Effect of Clay, Soil Organic Matter, and Soil pH on Initial and Residual Weed Control with Flumioxazin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flumioxazin Initial Control
2.2. Preparation of Soils
2.3. Flumioxazin Residual Control
2.4. Organic Matter Soils
2.5. pH Soils
3. Data Collection and Analysis
4. Results and Discussion
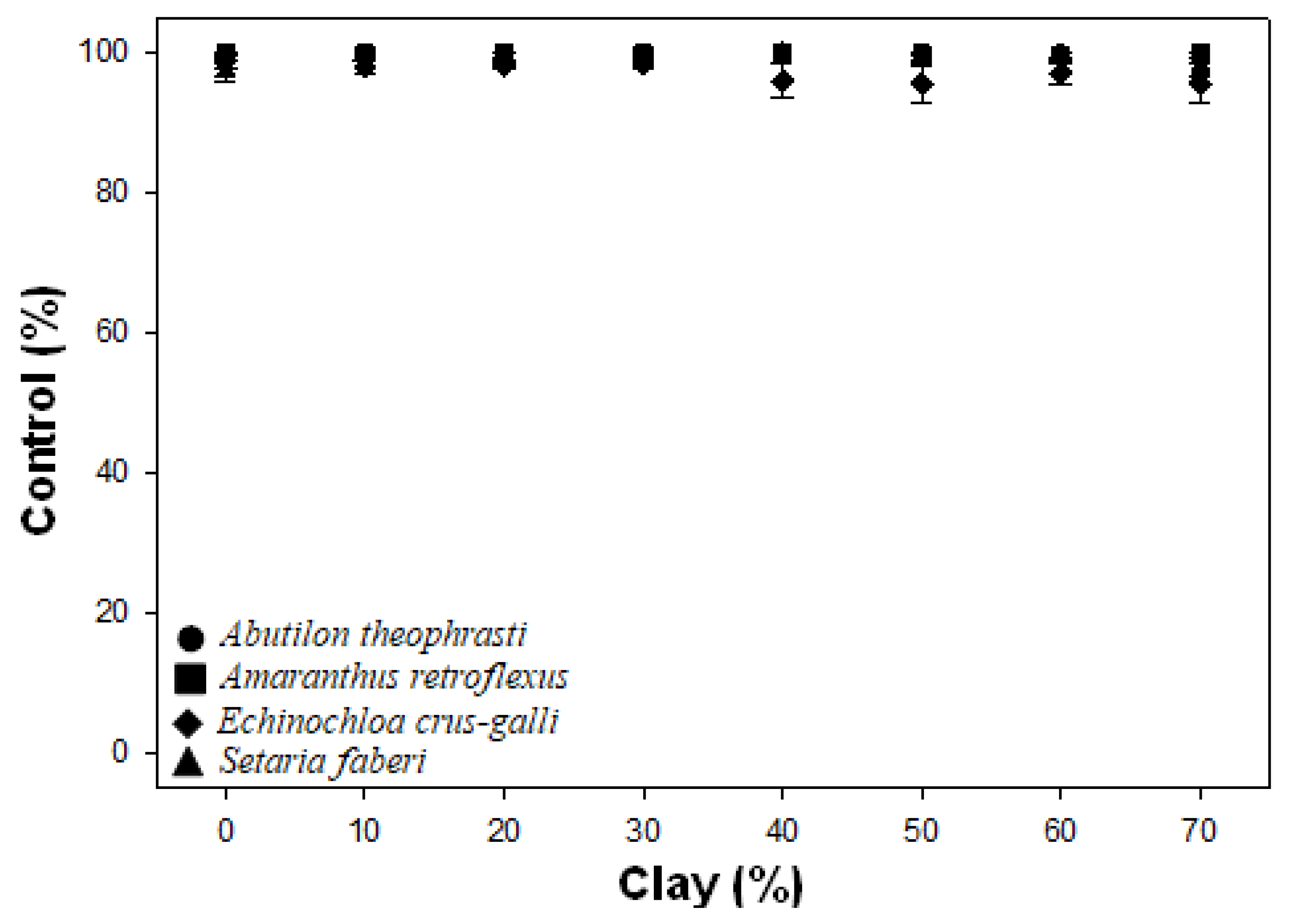
4.1. Flumioxazin Initial Control
4.2. Flumioxazin Residual Control
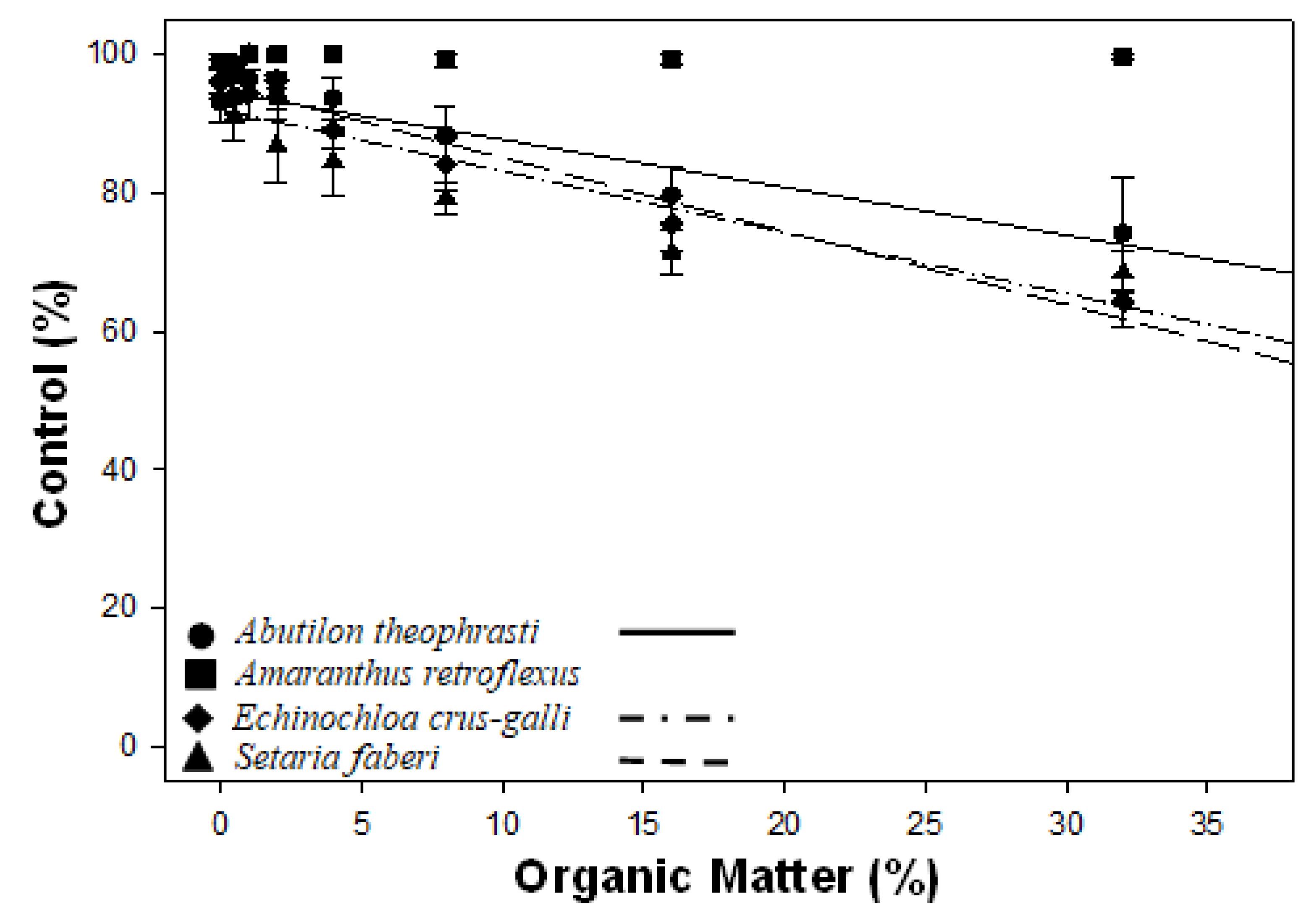
4.3. Organic Soils
4.4. Soil pH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrell, J.A.; Vencill, W.K.; Xia, K.; Grey, T.L. Sorption and desorption of flumioxazin to soil, clay minerals and ion-exchange resin. Pest Manag. Sci. 2005, 61, 40–46. [Google Scholar] [CrossRef]
- Wauchope, D.R.; Yeh, S.; Linders, J.B.; Kloskowski, R.; Tanka, K.; Rubin, B.; Katayama, A.; Kördel, W.; Gerstl, Z.; Lane, M.; et al. Pesticide soil sorption parameters: Theory, measurement, uses, limitations and reliability. Pest Manag. Sci. 2002, 58, 419–445. [Google Scholar] [CrossRef]
- Peter, C.J.; Weber, J.B. Adsorption, mobility, and efficacy of alachlor and metolachlor as influenced by soil properties. Weed Sci. 1985, 33, 874–881. [Google Scholar] [CrossRef]
- Walker, A. Activity and Selectivity in the field. In Interactions between Herbicides and the Soil; Hance, R.J., Ed.; Academic Press: New York, NY, USA, 1980; pp. 203–222. [Google Scholar]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science Principles and Practices, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 127–145. [Google Scholar]
- Weber, J.B.; Warren, R.L.; Swain, L.R.; Yelverton, F.H. Physicochemical property effects of three herbicides and three soils on herbicide mobility in field lysimeters. Crop. Prot. 2007, 26, 299–311. [Google Scholar] [CrossRef]
- Benoit, P.; Madrigal, I.; Preston, C.M.; Chenu, C.; Barriuso, E. Sorption and desorption of non-ionic herbicides onto particulate organic matter from the surface soils under different land uses. Eur. J. Soil Sci. 2008, 59, 178–189. [Google Scholar] [CrossRef]
- Walker, A.; Austin, C.R. Effect of recent cropping history and herbicide use on degradation rates of isoproturon in soils. Weed Res. 2003, 44, 5–11. [Google Scholar] [CrossRef]
- Niekamp, J.W.; Johnson, W.G. Weed management with sulfentrazone and flumioxazin in no-tillage soyabean (Glycine max). Crop Prot. 2001, 20, 215–220. [Google Scholar] [CrossRef]
- Wilson, D.E.; Nissen, S.J.; Thompson, A. Potato (Solanum tuberosum) variety and weed response to sulfentrazone and flumioxazin. Weed Technol. 2002, 16, 567–574. [Google Scholar] [CrossRef]
- Clewis, S.B.; Everman, W.J.; Jordan, D.L.; Wilcut, J.W. Weed management in North Carolina peanuts (Arachis hyogaea) with S-metoalchlor, diclosulam, flumioxazin, and sulfentrazone systems. Weed Technol. 2007, 21, 629–635. [Google Scholar] [CrossRef]
- Everman, W.J.; Clewis, S.B.; York, A.C.; Wilcut, J.W. Weed control and yield with flumioxazin, fomesafen, and S-metolachlor systems for glufosinate-resistant cotton residual weed wanagement. Weed Technol. 2009, 23, 391–397. [Google Scholar] [CrossRef]
- Hausman, N.E.; Tranel, P.J.; Riechers, D.E.; Maxwell, D.J.; Gonzini, L.C.; Hager, A.G. Responses of an HPPD inhibitor-resistant waterhemp (Amaranthus tuberculatus) population to soil-residual herbicides. Weed Technol. 2013, 27, 704–711. [Google Scholar] [CrossRef]
- Westhoven, A.M.; Stachler, J.M.; Loux, M.M.; Johnson, W.G. Management of glyphosate-tolerant common lambsquarters (Chenopodium album) in glyphosate-resistant soybean. Weed Technol. 2008, 22, 628–634. [Google Scholar] [CrossRef]
- Grey, T.L.; Wehtje, G.R. Residual herbicide weed control systems in peanut. Weed Technol. 2005, 19, 560–567. [Google Scholar] [CrossRef]
- Mahoney, K.J.; Shropshire, C.; Sikkema, P.H. Weed management in conventional-and no-till soybean using flumioxazin/pyroxasulfone. Weed Technol. 2014, 28, 298–306. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; McClelland, M.; Griffith, G.M. Conyza canadensis (L.) cronquist response to pre-plant application of residual herbicides in cotton (Gossypium hirsutum L.). Crop Prot. 2009, 28, 62–67. [Google Scholar] [CrossRef]
- Sebastian, D.J.; Nissen, S.J.; Westra, P.; Shaner, D.L.; Butters, G. Influence of soil properties and soil moisture on the efficacy of indaziflam and flumioxazin on Kochia scoparia L. Pest Manag. Sci. 2016, 73, 444–451. [Google Scholar] [CrossRef]
- Taylor-Lovell, S.; Wax, L.M.; Bollero, G. Preemergence flumioxazin and pendimethalin and postemergence herbicide systems for soybean (Glycine max). Weed Technol. 2002, 16, 502–511. [Google Scholar] [CrossRef]
- Calvet, R. Adsorption-desorption phenomena. In Interactions between Herbicides and the Soil; Hance, R.J., Ed.; Academic Press: New York, NY, USA, 1980; pp. 1–30. [Google Scholar]
- Koskinen, W.C.; Moorman, T.B. Effect of soil properties and processes on herbicide performance. In Weeds of Cotton Characterization and Control; McWhorter, C.G., Abernathy, J.R., Eds.; The Cotton Foundation: Memphis, TN, USA, 1992; pp. 365–402. [Google Scholar]
- Kah, M.; Brown, C.D. Absorption of ionisable pesticides in soils. Rev. Environ. Contam Toxicol. 2006, 188, 149–217. [Google Scholar]
- Sposito, G.; Skipper, N.T.; Sutton, R.; Park, S.; Soper, A.K.; Greathouse, J.A. Surface geochemistry of clay minerals. Proc. Natl. Acad. Sci. USA 1999, 96, 3358–3364. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.A.; Nascimento, O.R.; Martin-Neto, L. Hydrophobic interactions between spin-label 5-sasl and humic acid as revealed by ESR spectroscopy. Environ. Sci. Technol. 2001, 35, 761–765. [Google Scholar] [CrossRef]
- Alister, C.S.; Gómez, R.P.; Kogan, M. Dissipation and movement of flumioxazin in soil at four field sites in Chile. Pest Manag. Sci. 2008, 64, 579–583. [Google Scholar] [CrossRef]
- Boyd, N.S.; Sharpe, S.M.; Ramdas, K. Flumioxazin soil persistence under plastic mulch and effects of pretransplant applications on strawberry. Weed Technol 2021, 35, 319–323. [Google Scholar] [CrossRef]
- Price, A.J.; Koger, C.H.; Wilcut, J.W.; Miller, D.; Santen, E.V. Efficacy of residual and non-residual herbicides used in cotton production systems when applied with glyphosate, glufosinate, or MSMA. Weed Technol. 2008, 22, 459–466. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Schutte, B.J.; Lehnhoff, E.A.; Beck, L.L. Evaluation of POST-directed applications of flumioxazin in New Mexico chile pepper. Weed Technol. 2018, 33, 135–141. [Google Scholar] [CrossRef]
- Mueller-Warrant, G.W. Duration of control from preemergence herbicides for use in nonburned grass seed crops. Weed Technol. 1999, 13, 439–449. [Google Scholar] [CrossRef]
- Torrents, A.; Jayasundera, S. The sorption of non-ionic pesticides onto clay and the influence of natural organic carbon. Chemosphere 1997, 7, 1549–1565. [Google Scholar] [CrossRef]
- Ferrell, J.A.; Vencill, W.K. Flumioxazin soil persistence and mineralization in laboratory experiments. J. Agric. Food Chem. 2003, 51, 4719–4721. [Google Scholar] [CrossRef]
- André, C.L.; Rahe, J.E. Herbicide interactions with fungal root pathogens, with special reference to glyphosate. Annu. Rev. Phytopathol. 1992, 30, 579–602. [Google Scholar]
- Anderson, T.H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1992, 25, 393–395. [Google Scholar] [CrossRef]
- Corbin, F.T.; Upchurch, R.P. Influence of pH on detoxication of herbicides in soil. Weeds 1967, 15, 370–377. [Google Scholar] [CrossRef]
- Torstensson, L. Role of microorganisms in decomposition. In Interactions between Herbicides and the Soil; Hance, R.J., Ed.; Academic Press: New York, NY, USA, 1980; pp. 159–178. [Google Scholar]
- Kwon, J.; Armbrust, K.L.; Grey, T.L. Hydrolysis and photolysis of flumioxazin in aqueous buffer solutions. Pest Manag. Sci. 2004, 60, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.D.; Phillips, R.E. Diffusion of herbicides to seed. Weed Sci. 1971, 19, 128–132. [Google Scholar] [CrossRef]



| Soil | Sand | Silt | Clay | SOM | pH | CEC |
|---|---|---|---|---|---|---|
| Sand | 97.7 | 0.07 | 2.1 | 0.1 | 10 | 0.6 |
| 10% Clay | 88.2 | 0.4 | 11.2 | 0.2 | 9.3 | 1.5 |
| 20% Clay | 78.8 | 0.6 | 20.4 | 0.2 | 9 | 2.9 |
| 30% Clay | 66.5 | 2.6 | 30.7 | 0.2 | 8.8 | 3.7 |
| 40% Clay | 56.2 | 3.4 | 40.2 | 0.2 | 8.7 | 4.9 |
| 50% Clay | 43.7 | 5.4 | 50.6 | 0.3 | 8.7 | 5.4 |
| 60% Clay | 29.4 | 9.6 | 60.7 | 0.3 | 8.4 | 6.4 |
| 70% Clay | 18.8 | 9.6 | 71.1 | 0.5 | 8.3 | 6.9 |
| 0.5% SOM | 93.3 | 0.7 | 5.4 | 0.6 | 9.1 | 2.8 |
| 1% SOM | 92.7 | 0.7 | 5.4 | 1.2 | 8.8 | 3 |
| 2% SOM | 91.7 | 0.7 | 5.4 | 2.2 | 7.8 | 17.1 |
| 3% SOM | 90.9 | 0.7 | 5.4 | 3 | 7.8 | 24.2 |
| 4% SOM | 89.8 | 0.7 | 5.4 | 4.1 | 7.3 | 30 |
| 8% SOM | 85 | 1.7 | 5.4 | 7.9 | 7.1 | 35 |
| 16% SOM | 77.4 | 1.4 | 5.4 | 15.8 | 6.9 | 75.8 |
| 32% SOM | 62 | 0.9 | 5.4 | 31.7 | 6.6 | 121.6 |
| pH 4 | 68.4 | 10.8 | 16.8 | 4 | 4.07 | 6.6 |
| pH 5 | 69.8 | 11 | 15.4 | 3.8 | 4.93 | 7.2 |
| pH 6 | 68.2 | 12.6 | 14.3 | 4.9 | 6.07 | 5.5 |
| pH 7 | 70.1 | 10.4 | 16.8 | 2.7 | 7.07 | 7.1 |
| Soil | Species | Emergence | Model b | r2 |
|---|---|---|---|---|
| Clay | ||||
| ABUTH | 26 | NS | NS | |
| AMARE | 29 | NS | NS | |
| ECHCG | 88 | NS | NS | |
| SETFA | 18 | NS | NS | |
| SOM | ||||
| ABUTH | 41 | y = 94.64 − 0.69b | 0.31 | |
| AMARE | 70 | NS | NS | |
| ECHCG | 86 | y = 95.69 − 1.06b | 0.66 | |
| SETFA | 28 | y = 92.03 − 0.89b | 0.48 | |
| pH | ||||
| ABUTH | 32 | y = 139.24 (256.33/b) | 0.78 | |
| AMARE | 22 | NS | NS | |
| ECHCG | 65 | NS | NS | |
| SETFA | 20 | y = 126.43 − (200.38/b) | 0.53 |
| Soil | Sand | Silt | Clay | pH | SOM | CEC |
|---|---|---|---|---|---|---|
| Lab Soil pH 5 | 50.4 | 31 | 16.8 | 5.1 | 2.8 | 21 |
| Field Soil pH 5 | 49.8 | 36.8 | 16.8 | 4.9 | 2.3 | 6.6 |
| Field Soil pH 6 | 37.7 | 33.0 | 23.5 | 6 | 3.2 | 19.6 |
| Lab Soil pH 7 | 48.2 | 29.4 | 20.8 | 7 | 3 | 19.7 |
| Field Soil pH 7 | 40 | 29.6 | 27.8 | 7.1 | 2.6 | 12 |
| Lab Soil 0% SOM | 97.7 | 0.1 | 2.1 | 10 | 0.1 | 0.6 |
| Lab Soil 1% SOM | 92.7 | 0.7 | 5.4 | 8 | 1.2 | 3 |
| Lab Soil 3% SOM | 90.9 | 0.7 | 5.4 | 7.6 | 3 | 24.2 |
| Field Soil 3% SOM | 37.7 | 33 | 23.5 | 6 | 3.2 | 19.6 |
| Organic Soil (Muck) | 7.3 | 9.2 | 1.8 | 6.4 | 81.7 | 142.3 |
| 9 | Species | Emer | I50 | Model b | r2 |
|---|---|---|---|---|---|
| LS 0% SOM | |||||
| ABUTH | 36.7 | 5.7 | y = 100/1 + (x/5.67)9.85 | 0.98 | |
| AMARE | 39.7 | NS | NS | NS | |
| CHEAL | 33.5 | NS | NS | NS | |
| SETFA | 41.6 | 6.3 | y = 96.48/1 + (x/6.29)10.36 | 0.98 | |
| LS 1% SOM | |||||
| ABUTH | 37.5 | 5.4 | y = 96.86/1 + (x/5.51)4.29 | 0.95 | |
| AMARE | 38.9 | NS | NS | NS | |
| CHEAL | 35.5 | NS | NS | NS | |
| SETFA | 40.4 | 4.6 | y = 97.13 − 10.22x | 0.90 | |
| LS 3% SOM | |||||
| ABUTH | 38.9 | 4.4 | y = 93.63 − 10x | 0.97 | |
| AMARE | 38.3 | 11.6 | y = 100/1 + (x/11.57)3.63 | 0.72 | |
| CHEAL | 35.5 | 13 | y = 100/1 + (x/13.04)2.88 | 0.92 | |
| SETFA | 40.6 | 3.5 | y = 90.7 − 11.65x | ||
| FS 3% SOM | |||||
| ABUTH | 34.5 | 2.9 | y = 97.76/1 + (x/2.92)1.82 | 0.81 | |
| AMARE | 32.5 | NS | NS | NS | |
| CHEAL | 32.6 | NS | NS | NS | |
| SETFA | 38.5 | 2.8 | y = 92.1/1 + (x/3.03)2.4 | 0.94 | |
| Organic Soil | |||||
| ABUTH | 35 | 1.9 | y = 77.42/1 + (x/2.44)2.6 | 0.95 | |
| AMARE | 38.9 | 6.9 | y = 100/1 + (x/6.92)9.28 | 0.97 | |
| CHEAL | 34.8 | 7.3 | y = 100/1 + (x/7.27)7.29 | 0.98 | |
| SETFA | 37.5 | 1.7 | y = 80.09/1 + (x/1.99)3.12 | 0.93 |
| Scheme 50. | Species | Emer | I50 | Model b | r2 |
|---|---|---|---|---|---|
| LS pH 5 | |||||
| ABUTH | 34.9 | 4.49 | y = 94.37/1 + (x/4.53)14.76 | 0.95 | |
| AMARE | 33.1 | NS | NS | NS | |
| CHEAL | 32.6 | NS | NS | NS | |
| SETFA | 41.8 | 5.60 | y = 93.55/1 + (x/5.74)5.58 | 0.9 | |
| FS pH 5 | |||||
| ABUTH | 34.1 | 4.49 | y = 93.98/1 + (x/4.53)14.63 | 0.98 | |
| AMARE | 34.2 | NS | NS | NS | |
| CHEAL | 32 | NS | NS | NS | |
| SETFA | 39 | 5.03 | y = 95.68/1 + (x/5.11)5.55 | 0.96 | |
| FS pH 6 | |||||
| ABUTH | 34.5 | 2.85 | y = 97.76/1 + (x/2.92)1.82 | 0.81 | |
| AMARE | 32.5 | NS | NS | NS | |
| CHEAL | 32.6 | NS | NS | NS | |
| SETFA | 38.5 | 2.82 | y = 92.1/1 + (x/3.03)2.4 | 0.94 | |
| LS pH 7 | |||||
| ABUTH | 35.8 | 3.25 | y = 97.67/1 + (x/3.27)9.26 | 0.97 | |
| AMARE | 34 | 5.86 | y = 100/1 + (x/5.86)5.94 | 0.95 | |
| CHEAL | 34.3 | 5.93 | y = 100/1 + (x/5.93)6.69 | 0.97 | |
| SETFA | 42.9 | 3.83 | y = 90.63 − 10.61x | 0.96 | |
| FS pH 7 | |||||
| ABUTH | 35.2 | 2.78 | y = 99.13/1 + (x/2.79)3.31 | 0.98 | |
| AMARE | 32 | 5.64 | y = 100/1 + (x/5.64)5.71 | 0.88 | |
| CHEAL | 31.8 | 5.75 | y = 100/1 + (x/5.75)5.24 | 0.87 | |
| SETFA | 38 | 2.37 | y = 92.98/1 + (x/2.62)1.53 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glaspie, C.F.; Jones, E.A.L.; Penner, D.; Pawlak, J.A.; Everman, W.J. Effect of Clay, Soil Organic Matter, and Soil pH on Initial and Residual Weed Control with Flumioxazin. Agronomy 2021, 11, 1326. https://doi.org/10.3390/agronomy11071326
Glaspie CF, Jones EAL, Penner D, Pawlak JA, Everman WJ. Effect of Clay, Soil Organic Matter, and Soil pH on Initial and Residual Weed Control with Flumioxazin. Agronomy. 2021; 11(7):1326. https://doi.org/10.3390/agronomy11071326
Chicago/Turabian StyleGlaspie, Calvin F., Eric A. L. Jones, Donald Penner, John A. Pawlak, and Wesley J. Everman. 2021. "Effect of Clay, Soil Organic Matter, and Soil pH on Initial and Residual Weed Control with Flumioxazin" Agronomy 11, no. 7: 1326. https://doi.org/10.3390/agronomy11071326
APA StyleGlaspie, C. F., Jones, E. A. L., Penner, D., Pawlak, J. A., & Everman, W. J. (2021). Effect of Clay, Soil Organic Matter, and Soil pH on Initial and Residual Weed Control with Flumioxazin. Agronomy, 11(7), 1326. https://doi.org/10.3390/agronomy11071326





