Fungicide Application Affects Nitrogen Utilization Efficiency, Grain Yield, and Quality of Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design and Treatment
2.2. Soil Sampling and Fertilization
2.3. Phenotypic Evaluation
2.4. Statistical Analyses of the Evaluated Traits
2.5. Effects of Nitrogen and Fungicide on the Evaluated Traits
2.6. Estimation of the Breeding Progress Using the Wheat Diversity Panel
2.7. GY Performance and Stability Analysis
2.8. Cropping System Performance and Discriminating Level
3. Results
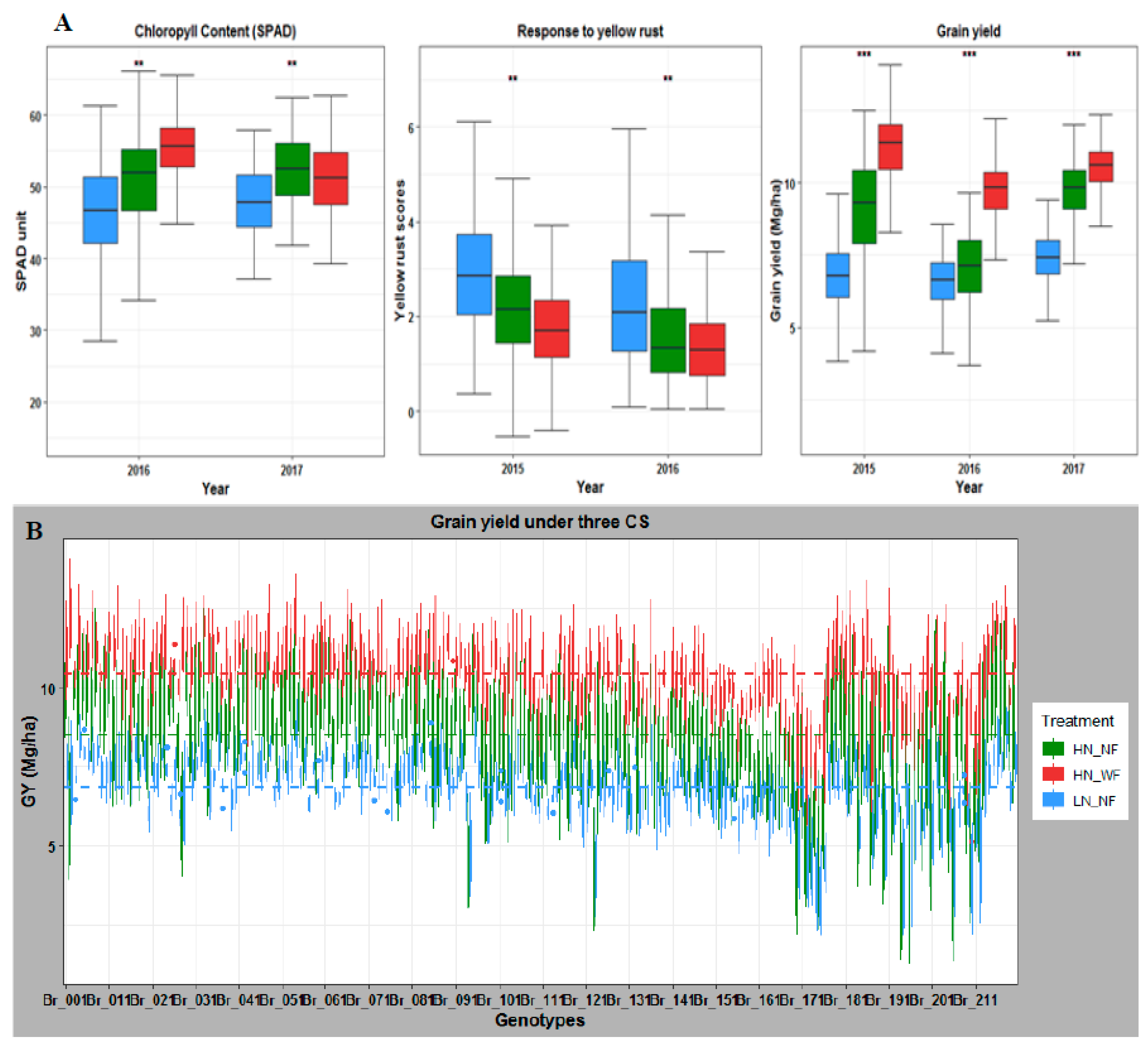
3.1. Nitrogen and Fungicide Application Effect on Phenotypic Traits
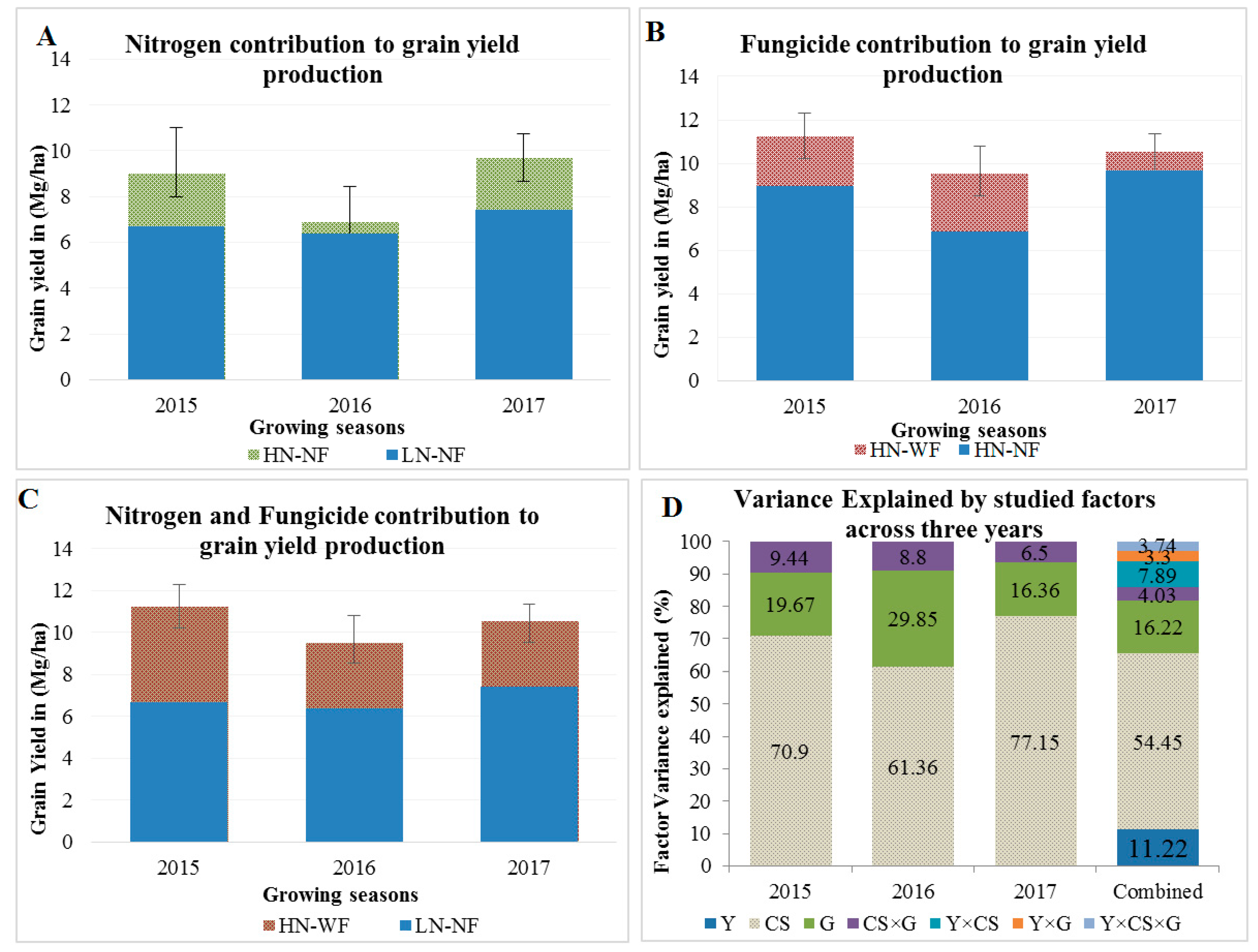
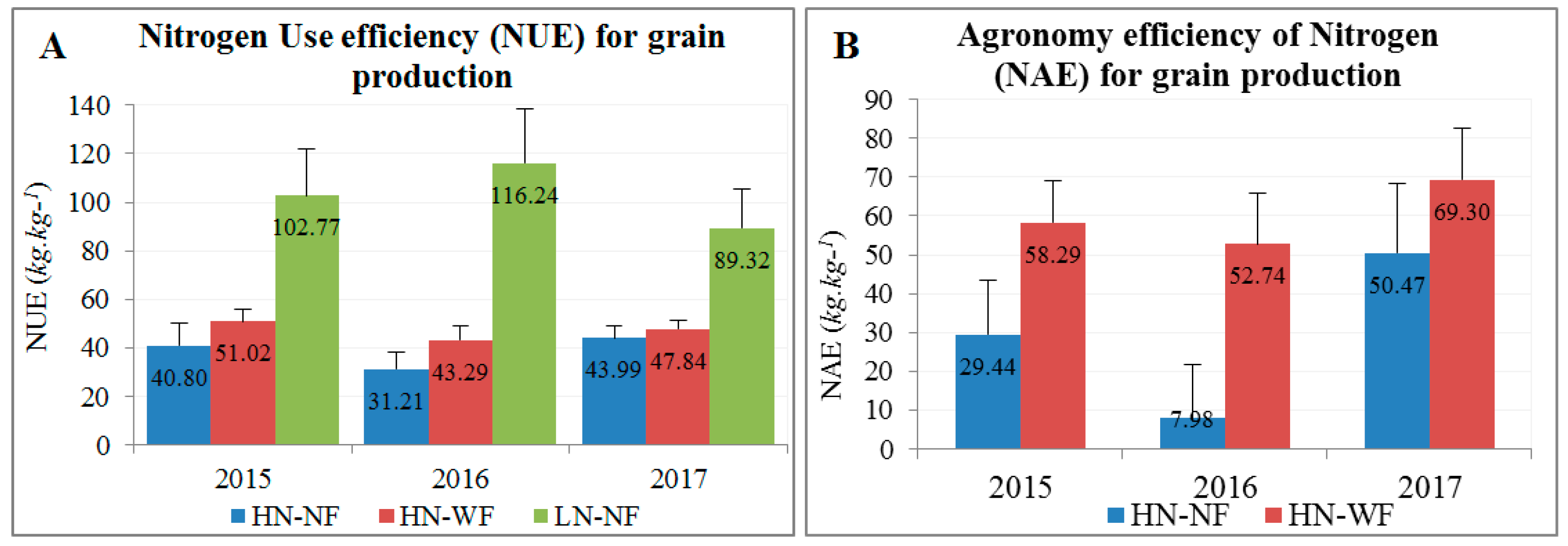
3.2. Application of Nitrogen and Fungicide Improve GY and Grain N Yield (GNY)
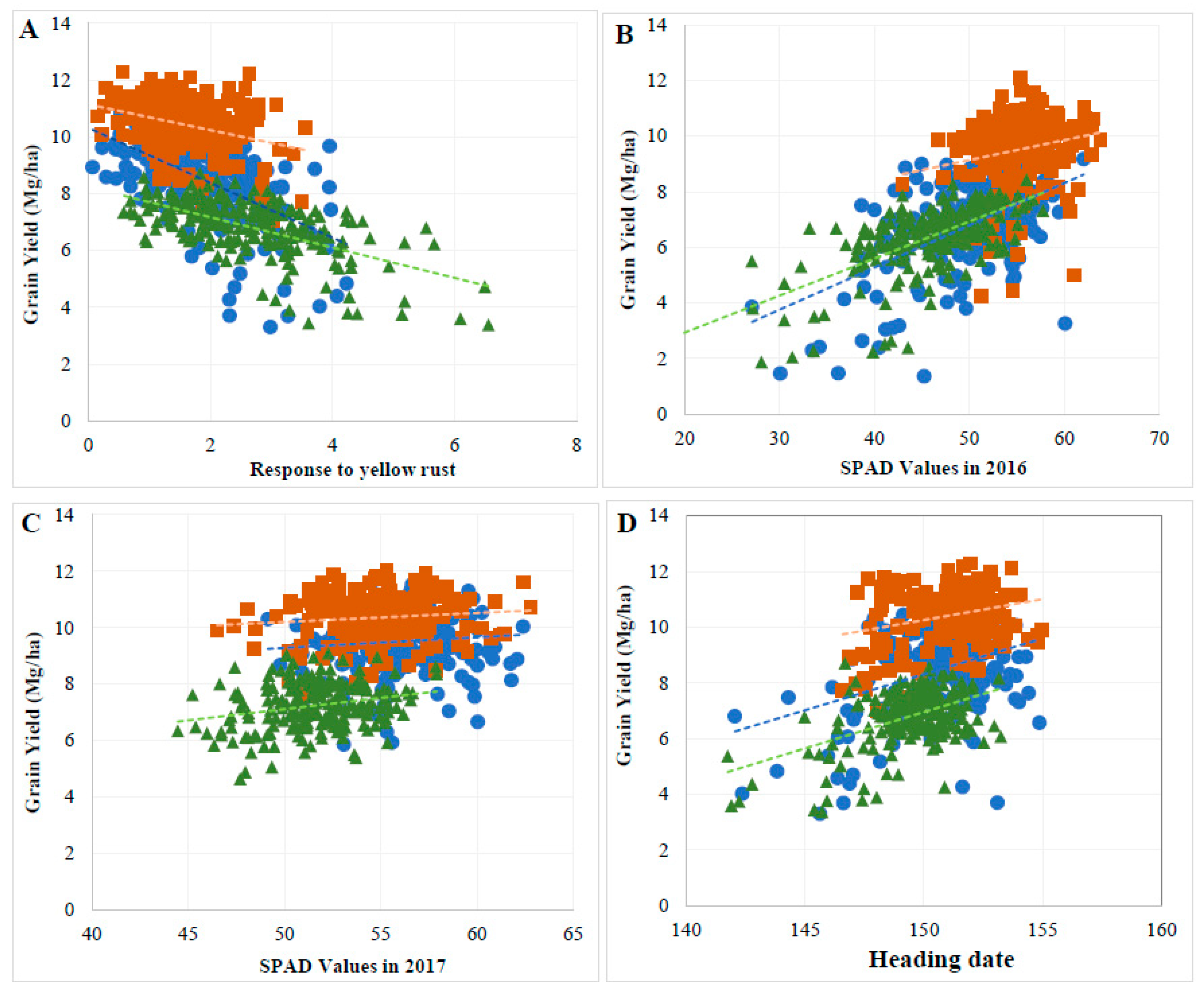
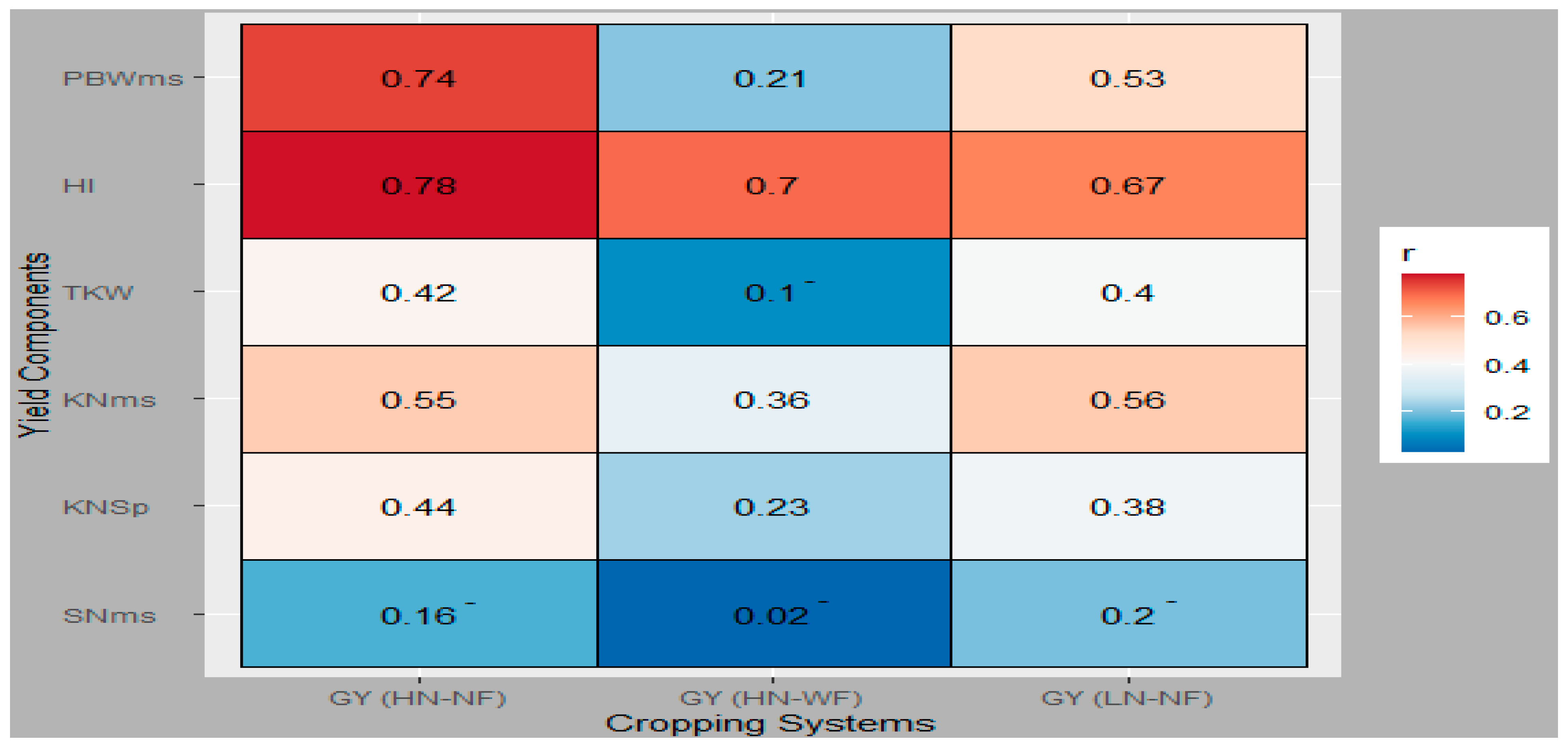
3.3. Genetic Relationship between GY and Evaluated Traits
3.4. GY Was Significantly Affected by Indirect Effects of Several Agronomic Traits
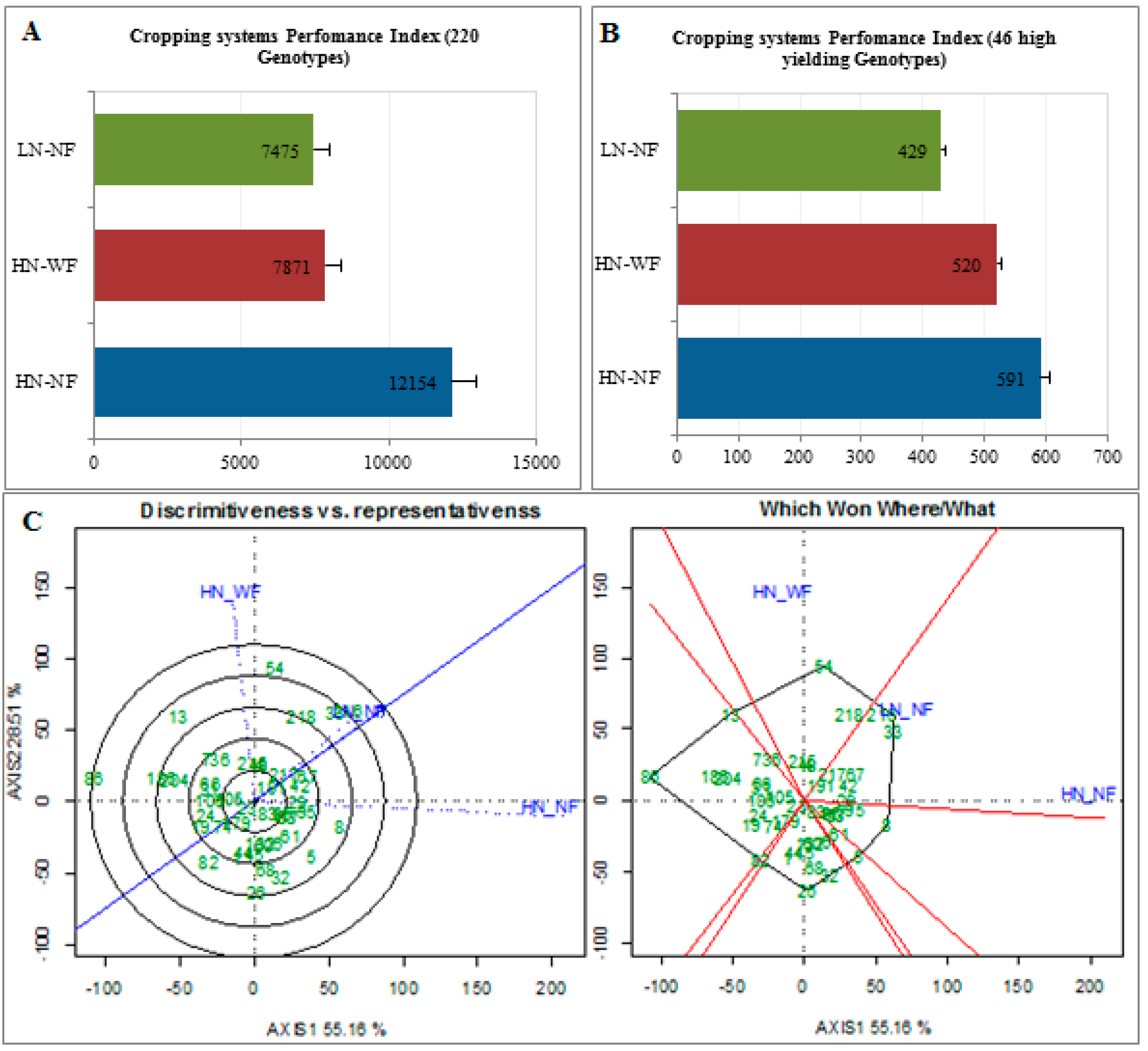
3.5. Few Cultivars Achieved Maximum Yield under HN-NF, the Most Discriminating CS
3.6. High Yielding Cultivars Showed Different Stability Level under the Three Cropping Systems
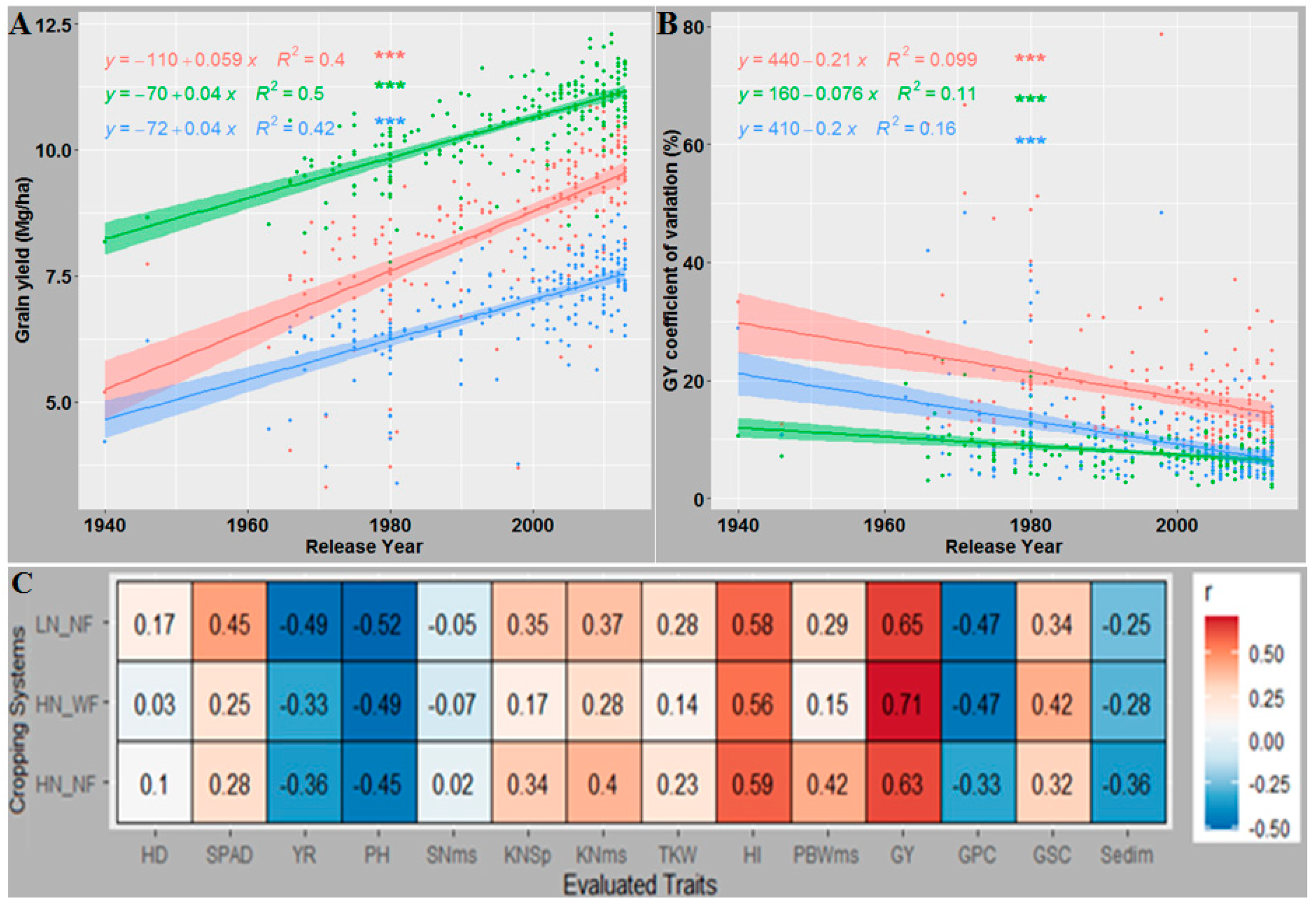
3.7. Newer Released Cultivars Outperformed Older Cultivars in Most Traits and GY under All CS
4. Discussion
4.1. Variation in Agronomic Traits and GY under the Effect of Nitrogen and Fungicide
4.2. Nitrogen and Fungicide Application Effects on N Flow from Soil to the Grain
4.3. Agronomic Traits Contribution to GY across Cropping Systems
4.4. Breeding Progress in Agronomic Traits and Yield Stability across Cropping Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyiga, B.C.; Ogbonnaya, F.C.; Sharma, R.C.; Baum, M.; Léon, J.; Ballvora, A. Genetic and Transcriptional Variations in NRAMP-2 and OPAQUE1 Genes Are Associated with Salt Stress Response in Wheat. Theor. Appl. Genet. 2019, 132, 323–346. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Liu, W.; Li, S.; Zhang, P.; Wang, C.; Lu, H.; Wang, L.; Xie, Y.; Ma, D.; Kang, G. Determining the Optimal N Input to Improve Grain Yield and Quality in Winter Wheat With Reduced Apparent N Loss in the North China Plain. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory, P.J. Crop Root Systems and Nutrient Uptake from Soils. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; John Wiley and Sons Inc.: Chichester, UK, 2011; pp. 21–45. [Google Scholar]
- Barraclough, P.B.; Lopez-Bellido, R.; Hawkesford, M.J. Genotypic Variation in the Uptake, Partitioning and Remobilisation of Nitrogen during Grain-Filling in Wheat. Field Crops Res. 2014, 156, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Kovacs, M.I.; Fowler, D.; Holley, R. Effects of Protein Content and Composition on White Noodle Making Quality: Color. Cereal Chem. 2004, 81, 777–784. [Google Scholar] [CrossRef]
- Yadav, S.N.; Peterson, W.; Easter, K.W. Do Farmers Overuse Nitrogen Fertilizer to the Detriment of the Environment? Environ. Resour. Econ. 1997, 9, 323–340. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011, 9, e1001124. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Genetic Variation in Traits for Nitrogen Use Efficiency in Wheat. J. Exp. Bot. 2017, 68, 2627–2632. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Y.; Ting, L. Top-Grain Filling Characteristics at an Early Stage of Maize (Zea mays L.) with Different Nitrogen Use Efficiencies. J. Integr. Agric. 2017, 16, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Buckwell, A.; Nadeu, E. Nutrient Recovery and Reuse (NRR) in European Agriculture: A Review of the Issues, Opportunities, and Actions; RISE Foundation: Brussels, Belgium, 2016. [Google Scholar]
- Hailu, D.; Fininsa, C. Epidemics of Stripe Rust (Puccinia Striiformis) on Common Wheat (Triticum Aestivum) in the Highlands of Bale, Southeastern Ethiopia. Crop Prot. 2007, 26, 1209–1218. [Google Scholar] [CrossRef]
- Danial, D.; Parlevliet, J. Effects of Nitrogen Fertilization on Disease Severity and Infection Type of Yellow Rust on Wheat Genotypes Varying in Quantitative Resistance. J. Phytopathol. 1995, 143, 679–681. [Google Scholar] [CrossRef]
- Bryson, R.; Paveley, N.; Clark, W.; Sylvester-Bradley, R.; Scott, R. Use of in-field measurements of green leaf area and incident radiation to estimate the effects of yellow rust epidemics on the yield of winter wheat. In Developments in Crop Science; Elsevier: Amsterdam, The Netherlands, 1997; Volume 25, pp. 77–86. ISBN 0378-519X. [Google Scholar]
- Carlton, R.R.; West, J.S.; Smith, P.; Fitt, B.D. A Comparison of GHG Emissions from UK Field Crop Production under Selected Arable Systems with Reference to Disease Control. Eur. J. Plant Pathol. 2012, 133, 333–351. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, J.R.; Barzman, M.; Booij, K.; Boonekamp, P.; Desneux, N.; Huber, L.; Kudsk, P.; Langrell, S.R.H.; Ratnadass, A.; Ricci, P.; et al. Robust Cropping Systems to Tackle Pests under Climate Change. A Review. Agron. Sustain. Dev. 2015, 35, 443–459. [Google Scholar] [CrossRef]
- Voss-Fels, K.P.; Stahl, A.; Wittkop, B.; Lichthardt, C.; Nagler, S.; Rose, T.; Chen, T.-W.; Zetzsche, H.; Seddig, S.; Baig, M.M. Breeding Improves Wheat Productivity under Contrasting Agrochemical Input Levels. Nat. Plants 2019, 5, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kahiluoto, H.; Kaseva, J.; Balek, J.; Olesen, J.E.; Ruiz-Ramos, M.; Gobin, A.; Kersebaum, K.C.; Takáč, J.; Ruget, F.; Ferrise, R. Decline in Climate Resilience of European Wheat. Proc. Natl. Acad. Sci. USA 2019, 116, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Jackson, M. Soil Chemical Analysis; Englewood Cliffs Inc.: Prentice Hall, NJ, USA, 1958; Volume 498, pp. 183–204. [Google Scholar]
- Apha. Standard Methods for the Examination of Water and Wastewater, 16th ed.; American Public Health Association: Washington, DC, USA, 1985. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; van den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Pask, A.; Pietragalla, J.; Mullan, D.; Reynolds, M. Physiological Breeding II: A Field Guide to Wheat Phenotyping; Cimmyt: Mexio city, Mexico, 2012; ISBN 970-648-182-6. [Google Scholar]
- Sheskin, D.J. Handbook of Parametric and Nonparametric Statistical Procedures; CRC Press: Washington, DC, USA, 2020; ISBN 1-00-008327-6. [Google Scholar]
- SAS Institute. Base SAS 9.4 Procedures Guide; SAS Institute: Cary, NC, USA, 2015; ISBN 1-62960-077-6. [Google Scholar]
- Piepho, H.-P.; Möhring, J. Computing Heritability and Selection Response from Unbalanced Plant Breeding Trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Foundation: Vienna, Austria, 2017. [Google Scholar]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Lichthardt, C.; Chen, T.-W.; Stahl, A.; Stützel, H. Co-Evolution of Sink and Source in the Recent Breeding History of Winter Wheat in Germany. Front. Plant Sci. 2020, 10, 1771. [Google Scholar] [CrossRef]
- Lin, C.; Binns, M. A Method of Analyzing Cultivar × Location × Year Experiments: A New Stability Parameter. Theor. Appl. Genet. 1988, 76, 425–430. [Google Scholar] [CrossRef]
- Pacheco, Á.; Vargas, M.; Alvarado, G.; Rodríguez, F.; Crossa, J.; Burgueño, J. GEA-R (Genotype × Environment Analysis with R for Windows) Version 4.1-, Hdl: 11529/10203; CIMMYT Research Data Software Repository Networks: Mexio city, Mexico, 2015; Volume 16. [Google Scholar]
- Francis, T.; Kannenberg, L. Yield Stability Studies in Short-Season Maize. I. A Descriptive Method for Grouping Genotypes. Can. J. Plant Sci. 1978, 58, 1029–1034. [Google Scholar] [CrossRef]
- Litke, L.; Gaile, Z.; Ruža, A. Effect of Nitrogen Fertilization on Winter Wheat Yield and Yield Quality. Agron. Res. 2018, 16, 500–509. [Google Scholar]
- Gooding, M.J.; Davies, W.P. Wheat Production and Utilization: Systems, Quality and the Environment; CAB International: Wallingford, England, 1997; ISBN 0-85199-155-6. [Google Scholar]
- Gooding, M.; Gregory, P.; Ford, K.; Pepler, S. Fungicide and Cultivar Affect Post-Anthesis Patterns of Nitrogen Uptake, Remobilization and Utilization Efficiency in Wheat. J. Agric. Sci. 2005, 143, 503–518. [Google Scholar] [CrossRef]
- Royo, C.; Alvaro, F.; Martos, V.; Ramdani, A.; Isidro, J.; Villegas, D.; Del Moral, L.F.G. Genetic Changes in Durum Wheat Yield Components and Associated Traits in Italian and Spanish Varieties during the 20th Century. Euphytica 2007, 155, 259–270. [Google Scholar] [CrossRef]
- FAO The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organisation: Rome, Italy, 2017; ISBN 9789251095515.
- Te Beest, D.; Paveley, N.; Shaw, M.; Van Den Bosch, F. Disease–Weather Relationships for Powdery Mildew and Yellow Rust on Winter Wheat. Phytopathology 2008, 98, 609–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, W.E.; Brandt, S.; Hutt-Taylor, K. Response of Oat Grain Yield and Quality to Nitrogen Fertilizer and Fungicides. Agron. J. 2020, 112, 1021–1034. [Google Scholar] [CrossRef] [Green Version]
- Neumann, S.; Paveley, N.; Beed, F.D.; Sylvester-Bradley, R. Nitrogen per Unit Leaf Area Affects the Upper Asymptote of Puccinia Striiformis f. Sp. Tritici Epidemics in Winter Wheat. Plant Pathol. 2004, 53, 725–732. [Google Scholar] [CrossRef]
- Tripathi, S.; Sayre, K.; Kaul, J.; Narang, R. Lodging Behavior and Yield Potential of Spring Wheat (Triticum Aestivum L.): Effects of Ethephon and Genotypes. Field Crops Res. 2004, 87, 207–220. [Google Scholar] [CrossRef]
- Guttieri, M.J.; Stephen Baenziger, P.; Frels, K.; Carver, B.; Arnall, B.; Waters, B.M. Variation for Grain Mineral Concentration in a Diversity Panel of Current and Historical Great Plains Hard Winter Wheat Germplasm. Crop Sci. 2015, 55, 1035–1052. [Google Scholar] [CrossRef]
- Tucker, M. Primary Nutrients and Plant Growth. Essent. Plant Nutr. 2004, 126. [Google Scholar]
- Bojović, B.; Marković, A. Correlation between Nitrogen and Chlorophyll Content in Wheat (Triticum aestivum L.). Kragujev. J. Sci. 2009, 31, 69–74. [Google Scholar]
- Gregory, P.J.; Gooding, M.; Ford, K.; Clarke, M.P.; Pepler, S. Managing Roots, Nitrogen and Fungicides to Improve Yield and Quality of Wheat. HGCA Proj. Rep. 2005, 359. [Google Scholar]
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.; Tirol-Padre, A.; Reddy, C.; Cassman, K.; Verma, S.; Powlson, D.; Van Kessel, C.; De Richter, D.; Chakraborty, D.; Pathak, H. Global Nitrogen Budgets in Cereals: A 50-Year Assessment for Maize, Rice, and Wheat Production Systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angus, J. Nitrogen Supply and Demand in Australian Agriculture. Aust. J. Exp. Agric. 2001, 41, 277–288. [Google Scholar] [CrossRef]
- Roper, M.; Gupta, V. Enhancing Non-Symbiotic N2 Fixation in Agriculture. Open Agric. J. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Koksal, Y.; Sozen, E.; Ci, E.A. Heritability and Correlation of Yield and Quality Traits in Durum Wheat (Triticum durum). Indian J. Agric. Sci. 2007, 77, 565–568. [Google Scholar]
- Stuber, C.; Johnson, V.A.; Schmidt, J.W. Grain Protein Content and Its Relationship to Other Plant and Seed Characters in the Parents and Progeny of a Cross of Triticum aestivum L. Crop Sci. 1962, 2, 506–508. [Google Scholar] [CrossRef] [Green Version]
- Miezan, K.; Heyne, E.; Finney, K. Genetic and Environmental Effects on the Grain Protein Content in Wheat 1. Crop Sci. 1977, 17, 591–593. [Google Scholar] [CrossRef]
- Oury, F.-X.; Godin, C. Yield and Grain Protein Concentration in Bread Wheat: How to Use the Negative Relationship between the Two Characters to Identify Favourable Genotypes? Euphytica 2007, 157, 45–57. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.-M.; Jeuffroy, M.-H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the Grain Protein Concentration–Grain Yield Negative Relationship Is Highly Correlated to Post-Anthesis N Uptake in Winter Wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef] [Green Version]
- Michel, S.; Löschenberger, F.; Ametz, C.; Pachler, B.; Sparry, E.; Bürstmayr, H. Combining Grain Yield, Protein Content and Protein Quality by Multi-Trait Genomic Selection in Bread Wheat. Theor. Appl. Genet. 2019, 132, 2767–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansouri, A.; Oudjehih, B.; Benbelkacem, A.; Fellahi, Z.E.A.; Bouzerzour, H. Variation and Relationships among Agronomic Traits in Durum Wheat [Triticum turgidum (L.) Thell. ssp. Turgidum Conv. Durum (Desf.) MacKey] under South Mediterranean Growth Conditions: Stepwise and Path Analyses. Int. J. Agron. 2018, 2018. [Google Scholar] [CrossRef]
- Islam, M.R.; Haque, K.S.; Akter, N.; Karim, M.A. Leaf Chlorophyll Dynamics in Wheat Based on SPAD Meter Reading and Its Relationship with Grain Yield. Sci. Agric. 2014, 8, 13–18. [Google Scholar]
- Sanchez-Bragado, R.; Elazab, A.; Zhou, B.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T.; Araus, J.L. Contribution of the Ear and the Flag Leaf to Grain Filling in Durum Wheat Inferred from the Carbon Isotope Signature: Genotypic and Growing Conditions Effects. J. Integr. Plant Biol. 2014, 56, 444–454. [Google Scholar] [CrossRef]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat Stripe (Yellow) Rust Caused by P Uccinia Striiformis f. Sp. Tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef]
- Mashilo, J.; Tshikunde, N.M.; Shimelis, H.; Odindo, A. Agronomic and Physiological Traits, and Associated Quantitative Trait Loci (QTL) Affecting Yield Response in Wheat (Triticum aestivum L.): A Review. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Maeoka, R.E.; Sadras, V.O.; Ciampitti, I.A.; Diaz, D.R.; Fritz, A.K.; Lollato, R.P. Changes in the Phenotype of Winter Wheat Varieties Released between 1920 and 2016 in Response to In-Furrow Fertilizer: Biomass Allocation, Yield, and Grain Protein Concentration. Front. Plant Sci. 2020, 10, 1786. [Google Scholar] [CrossRef] [Green Version]
- Kadhem, F.; Baktash, F. AMMI Analysis of Adaptability and Yield Stability of Promising Lines of Bread Wheat (Triticum aestavum L.). Iraq. J. Agric. Sci. 2016, 47, 35–43. [Google Scholar]
- Kaggwa, R.J. Field Root Biomass, Morphology and Nitrogen Use Efficiency of Pavon 76 and Its Wheat-Rye (1RS) Translocations; The University of Arizona: Tucson, AZ, USA, 2013. [Google Scholar]
- Zhe, Y.; Lauer, J.G.; Borges, R.; de Leon, N. Effects of Genotype × Environment Interaction on Agronomic Traits in Soybean. Crop Sci. 2010, 50, 696–702. [Google Scholar] [CrossRef]
- Gauch, H.G., Jr. Statistical Analysis of Yield Trials by AMMI and GGE. Crop Sci. 2006, 46, 1488–1500. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koua, A.P.; Baig, M.M.; Oyiga, B.C.; Léon, J.; Ballvora, A. Fungicide Application Affects Nitrogen Utilization Efficiency, Grain Yield, and Quality of Winter Wheat. Agronomy 2021, 11, 1295. https://doi.org/10.3390/agronomy11071295
Koua AP, Baig MM, Oyiga BC, Léon J, Ballvora A. Fungicide Application Affects Nitrogen Utilization Efficiency, Grain Yield, and Quality of Winter Wheat. Agronomy. 2021; 11(7):1295. https://doi.org/10.3390/agronomy11071295
Chicago/Turabian StyleKoua, Ahossi Patrice, Mirza Majid Baig, Benedict Chijioke Oyiga, Jens Léon, and Agim Ballvora. 2021. "Fungicide Application Affects Nitrogen Utilization Efficiency, Grain Yield, and Quality of Winter Wheat" Agronomy 11, no. 7: 1295. https://doi.org/10.3390/agronomy11071295
APA StyleKoua, A. P., Baig, M. M., Oyiga, B. C., Léon, J., & Ballvora, A. (2021). Fungicide Application Affects Nitrogen Utilization Efficiency, Grain Yield, and Quality of Winter Wheat. Agronomy, 11(7), 1295. https://doi.org/10.3390/agronomy11071295





