Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microgreen Cultivation and Experimental Design
2.2. Measurements and Observations
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physiology Measurements
3.2. Fresh and Dry Weight Production
3.3. Color Parameters and Texture at Harvest and Postharvest
3.4. Respiration Rates as O2 Consumption and CO2 Production at Harvest and During Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, microgreens and “Baby Leaf” vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional Characterization and Shelf-Life of Packaged Microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, Shelf Life, and Bioactive Components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The Effects of LED Illumination Spectra and Intensity on Carotenoid Content in Brassicaceae Microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-Scale Vegetable Production and the Rise of Microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed Treatments to Advance Greenhouse Establishment of Beet and Chard Microgreens. J. Hortic. Sci. Biotechnol. 2004, 79, 565–570. [Google Scholar] [CrossRef]
- Saengha, W.; Karirat, T.; Buranrat, B.; Matra, K.; Deeseenthum, S.; Katisart, T.; Luang-In, V. Cold Plasma Treatment on Mustard Green Seeds and Its Effect on Growth, Isothiocyanates, Antioxidant Activity and Anticancer Activity of Microgreens. Int. J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Luang-In, V.; Saengha, W.; Karirat, T.; Buranrat, B.; Matra, K.; Deeseenthum, S.; Katisart, T. Effect of Cold Plasma and Elicitors on Bioactive Contents, Antioxidant Activity and Cytotoxicity of Thai Rat-Tailed Radish Microgreens. J. Sci. Food Agric. 2021, 101, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yang, T.; Liu, X.; Luo, Y. Effects of Pre- and Postharvest Calcium Treatments on Shelf Life and Postharvest Quality of Broccoli Microgreens. HortScience Horts 2015, 50, 1801–1808. [Google Scholar] [CrossRef] [Green Version]
- Niroula, A.; Amgain, N.; Kc, R.; Adhikari, S.; Acharya, J. Pigments, Ascorbic Acid, Total Polyphenols and Antioxidant Capacities in Deetiolated Barley (Hordeum Vulgare) and Wheat (Triticum Aestivum) Microgreens. Food Chem. 2021, 354, 129491. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, D.J.; Katsenios, N.; Efthimiadou, A.; Karkanis, A.; Efthimiadis, P. Investigation of Pulsed Electromagnetic Field as a Novel Organic Pre-Sowing Method on Germination and Initial Growth Stages of Cotton. Electromagn. Biol. Med. 2012, 31, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Hajnorouzi, A.; Vaezzadeh, M.; Ghanati, F.; Jamnezhad, H.; Nahidian, B. Growth Promotion and a Decrease of Oxidative Stress in Maize Seedlings by a Combination of Geomagnetic and Weak Electromagnetic Fields. J. Plant Physiol. 2011, 168, 1123–1128. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Ranjitha Kumari, B.D. Pulsed Magnetic Field: A Contemporary Approach Offers to Enhance Plant Growth and Yield of Soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Flórez, M.; Carbonell, M.V.; Martínez, E. Exposure of Maize Seeds to Stationary Magnetic Fields: Effects on Germination and Early Growth. Environ. Exp. Bot. 2007, 59, 68–75. [Google Scholar] [CrossRef]
- Katsenios, N.; Sparangis, P.; Kakabouki, I.; Efthimiadou, A. Influence of Pulsed Electromagnetic Field as a Pre-Sowing Treatment on Germination, Plant Growth and Yield of Broad Beans. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1398–1412. [Google Scholar] [CrossRef]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red Beet (Beta Vulgaris) and Amaranth (Amaranthus Sp.) Microgreens: Effect of Storage and in Vitro Gastrointestinal Digestion on the Untargeted Metabolomic Profile. Food Chem. 2020, 332, 127415. [Google Scholar] [CrossRef]
- Xiao, Z.; Luo, Y.; Lester, G.E.; Kou, L.; Yang, T.; Wang, Q. Postharvest Quality and Shelf Life of Radish Microgreens as Impacted by Storage Temperature, Packaging Film, and Chlorine Wash Treatment. LWT Food Sci. Technol. 2014, 55, 551–558. [Google Scholar] [CrossRef]
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and Microgreens: Trends, Opportunities, and Horizons for Novel Research. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Blackwell Wissenschafts-Verlag: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Katsenios, N.; Bilalis, D.; Efthimiadou, A.; Aivalakis, G.; Nikolopoulou, A.-E.; Karkanis, A.; Travlos, I. Role of Pulsed Electromagnetic Field on Enzyme Activity, Germination, Plant Growth and Yield of Durum Wheat. Biocatal. Agric. Biotechnol. 2016, 6, 152–158. [Google Scholar] [CrossRef]
- Shine, M.B.; Guruprasad, K.N.; Anand, A. Enhancement of Germination, Growth, and Photosynthesis in Soybean by Pre-Treatment of Seeds with Magnetic Field. Bioelectromagnetics 2011, 32, 474–484. [Google Scholar] [CrossRef]
- Martinez, E.; Carbonell, M.V.; Florez, M. Magnetic Biostimulation of Initial Growth Stages of Wheat (Triticum aestivum, L.). Electromagn. Biol. Med. 2002, 21, 43–53. [Google Scholar] [CrossRef]
- Fischer, G.; Tausz, M.; Köck, M.; Grill, D. Effects of Weak 16 3/2 Hz Magnetic Fields on Growth Parameters of Young Sunflower and Wheat Seedlings. Bioelectromagnetics 2004, 25, 638–641. [Google Scholar] [CrossRef]
- Rochalska, M. Influence of Frequent Magnetic Field on Chlorophyll Content in Leaves of Sugar Beet Plants. Nukleonika 2005, 50 (Suppl. 2), 25–28. [Google Scholar]
- Turker, M.; Temirci, C.; Battal, P.; Erez, M.E. The Effects of an Artificial and Static Magnetic Field on Plant Growth, Chlorophyll and Phytohormone Levels in Maize and Sunflower Plants. Phyton Ann. Rei Bot. 2007, 14, 271–284. [Google Scholar]
- Giannoglou, M.; Xanthou, Z.-M.; Chanioti, S.; Stergiou, P.; Christopoulos, M.; Dimitrakellis, P.; Efthimiadou, A.; Gogolides, Ε.; Katsaros, G. Effect of Cold Atmospheric Plasma and Pulsed Electromagnetic Fields on Strawberry Quality and Shelf-Life. Innov. Food Sci. Emerg. Technol. 2021, 68, 102631. [Google Scholar] [CrossRef]
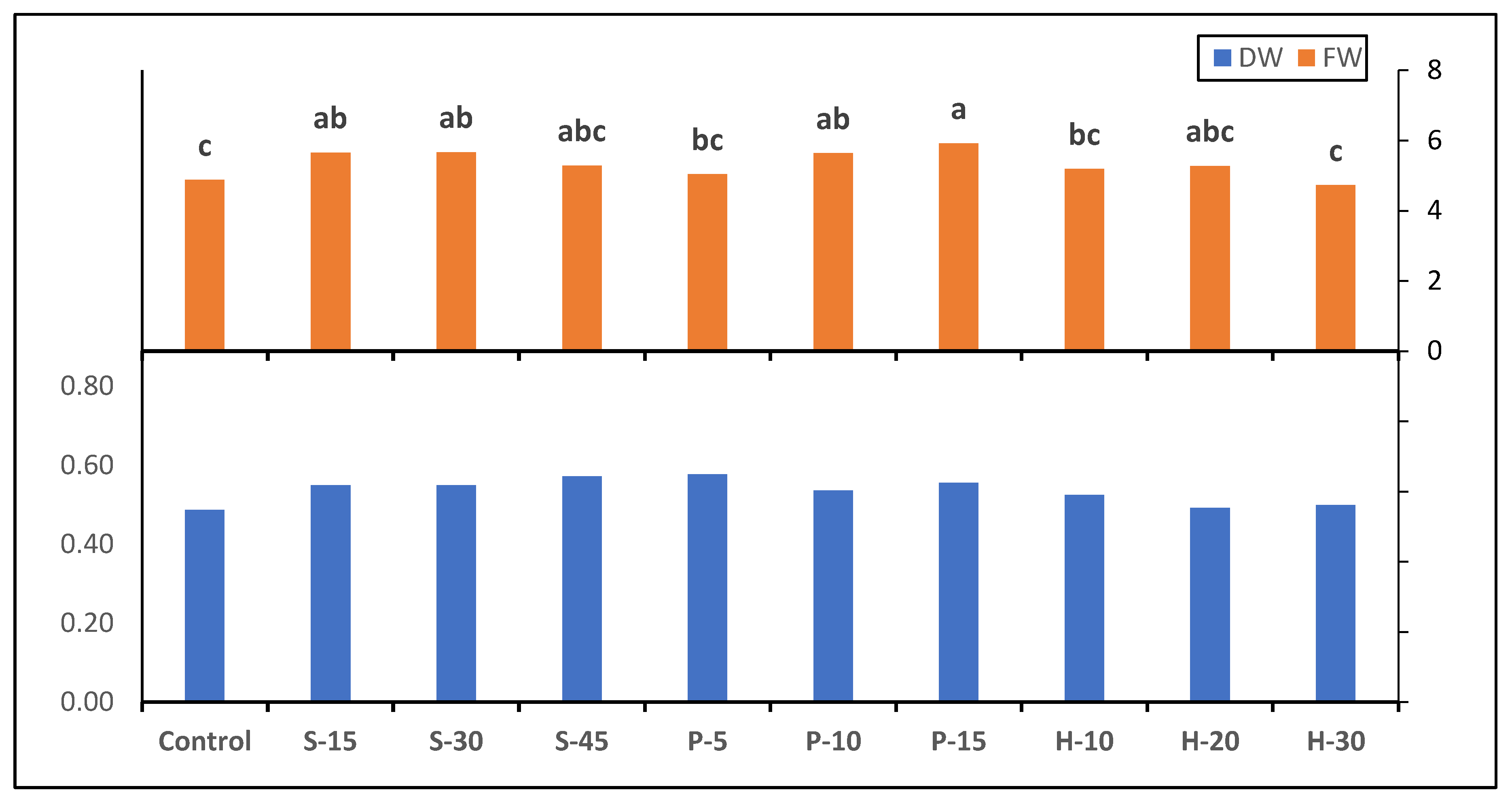

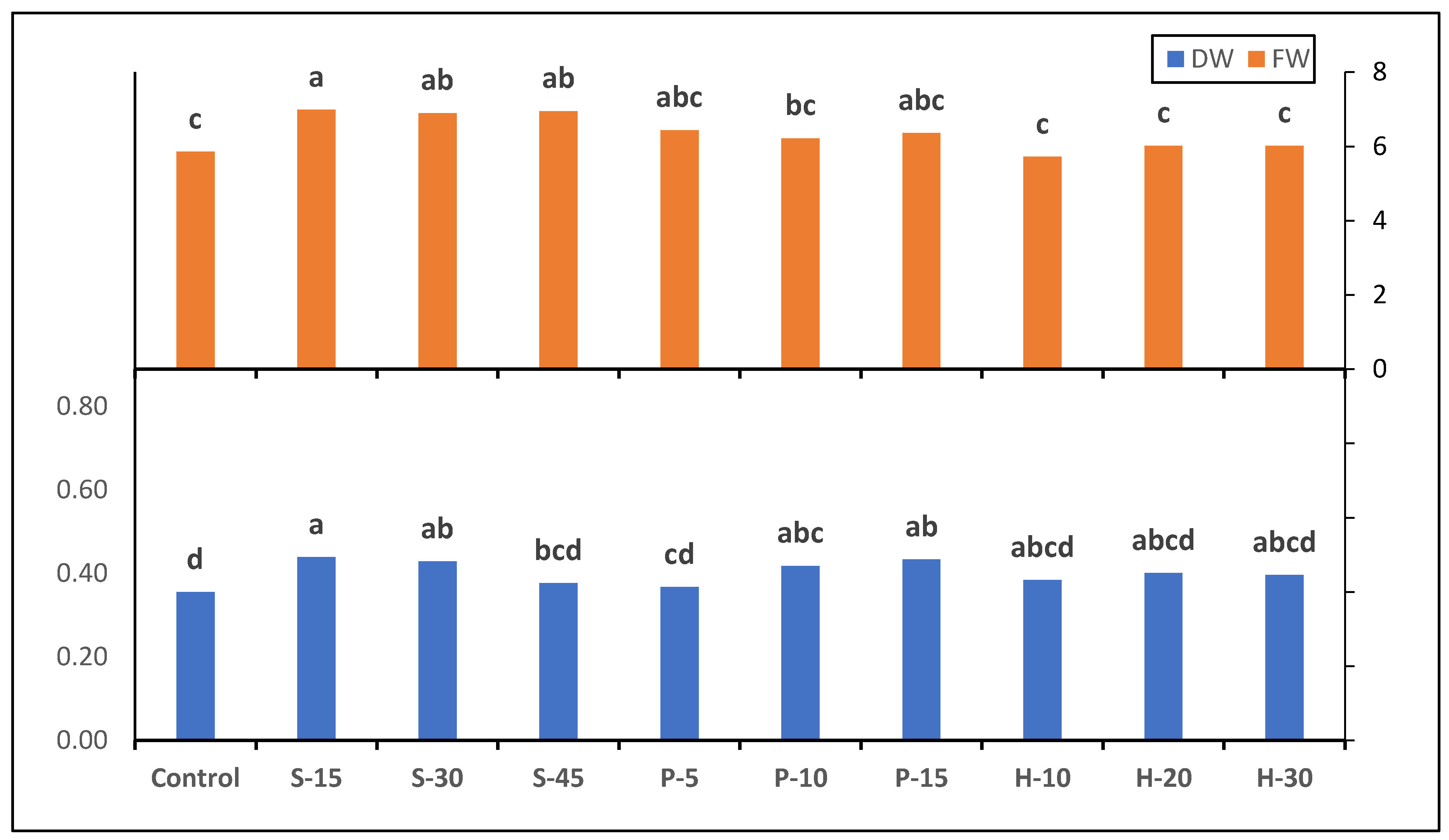
| Photosynthetic Rate (μmol CO2 m−2 s−1) | Transpiration Rate (mmol H2O m−2 s−1) | Stomatal Conductance (mol m−2 s−1) | |
|---|---|---|---|
| Kale | |||
| Control | 7.17 d | 0.92 d | 0.04 b |
| S-15 | 8.58 bc | 1.15 b | 0.08 a |
| S-30 | 9.11 a | 1.31 a | 0.08 a |
| S-45 | 8.38 c | 1.03 c | 0.09 a |
| P-5 | 6.75 de | 0.90 d | 0.08 a |
| P-10 | 6.62 e | 0.88 d | 0.08 a |
| P-15 | 6.86 de | 0.89 d | 0.07 a |
| H-10 | 8.58 bc | 1.20 b | 0.09 a |
| H-20 | 8.77 abc | 1.17 b | 0.09 a |
| H-30 | 8.95 ab | 1.21 b | 0.09 a |
| Ftreat | 47.456 *** | 29.273 *** | 3.870 ** |
| Wheat | |||
| Control | 10.70 d | 1.62 c | 0.22 b |
| S-15 | 13.12 c | 2.30 b | 0.28 a |
| S-30 | 14.56 ab | 2.28 b | 0.30 a |
| S-45 | 14.02 abc | 2.34 b | 0.30 a |
| P-5 | 13.90 abc | 2.44 b | 0.32 a |
| P-10 | 14.68 a | 2.26 b | 0.32 a |
| P-15 | 14.04 abc | 2.30 b | 0.34a |
| H-10 | 14.08 abc | 2.36 b | 0.32 a |
| H-20 | 13.78 abc | 2.84 a | 0.34 a |
| H-30 | 13.62 bc | 2.92 a | 0.34 a |
| Ftreat | 14.763 *** | 23.967 *** | 4.222 ** |
| Spinach | |||
| Control | 14.68 c | 1.66 d | 0.18 d |
| S-15 | 15.12 bc | 1.82 c | 0.30 c |
| S-30 | 15.78 b | 1.80 c | 0.30 c |
| S-45 | 15.22 bc | 1.92 b | 0.30 c |
| P-5 | 15.40 bc | 1.94 b | 0.28 c |
| P-10 | 15.82 b | 1.94 b | 0.30 c |
| P-15 | 15.14 bc | 2.00 b | 0.30 c |
| H-10 | 19.08 a | 2.28 a | 0.32 bc |
| H-20 | 19.24 a | 2.30 a | 0.38 a |
| H-30 | 18.50 a | 2.30 a | 0.36 ab |
| Ftreat | 33.086 *** | 67.228 *** | 11.648 *** |
| At Harvest | Postharvest (8 DAH) | |||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | a* | Texture (g) | L* | a* | b* | Texture (g) | |
| Kale | ||||||||
| Control | 44.04 | −16.53 | 20.69 | 73.25 | 45.51 | −17.27 | 23.26 | 75.22 |
| S-15 | 44.41 | −16.50 | 20.79 | 86.29 | 45.77 | −17.07 | 23.26 | 89.67 |
| S-30 | 44.69 | −16.01 | 20.81 | 80.69 | 44.71 | −16.42 | 21.71 | 90.92 |
| S-45 | 44.09 | −16.20 | 20.62 | 88.08 | 45.90 | −17.08 | 23.25 | 84.76 |
| P-5 | 45.21 | −16.29 | 20.84 | 86.29 | 45.44 | −15.63 | 22.49 | 84.40 |
| P-10 | 44.87 | −16.43 | 21.08 | 80.69 | 45.21 | −16.77 | 22.90 | 86.62 |
| P-15 | 44.97 | −16.31 | 20.41 | 84.54 | 44.44 | −16.45 | 21.65 | 81.34 |
| H-10 | 45.26 | −16.54 | 20.83 | 84.42 | 45.39 | −16.99 | 23.15 | 79.20 |
| H-20 | 44.48 | −15.99 | 20.08 | 86.56 | 44.90 | −16.53 | 22.08 | 80.39 |
| H-30 | 44.49 | −15.88 | 20.11 | 81.59 | 45.07 | −16.70 | 21.87 | 89.59 |
| Ftreat | 1.253 ns | 1.432 ns | 1.038 ns | 1.135 ns | 0.466 ns | 1.005 ns | 1.200 ns | 1.876 ns |
| Wheat | ||||||||
| Control | 43.11a | −18.78 ab | 22.66 | 163.28 | 42.21 | −17.66ab | 23.38 | 164.61 |
| S-15 | 43.25a | −19.13 bcd | 22.82 | 172.36 | 43.55 | −18.03abc | 24.32 | 177.17 |
| S-30 | 43.08a | −19.20 bcd | 22.59 | 173.63 | 43.95 | −18.89abc | 24.70 | 170.28 |
| S-45 | 42.85a | −19.26 cd | 23.06 | 174.94 | 44.57 | −19.49c | 25.71 | 176.60 |
| P-5 | 42.70a | −18.90 abcd | 22.70 | 172.13 | 44.61 | −19.46c | 25.20 | 167.92 |
| P-10 | 42.05ab | −19.21 bcd | 22.95 | 151.97 | 44.93 | −18.91abc | 25.39 | 172.71 |
| P-15 | 42.43a | −19.32 cd | 23.01 | 165.30 | 42.96 | −18.57abc | 24.14 | 160.36 |
| H-10 | 43.42a | −19.36 d | 23.36 | 171.60 | 44.23 | −17.39a | 23.06 | 154.64 |
| H-20 | 42.97a | −18.88 abc | 22.71 | 146.90 | 43.32 | −17.94abc | 24.66 | 167.80 |
| H-30 | 40.84b | −18.48 a | 22.80 | 151.90 | 44.42 | −19.03bc | 24.83 | 165.50 |
| Ftreat | 2.980 * | 4.137 ** | 0.859 ns | 1.619 ns | 0.630 ns | 0.041 * | 0.459 ns | 0.743 ns |
| Spinach | ||||||||
| Control | 41.59 | −17.51 | 23.59 a | 43.64 | 41.75 | −17.73 b | 23.20 | 49.02 |
| S-15 | 42.67 | −17.60 | 23.07 ab | 50.64 | 41.99 | −17.57 b | 22.74 | 47.94 |
| S-30 | 41.27 | −16.94 | 21.69 cd | 44.83 | 40.04 | −17.80 b | 23.30 | 49.63 |
| S-45 | 41.92 | −17.35 | 22.49 abc | 49.53 | 40.85 | −16.75 ab | 21.55 | 53.89 |
| P-5 | 42.76 | −17.61 | 22.98 abc | 50.64 | 39.96 | −16.52 ab | 21.79 | 58.09 |
| P-10 | 42.60 | −16.97 | 21.67 cd | 44.83 | 40.28 | −16.94 ab | 23.13 | 54.34 |
| P-15 | 42.75 | −17.66 | 23.10 ab | 45.89 | 39.41 | −16.68 ab | 22.65 | 49.73 |
| H-10 | 41.50 | −17.21 | 22.26 abc | 46.99 | 41.95 | −17.57 b | 22.92 | 50.88 |
| H-20 | 42.03 | −17.17 | 22.00 bc | 48.54 | 40.44 | −16.64 ab | 22.56 | 53.10 |
| H-30 | 41.46 | −16.57 | 20.56 d | 48.07 | 39.59 | −15.95 a | 21.72 | 57.06 |
| Ftreat | 1.729 ns | 2.176 ns | 4.674 ** | 1.317 ns | 1.142 ns | 2.416 * | 0.983 ns | 0.461 ns |
| At Harvest | Postharvest (8 DAH) | Weight Loss (%) | |||
|---|---|---|---|---|---|
| Respiration as O2 Consumption (mL/kg/h) | Respiration as CO2 Production (mL/kg/h) | Respiration as O2 Consumption (mL/kg/h) | Respiration as CO2 Production (mL/kg/h) | ||
| Kale | |||||
| Control | 132.38 | 88.25 | 70.06 | 86.86 | 1.923 |
| S-15 | 84.02 | 98.37 | 76.48 | 84.23 | 2.090 |
| S-30 | 122.84 | 122.84 | 72.95 | 104.36 | 2.148 |
| S-45 | 141.27 | 141.27 | 98.67 | 110.01 | 2.076 |
| P-5 | 103.53 | 103.53 | 66.77 | 103.30 | 2.208 |
| P-10 | 110.56 | 122.06 | 71.70 | 96.89 | 2.136 |
| P-15 | 96.74 | 96.74 | 75.59 | 92.08 | 2.103 |
| H-10 | 93.72 | 121.66 | 80.44 | 80.44 | 2.251 |
| H-20 | 110.22 | 101.47 | 75.94 | 83.22 | 1.923 |
| H-30 | 144.91 | 144.91 | 86.08 | 104.95 | 2.666 |
| Ftreat | 2.082ns | 2.074 ns | 0.599 ns | 0.821 ns | 0.247 ns |
| Wheat | |||||
| Control | 65.53 b | 78.89 cd | 89.10 | 65.97 bc | 1.229 |
| S-15 | 64.29 b | 75.82 cd | 85.76 | 37.18 c | 1.447 |
| S-30 | 62.65 b | 78.54 cd | 73.48 | 36.41 c | 1.485 |
| S-45 | 61.02 b | 67.99 d | 101.15 | 75.81 abc | 1.608 |
| P-5 | 75.85 ab | 89.90 bcd | 100.77 | 88.45 ab | 1.599 |
| P-10 | 87.25 ab | 99.43 abc | 101.94 | 91.17 ab | 2.034 |
| P-15 | 82.93 ab | 97.76 abc | 108.73 | 94.17 ab | 1.999 |
| H-10 | 80.47 ab | 104.65 ab | 74.38 | 76.40 abc | 1.927 |
| H-20 | 87.05 ab | 104.33 ab | 142.93 | 119.40 a | 2.194 |
| H-30 | 98.14 a | 121.26 a | 113.86 | 107.08 ab | 2.688 |
| Ftreat | 2.563 * | 5.195 ** | 1.237 ns | 3.894 * | 1.982 ns |
| Spinach | |||||
| Control | 60.42 | 60.42 e | 34.66 b | 10.93 d | 2.272 |
| S-15 | 57.66 | 63.35 e | 47.43 b | 20.64 d | 2.397 |
| S-30 | 61.69 | 80.32 cde | 37.82 b | 24.95 cd | 2.588 |
| S-45 | 71.44 | 77.17 de | 39.72 b | 39.72 bc | 2.069 |
| P-5 | 62.84 | 80.70 cde | 52.89 b | 41.26 bc | 2.037 |
| P-10 | 63.48 | 103.78 abc | 45.33 b | 45.33 b | 1.660 |
| P-15 | 72.75 | 78.49 cde | 43.65 b | 50.23 b | 1.374 |
| H-10 | 70.26 | 92.33 bcd | 51.72 b | 45.33 b | 2.202 |
| H-20 | 96.57 | 119.57 a | 50.76 b | 56.97 b | 1.704 |
| H-30 | 75.84 | 108.70 ab | 91.47 a | 91.47 a | 2.224 |
| Ftreat | 1.379 ns | 5.763 ** | 5.116 ** | 13.541 *** | 1.121 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsenios, N.; Christopoulos, M.V.; Kakabouki, I.; Vlachakis, D.; Kavvadias, V.; Efthimiadou, A. Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens. Agronomy 2021, 11, 1364. https://doi.org/10.3390/agronomy11071364
Katsenios N, Christopoulos MV, Kakabouki I, Vlachakis D, Kavvadias V, Efthimiadou A. Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens. Agronomy. 2021; 11(7):1364. https://doi.org/10.3390/agronomy11071364
Chicago/Turabian StyleKatsenios, Nikolaos, Miltiadis V. Christopoulos, Ioanna Kakabouki, Dimitrios Vlachakis, Victor Kavvadias, and Aspasia Efthimiadou. 2021. "Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens" Agronomy 11, no. 7: 1364. https://doi.org/10.3390/agronomy11071364
APA StyleKatsenios, N., Christopoulos, M. V., Kakabouki, I., Vlachakis, D., Kavvadias, V., & Efthimiadou, A. (2021). Effect of Pulsed Electromagnetic Field on Growth, Physiology and Postharvest Quality of Kale (Brassica oleracea), Wheat (Triticum durum) and Spinach (Spinacia oleracea) Microgreens. Agronomy, 11(7), 1364. https://doi.org/10.3390/agronomy11071364










