural commodities produced in this region [
2]. Mineral soils used for sugarcane production in South Florida are low in soil organic matter (OM) (10–40 g kg
−1) and clay contents (0–30 g kg
−1), thus resulting in low water and nutrient holding capacities [
3]. These mineral soils account for approximately 28% of the total sugarcane acreage in South Florida [
4]. Recently, the proportion of sugarcane grown on mineral soils has increased and there is a need for sugarcane expansion on these soils if production is to be increased. Typically, sugarcane growers relied on inorganic fertilizers to improve yield, which ultimately increases production costs and the risk of water pollution [
5]. Therefore, sugarcane producers and environmental researchers in Florida and elsewhere are interested in examining innovative nutrient management practices involving organic sources to reduce fertilizer costs and environmental risks.
Organic materials such as crop residues and by-products from industries are a valuable source of OM and plant nutrients, which bring many benefits when they are applied to mineral soils, such as decreasing soil bulk density (BD), increasing water holding capacity (WHC) and enhancing soil structure, increasing cation exchange capacity (CEC) and nutrient cycling, increasing macronutrients and micronutrients in soils, and controlling erosion [
5,
6,
7]. The improved soil physical, chemical, and biological properties assist in increasing crop quality and yield. The enhanced soil structure and lower BD provide a better soil environment for root growth. The improved CEC helps to retain more essential nutrients in soils for crop uptake and limit leaching. Moreover, organic amendments help to improve crop physiological responses to stress, stimulate crop growth, and protect against plant disease [
8].
In South Florida, locally derived organic wastes from the sugarcane industry are available, with the potential to be used as soil amendments for sugarcane grown on mineral soils. Bagasse, one of the industry by-products generated and accumulated in the mills, is a fibrous material left after the extraction of sugar juice from sugarcane. Although it is currently used as a fuel for sugar mills in this region, there is still large amount of bagasse left in need of disposal. Bagasse contains approximately 56.17% moisture, 33.91% carbon (C), 0.51% nitrogen (N), 0.26 g kg
−1 phosphorous (P), 1 g kg
−1 potassium (K), and 0.4 g kg
−1 silicon (Si) and has a C/N ratio of 65.88. The composition, nutrient levels, and benefits of bagasse vary depending on sugarcane variety, locality, mill efficiency, soil type, and climate factors. The use of bagasse has shown positive effects in terms of crop growth and yield [
9,
10,
11,
12,
13]. In Sudan, it has been reported that bagasse applied at 45 ton ha
−1 increased sunflower yield by 21.6% and 29.3% in the winter of 2004 and autumn of 2005, respectively [
12]. This study also showed that the depth of roots, plant height, and head diameter of sunflower were significantly increased by adding bagasse to the cracking clay soil. Sugarcane yield was increased by 1.83 ton ha
−1 over the control when bagasse was mixed into a vertisol in Colombia at 60 ton ha
−1 [
9].
While several studies have examined the effects of bagasse on crop growth and yields, no studies have been done in South Florida using bagasse as a soil organic amendment for sugarcane production on mineral soils. Some of the previous work in this region included studying the effects of other sugarcane industry by-products such as mill mud and mill ash on sugarcane nutrition and yield [
2,
5,
14]. These studies indicated that the sugarcane yields were significantly improved due to mill mud application on mineral soils in South Florida.
The objective of this study was to investigate how sugarcane grown on mineral soils responded when treated with different rates of bagasse (5 cm bagasse, 10 cm bagasse, and 10 cm bagasse plus N) compared with the control (no bagasse application). The specific objectives of this farm trial were to: (1) compare the commercial sugarcane, sucrose, and sugar yield in the soils amended with bagasse applied at various rates with the control; (2) determine the nutritional effects of bagasse on sugarcane leaf tissue contents.
2. Materials and Methods
2.1. Study Area and Experimental Design
The field trial was conducted in Clewiston, South Florida (26°41′18.3″ N 80°57′01.6″ W), on mineral soils, predominantly comprised of Margate sand (Siliceous, hyperthermic Mollic Psammaquents). Mean annual precipitation in this region is 1154 mm, and average maximum and minimum temperatures are 28.7 °C and 18.5 °C, respectively (U.S. Climate Data).
Basic physiochemical properties of the mineral soil at this experiment site are shown in
Table 1. The soil pH was determined using a 1:1 water to soil (
v/
v) mixture, and BD was calculated by dividing the mass of soil by a fixed volume. Organic matter (OM) was measured by loss on ignition (LOI) method. Water holding capacity (WHC) was determined by a modified method described by measuring the amount of water retained in soil after saturation [
15]. Cation exchange capacity (CEC) was determined by the ammonium acetate method [
16], and ammonium concentration was analyzed by flow injection analysis on Lachat analyzer (Hach Company, Loveland, CO, USA). Total Kjeldahl Nitrogen (TKN) was analyzed by digesting soil samples followed by colorimetric determination (EPA method 351.2). Total phosphorus (P) was measured by ashing soil samples and extracting using 6 M hydrochloric acid (HCl) and analyzed with an Agilent 5110 inductively coupled plasma-optical emission spectrometer (ICP-OES) (Santa Clara, CA, USA). Plant available nutrient concentrations including P, potassium (K), calcium (Ca), iron (Fe), and magnesium (Mg) of the mineral soil were determined using Mehlich-3 (M3) extraction method.
The experiment was conducted in a completely randomized design with three treatments plus a control. The three treatments included 5 cm bagasse (85 ton ha−1, wet weight), 10 cm bagasse (170 ton ha−1, wet weight), and 10 cm bagasse plus N (336 kg ha−1 ammonium nitrate). The control was no bagasse and no extra N added. The rates of bagasse were selected in conjunction with discussions with farm mangers and bagasse applicators, taking into account the feasibility and logistics of field-scale bagasse application in a commercial setting. The 5 cm of bagasse is the minimum amount that can be applied uniformly in the fields. Each treatment and the control were replicated three times. The ammonium nitrate fertilizer was applied in two evenly divided split applications: the first was right after bagasse application and the second application was approximately one month before planting sugarcane. The C/N ratio of the 10 cm bagasse plus N treatment would be decreased to approximately 58.75 after N application.
Bagasse was applied only once to the soil surface and incorporated into top 15 cm soil with a disk on June 2017, with no posterior application over the entire experimental period. Calcium silicate was applied uniformly to the experimental plots on August 2017 based on soil-test silicon (Si). Sugarcane was planted in November 2017 and grown for approximately 4 years, including plant cane (approximately 13 months) and two ratoons (16 months and 13 months, respectively). Bagasse was obtained from the U. S. Sugar Corporation sugarcane mill in Clewiston, Florida. Basic physiochemical properties of bagasse in this experiment site are shown in
Table 2. The pH of bagasse was determined by a 1:1 water to bagasse (
v/
v) mixture; total carbon (C) of bagasse and total N were determined by a Costech ECS 4010 elemental analyzer, and then the C:N ratio was calculated from these analyses. All total nutrient contents were measured by ashing bagasse samples and then extracting using 6 M HCl and analyzing with ICP. More information about physicochemical properties of bagasse can be found in [
17].
All 12 experimental plots received standard fertilizer, pesticide and herbicide applications over the entire cane production cycle (plant cane and two ratoons) based on recommendations for commercial sugarcane production on mineral soils in South Florida. Nutrient applications include N, P, K, Fe, Mg, boron (B), copper (Cu), manganese (Mn), and zinc (Zn). N and K fertilizers were split into three applications in the plant cane year and two applications in ratoon year 1 and 2.
2.2. Leaf Tissue Analyses
Sugarcane leaf samples were collected during the grand-growth period in June or July of each crop year. Top visible dewlap (TVD) leaves were collected randomly from all 12 field plots. Sugarcane leaf blades were separated from leaf midribs, rinsed with DI water, dried in the oven at 60 °C until up to a constant weight, ground to pass through 1 mm sieve, and analyzed for different nutrient concentrations. Leaf K, P, Ca, Mg, Cu, Fe, Mn, and Zn were determined by the nitric acid and hydrogen peroxide digestion method, and tissue N and Si were analyzed by the autoclave digestion method.
Leaf tissue analysis is a diagnostic tool for detecting nutritional problems and achieving a high degree of precision in nutrient management in sugarcane production system in Florida [
18,
19]. The critical nutrient level (CNL) approach was used in leaf tissue analysis, which determines whether nutrient concentrations in sugarcane leaves are adequate for optimal growth. The leaf nutrient concentrations were grouped into six categories as described in [
19]: very deficient (VD), deficient (D), marginal (M), sufficient (S), sufficient plus (SP), and high (H) (refer
Appendix A).
2.3. Yield Measurements
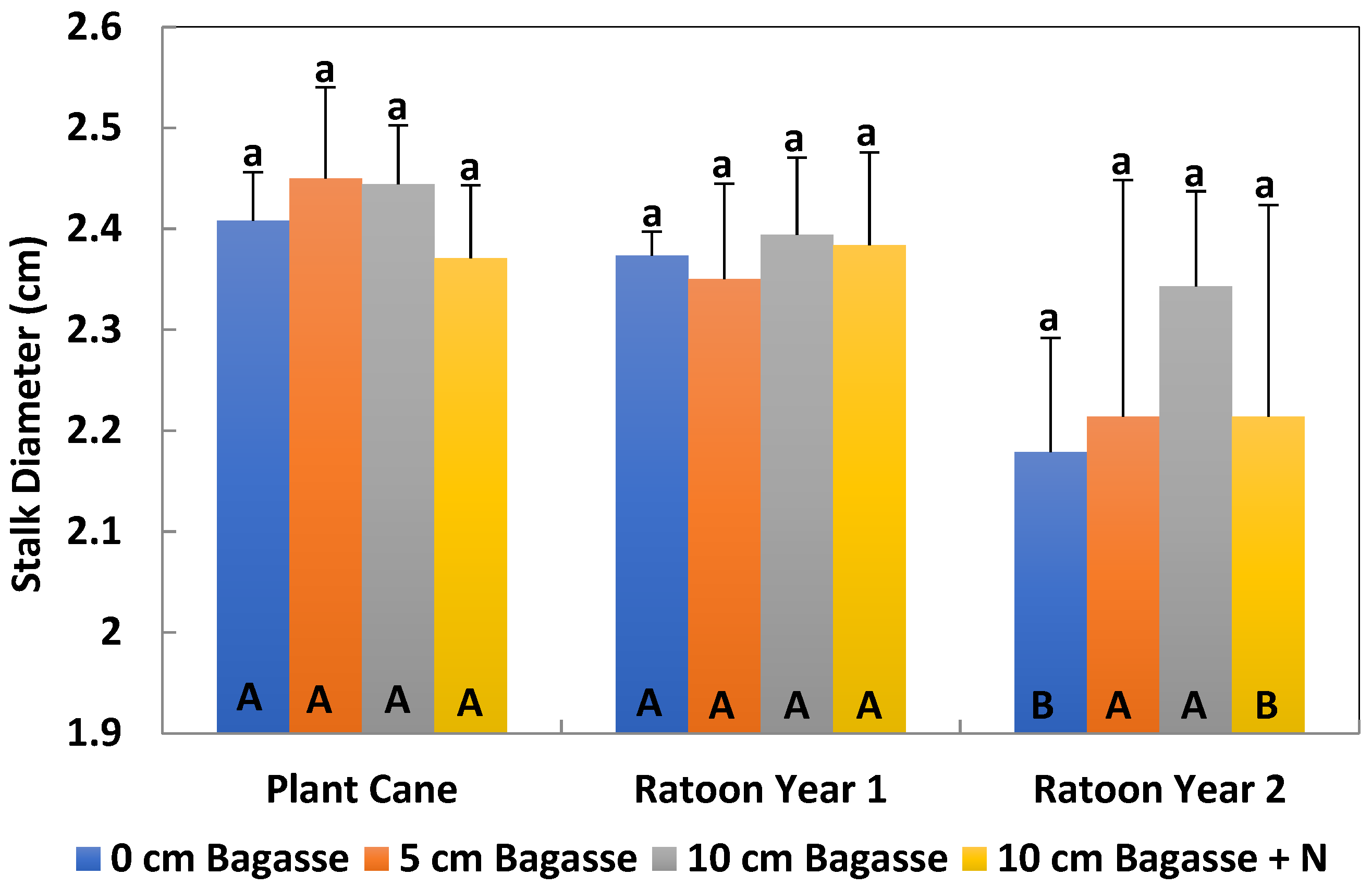
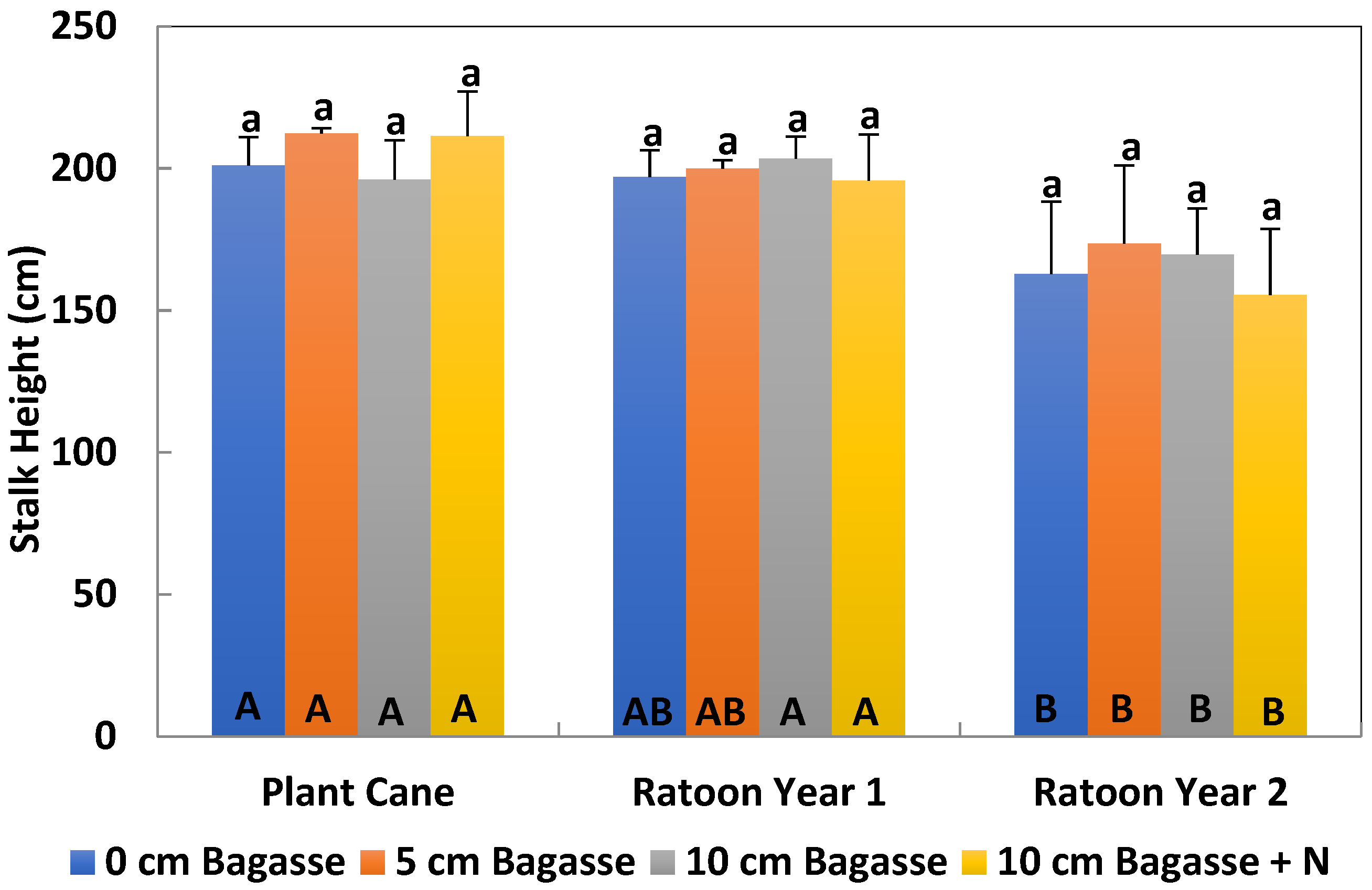
Stalk heights and diameters were measured in August–September of each crop year. Five random millable stalks were measured at four different locations in each experimental plot for a total of 20 millable stalks. These locations were near the four corners of each plot. The selected locations were 15 rows away from plot sides (ditch). Sugarcane height was measured from the bottom of cane to the first clearly visible dewlap, and the diameter was measured in the middle of the stalk.
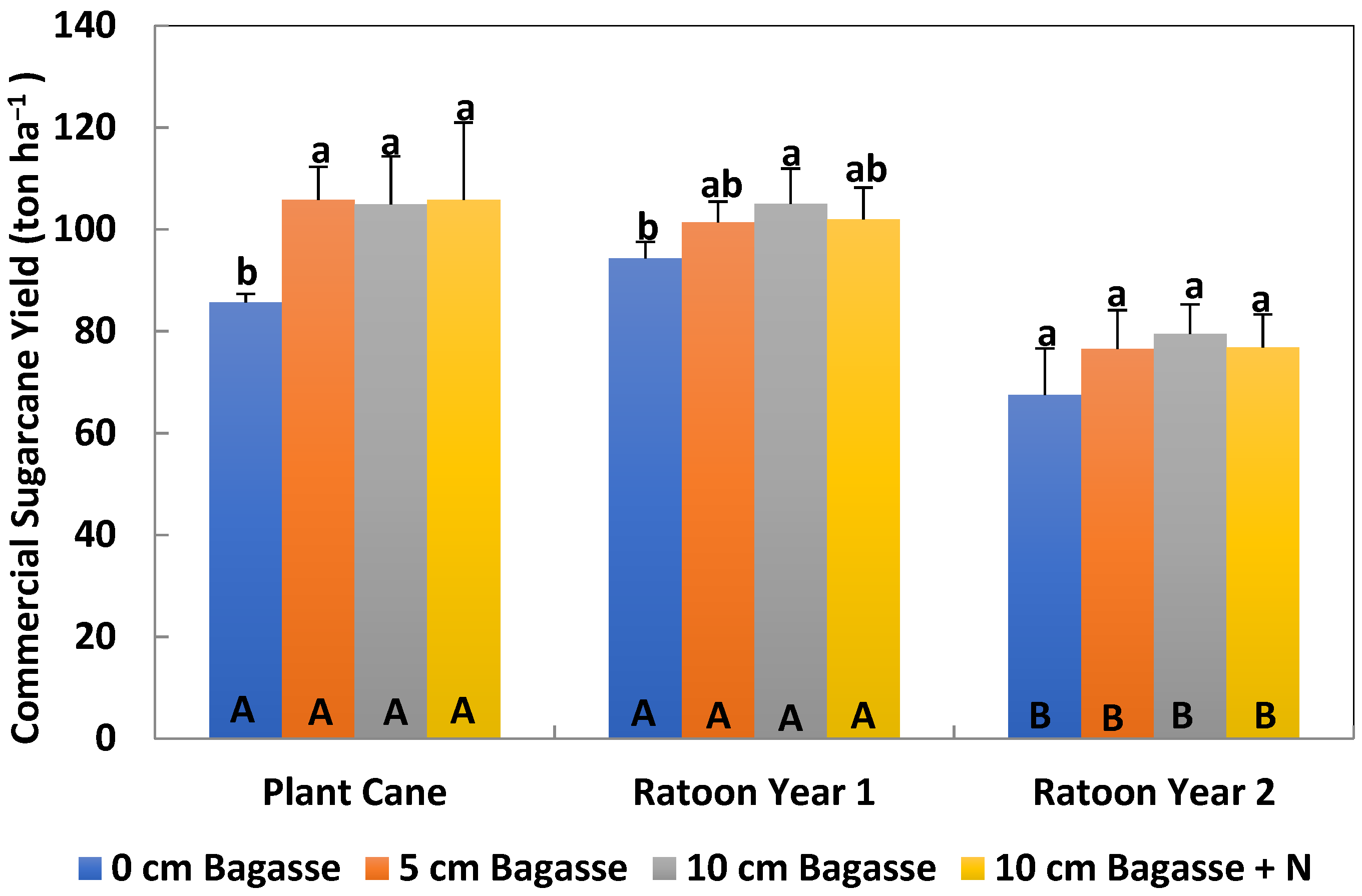
Sugarcane plant cane was harvested in December 2018, the first ratoon crop was harvested on April 2020, and the second ratoon crop was harvested on May 2021. The sugarcane was harvested commercially from each experimental plot and the sugarcane yield was recorded.
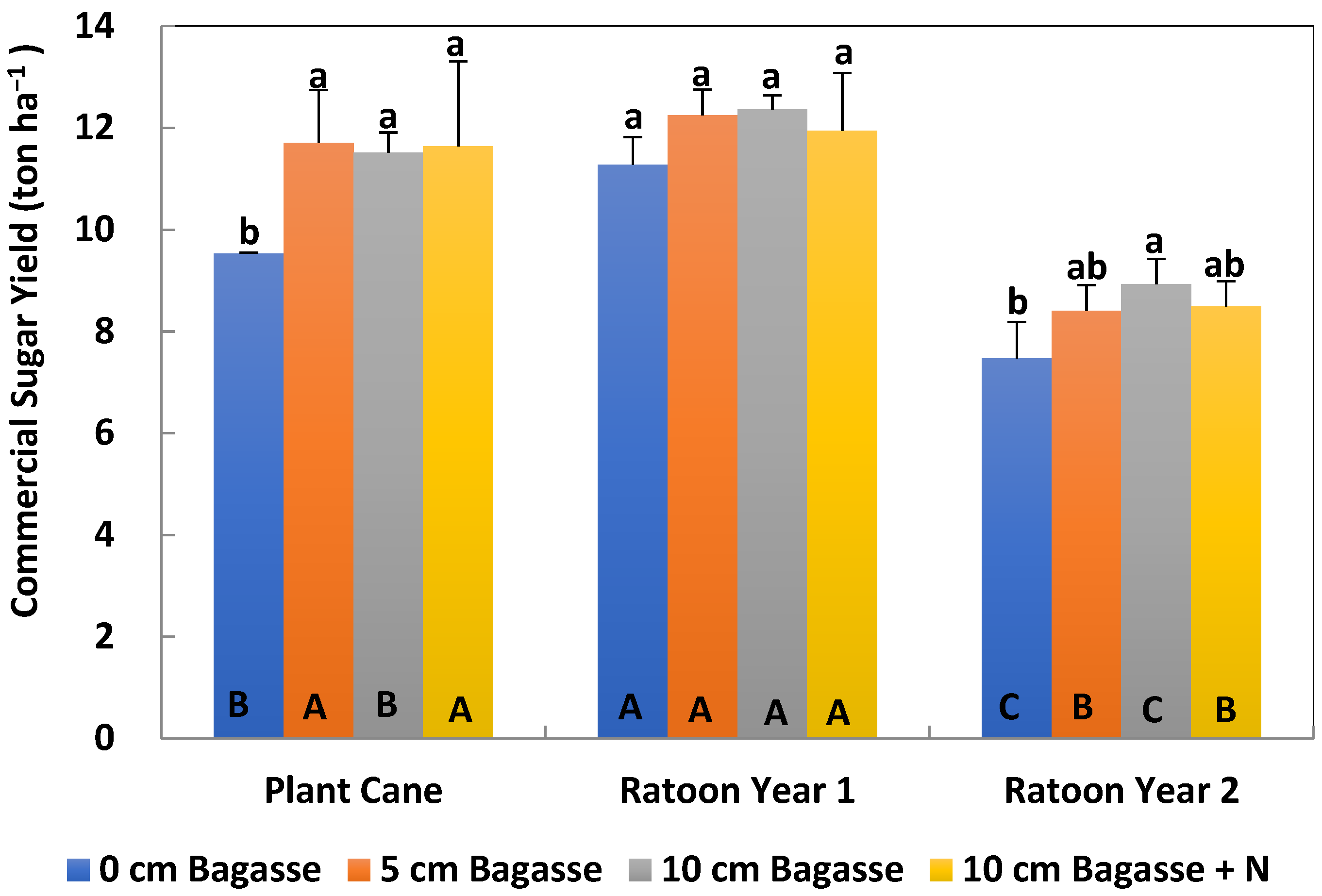
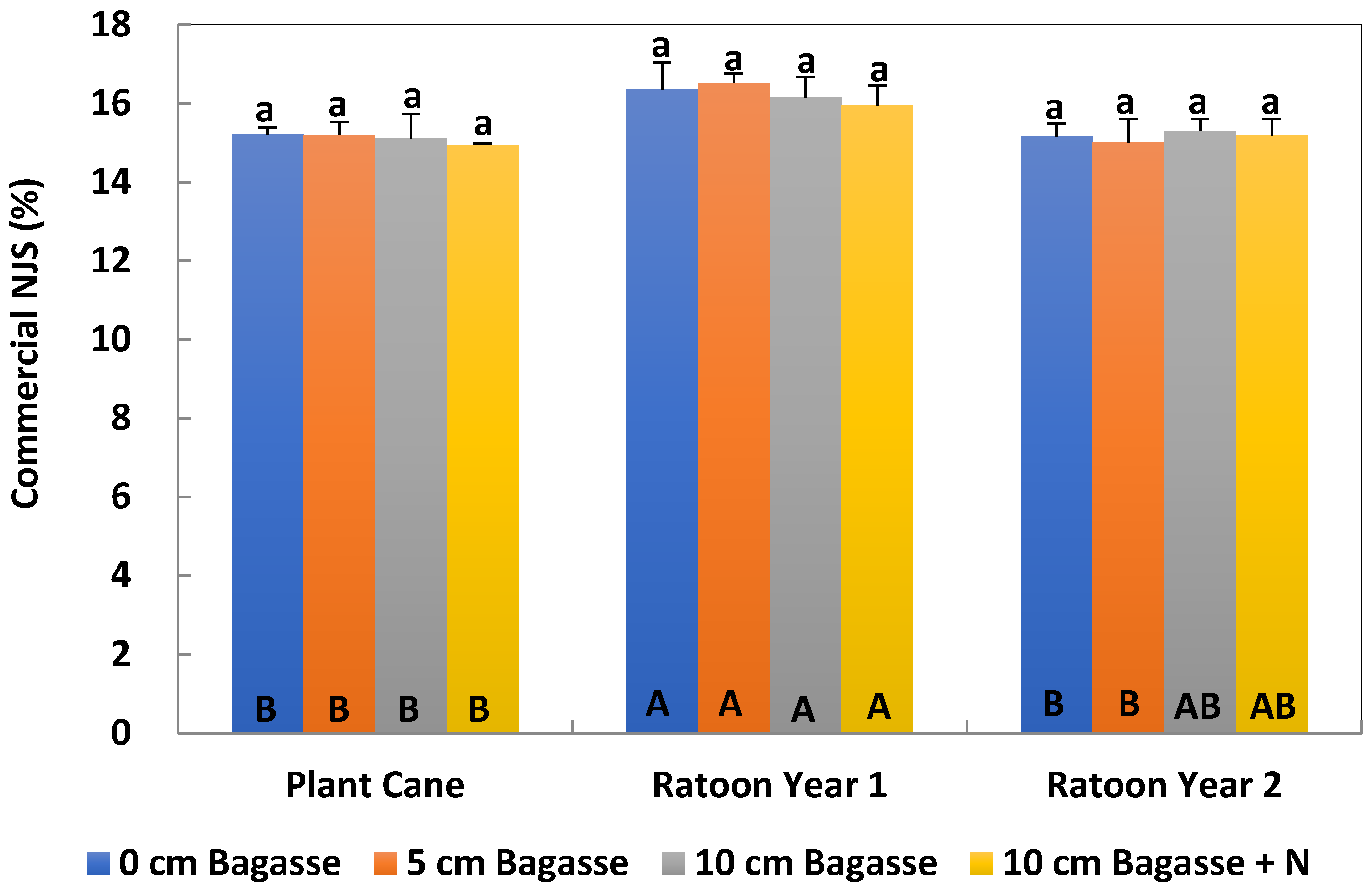
After the cane was harvested, stalks were milled and the crusher juice was immediately analyzed for brix and pol. Brix is a measurement of percent soluble solids, while pol is a unitless measurement of the polarization of the sugar solution. Brix and pol measurements were calculated by the theoretical recoverable sugar method calculator [
20], which determined percentage sucrose, and was expressed as normal juice sucrose (NJS%). The sugar yield was also calculated.
2.4. Statistical Analyses
Analyses of variance for stalk heights and diameters, sugarcane yield, percentage normal juice sucrose (NJS%), sugar yield, and leaf nutrient concentrations were performed using SAS/GLIMMIX in SAS 9.4 (SAS Institute, 2011) to determine statistically significant differences among treatments. Treatment (bagasse applied at different rates) and crop cycle were analyzed as the fixed effects, and plots were considered as random effects. Least significant mean differences (p < 0.05) were used to determine the significant treatment effects. Sugarcane yield parameters data were normally distributed, while leaf nutrient concentrations were not normally distributed. Thus, Spearman correlation analyses were used to determine the relations between sugarcane yield parameters and leaf nutrient concentrations.