Metabolic Adjustment of Glycine max (L.) Merril in the Presence of Nitrate and Bradyrhizobium japonicum
Abstract
1. Introduction
2. Materials and Methods
3. Results
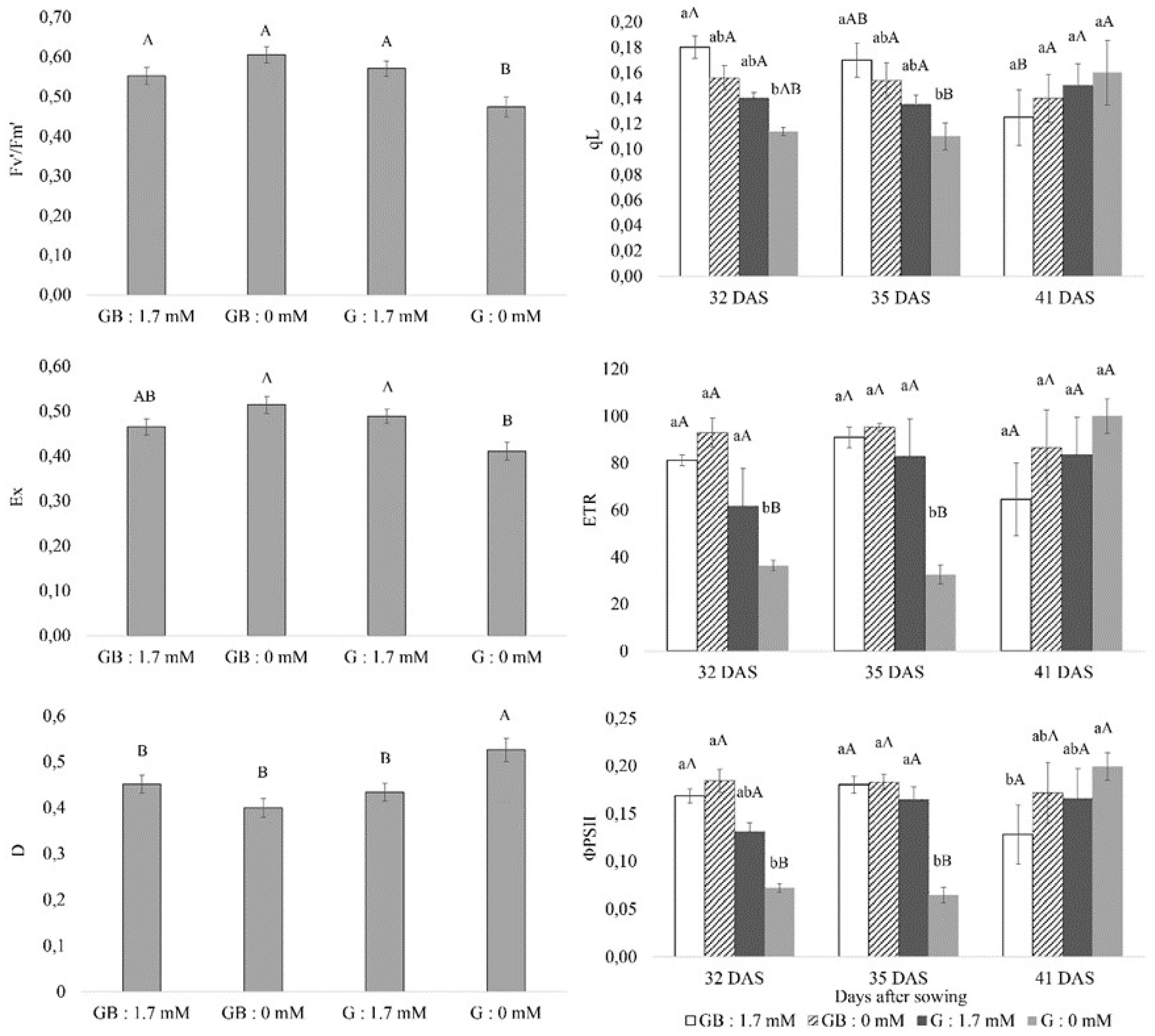
3.1. Chlorophyll a Fluorescence
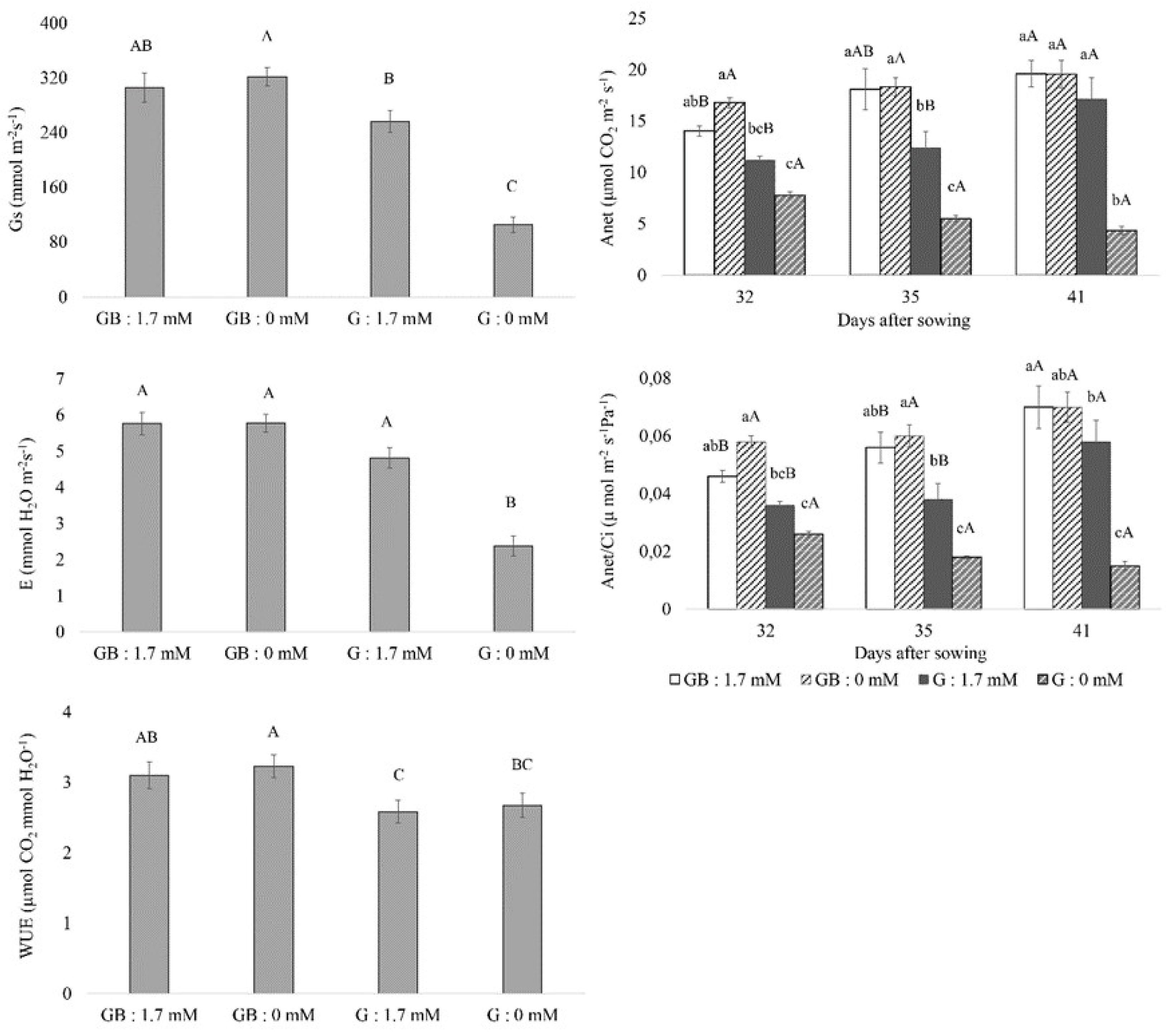
3.2. Gas Exchange
3.3. Dry Mass Production
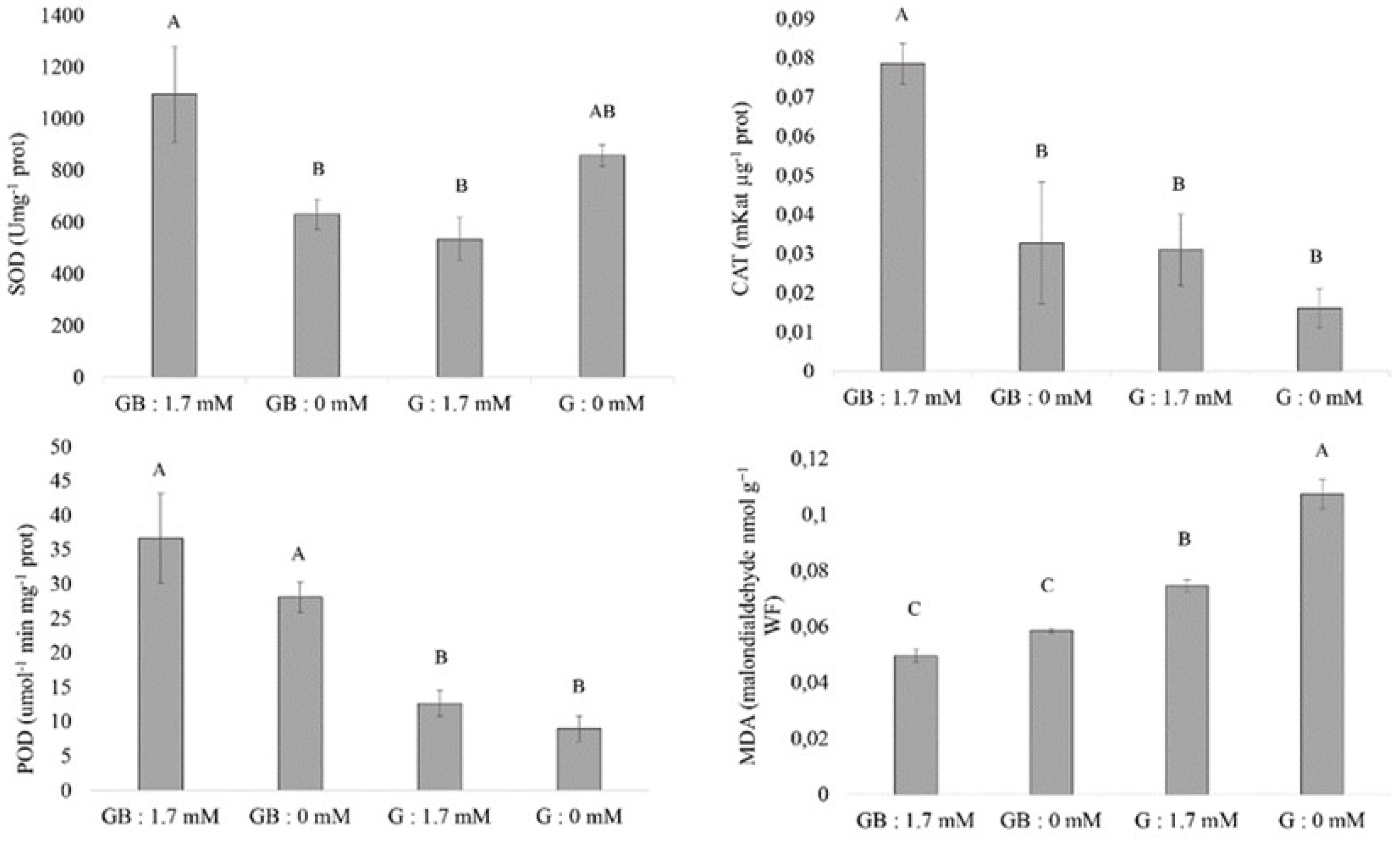
3.4. Antioxidant Enzymes and Lipid Peroxidation
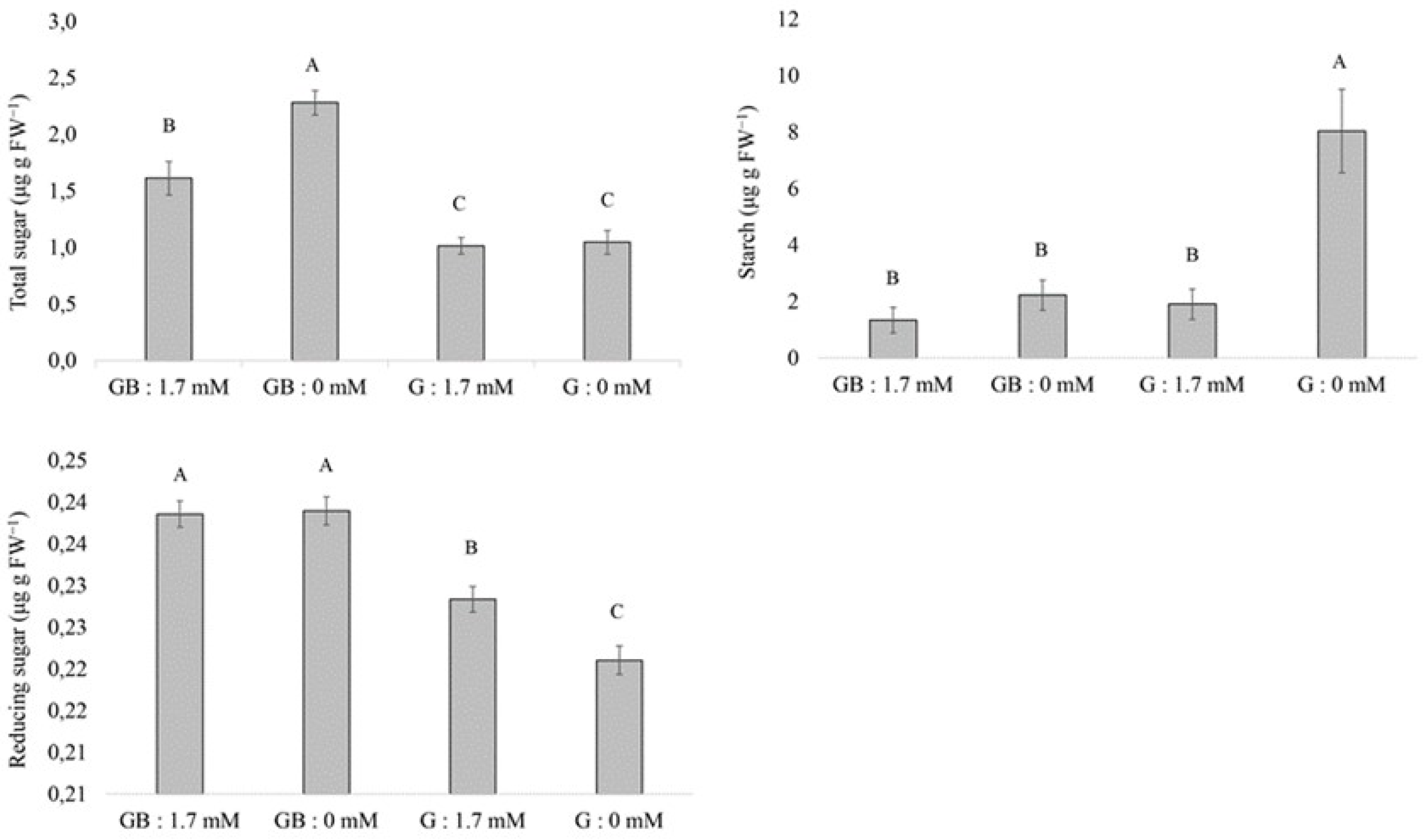
3.5. Quantification of Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zuffo, A.M.; Steiner, F.; Busch, A.; Zoz, T. Response of early soybean cultivars to nitrogen fertilization associated with Bradyrhizobium japonicum inoculation. Pesqui. Agropecu. Trop. 2018, 48, 436–446. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Menge, D.N.L.; Reed, S.C.; Cleveland, C.C. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368. [Google Scholar] [CrossRef]
- Fernandez-Göbel, T.F.; Deanna, R.; Muñoz, N.B.; Robert, G.; Asurmendi, S.; Lascano, R. Redox Systemic Signaling and Induced Tolerance Responses During Soybean—Bradyrhizobium japonicum Interaction: Involvement of Nod Factor Receptor and Autoregulation of Nodulation. Front. Plant Sci. 2019, 10, 141. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Fagan, E.B.; Medeiros, S.L.P.; Manfron, P.A.; Casaroli, D.; Simon, J.; Neto, D.D.; van Lier, Q.d.J.; Santos, O.S.; Mülle, L. Fisiologia da fixação biológica do nitrogênio em soja. Rev. da FZVA 2007, 14, 89–106. [Google Scholar]
- Barbosa, M.R.; Medeiros De Araújo, M.; Ii, S.; Willadino Iii, L.; Ulisses, C.; Terezinha, I.; Camara, R. Plant generation and enzymatic detoxification of reactive oxygen species. Cienc. Rural 2014, 44, 453–460. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Silveira, J.A.G.; Bonifacio, A.; Figueiredo, M.D.V.B. Metabolism of nitrogen and carbon: Optimization of biological nitrogen fixation and cowpea development. Soil Biol. Biochem. 2013, 67, 226–234. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, H.Y.; Bai, C.M.; Qi, M.F.; Xu, C.Q.; Hao, J.H.; Li, Y.; Li, T.L. Grafting helps improve photosynthesis and carbohydrate metabolism in leaves of muskmelon. Int. J. Biol. Sci. 2011, 7, 1161–1170. [Google Scholar] [CrossRef][Green Version]
- Brazilian Agricultural Research Corporation. Embrapa Tecnologias de Produção de Soja—Região Central do Brasil 2014; Embrapa: Brasília, Brazil, 2013; p. 242. [Google Scholar]
- Heckmann, M.O.; Drevon, J.J. Inhibition of Symbiotic Nitrogen Fixation by Nitrate. In Physiological Limitations and the Genetic Improvement of Symbiotic Nitrogen Fixation; O’Gara, F., Manian, S., Drevon, J.J., Eds.; Springer: Dordrecht, The Netherlands, 1988; Volume 3, pp. 97–106. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 1–32. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Ma, K.; Chen, L. Temperature-Dependent Gas Exchange and Stomatal/Non-Stomatal Limitation to CO2 Assimilation of Quercus Liaotungensis under Midday High Irradiance. Photosynth. Res. 2001, 39, 383–388. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Peixoto, H.P.P.; Cambraia, J.; Sant, A.R.; Mosquim, R.; Moreira, M.A. Aluminum Effects on Lipid Peroxidation and on the Activities of Enzymes of Oxidative Metabolism in Sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in soluble carbohydrates during storage of Caesalpinia echinata LAM. (Brazilwood) seeds, an endangered leguminous tree from the Brazilian Atlantic Forest. Brazilian J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative Determination of Carbohydrates with Dreywood’ s Anthrone Reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Wilhelm, C.; Selmar, D. Energy dissipation is an essential mechanism to sustain the viability of plants: The physiological limits of improved photosynthesis. J. Plant Physiol. 2011, 168, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Parente, T.L.; Lazarini, E.; Caioni, S.; Pivetta, R.S.; Souza, L.G.M.; Bossolani, J.W. Adubação nitrogenada em genótipos de soja associada à inoculação em semeadura direta no Cerrado. Brazilian J. Agric. Sci. 2015, 10, 249–255. [Google Scholar] [CrossRef][Green Version]
- Kaschuk, G.; Nogueira, M.A.; de Luca, M.J.; Hungria, M. Response of determinate and indeterminate soybean cultivars to basal and topdressing N fertilization compared to sole inoculation with Bradyrhizobium. Field Crop. Res. 2016, 195, 21–27. [Google Scholar] [CrossRef]
- Nishida, H.; Susaki, T. Nitrate-mediated control of root nodule symbiosis. Curr. Opin. Plant Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Kaschuk, G.; Yin, X.; Hungria, M.; Leffelaar, P.A.; Giller, K.E.; Kuyper, T.W. Photosynthetic adaptation of soybean due to varying effectiveness of N2 fixation by two distinctBradyrhizobium japonicum strains. Environ. Exp. Bot. 2012. [Google Scholar] [CrossRef]
- Cruz, J.L.; Alves, A.A.C.; LeCain, D.R.; Ellis, D.D.; Morgan, J.A. Effect of elevated CO2 concentration and nitrate: Ammonium ratios on gas exchange and growth of cassava (Manihot esculenta Crantz). Plant Soil 2014, 374, 33–43. [Google Scholar] [CrossRef]
- Campos, F.G.; Vieira, M.A.R.; Amaro, A.C.E.; DelaCruz-Chacón, I.; Marques, M.O.M.; Ferreira, G.; Boaro, C.S.F. Nitrogen in the defense system of Annona emarginata (Schltdl.) H. Rainer. PLoS ONE 2019, 14, e0217930. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- May, W.E.; Brandt, S.A.; Gan, Y.; Kutcher, H.R.; Holzapfel, C.B.; Lafond, G.P. Adaptation of oilseed crops across Saskatchewan. Can. J. Plant Sci. 2010, 90, 667–677. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Petter, F.A.; Pacheco, L.P.; de Neto, F.A.; Santos, G.G. Respostas de cultivares de soja à adubação nitrogenada tardia em solos de cerrado. Rev. Caatinga 2012, 25, 67–72. [Google Scholar]
- da Silva, A.F.; de Carvalho, M.A.C.; Schoninger, E.L.; Monteiro, S.; Caione, G.; Santos, P.A. Doses de inoculante e nitrogênio na semeadura da soja em área de primeiro cultivo. Biosci. J. 2011, 27, 404–412. [Google Scholar]
- Gericó, T.G.; Tavanti, R.F.R.; de Oliveira, S.C.; Lourenzani, A.E.B.S.; de Lima, J.P.; Ribeiro, R.P.; dos Santos, L.C.C.; dos Reis, A.R. Bradyrhizobium sp. enhance ureide metabolism increasing peanuts yield. Arch. Microbiol. 2020, 202, 645–656. [Google Scholar] [CrossRef]
| Solution | Molar | Initial Step | With Nitrate | Without Nitrate |
|---|---|---|---|---|
| Nutritional Solution (mL L) | ||||
| Macronutrients | ||||
| KHPO | 1.00 | - | 25 | - |
| K2SO | 0.50 | 125 | - | 125 |
| KNO | 1.00 | - | 125 | - |
| Ca(NO) | 1.00 | - | 125 | - |
| Ca(HPO) | 0.05 | 500 | - | 250 |
| MgSO | 1.00 | 50 | 50 | 25 |
| CaSO | 0.01 | - | - | 5000 |
| Micronutrients | - | 25 | 25 | 25 |
| Fe EDTA | - | 25 | 25 | 25 |
| Treatments | DLBM | DSM + P | DNM | DRM | DRM + N | TDM |
|---|---|---|---|---|---|---|
| GB: 1.7 mM | 1.45 ± 0.06 a | 1.16 ± 0.02 a | 0.08 ± 0.01 b | 1.00 ± 0.02 a | 1.09 ± 0.05 a | 3.73 ± 0.01 a |
| GB: 0 mM | 0.78 ± 0.03 b | 0.76 ± 0.04 b | 0.15 ± 0.016 a | 0.60 ± 0.03 b | 0.75 ± 0.04 b | 2.32 ± 0.09 c |
| G: 1.7 mM | 0.92 ± 0.04 b | 0.85 ± 0.01 b | - | 0.90 ± 0.02 a | 0.90 ± 0.02 b | 2.72 ± 0.05 b |
| G: 0 mM | 0.32 ± 0.01 c | 0.40 ± 0.01 c | - | 0.46 ± 0.02 b | 0.46 ± 0.02 c | 1.17 ± 0.02 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Júnior, A.M.; Campos, F.G.; Barzotto, G.R.; Pagassini, J.A.V.; Vieira, M.A.R.; Boaro, C.S.F. Metabolic Adjustment of Glycine max (L.) Merril in the Presence of Nitrate and Bradyrhizobium japonicum. Agronomy 2021, 11, 1518. https://doi.org/10.3390/agronomy11081518
Júnior AM, Campos FG, Barzotto GR, Pagassini JAV, Vieira MAR, Boaro CSF. Metabolic Adjustment of Glycine max (L.) Merril in the Presence of Nitrate and Bradyrhizobium japonicum. Agronomy. 2021; 11(8):1518. https://doi.org/10.3390/agronomy11081518
Chicago/Turabian StyleJúnior, Alberto Mongolo, Felipe Girotto Campos, Gustavo Ribeiro Barzotto, Jonas Akenaton Venturineli Pagassini, Maria Aparecida Ribeiro Vieira, and Carmen Sílvia Fernandes Boaro. 2021. "Metabolic Adjustment of Glycine max (L.) Merril in the Presence of Nitrate and Bradyrhizobium japonicum" Agronomy 11, no. 8: 1518. https://doi.org/10.3390/agronomy11081518
APA StyleJúnior, A. M., Campos, F. G., Barzotto, G. R., Pagassini, J. A. V., Vieira, M. A. R., & Boaro, C. S. F. (2021). Metabolic Adjustment of Glycine max (L.) Merril in the Presence of Nitrate and Bradyrhizobium japonicum. Agronomy, 11(8), 1518. https://doi.org/10.3390/agronomy11081518







