Abstract
In this study, fermentation-based organic fertilizer (OF) was produced from the aboveground parts of Fallopia japonica (Houtt.) Ronse Decr. The quantity of N in OF (17.2 kg t−1 fresh lactic-fermented OF) was higher than average in cattle farmyard manure, but on a comparable level to solid poultry and rabbit manure. The OF was applied on a field to evaluate its effect on Chinese cabbage. The applied nutrients with OF N159 were 159, 19 and 100 kg ha−1 for N, P, and K, respectively. The applied nutrients with OF N317 were 317, 38, and 200 kg ha−1 for N, P, and K, respectively. The average mass of marketable Chinese cabbage (Brassica pekinensis Rupr.) single heads ranged from 253 g with N0 treatment to 602 g with N317 treatment. The nutrient recovery efficiency REN,P,K was 37, 20, and 50% for N317 and 55, 48, and 77% for N159. The OF was found to be a suitable alternative to farmyard manure. Additionally, OF produced from F. japonica could complement existing approaches to limit the spread of invasive species in cities. Further research should focus on perennial crop rotations and cropping patterns, different soil types, and a greater variety of crops and consider the possible retention of urban farmers using fertilizer from invasive plants.
1. Introduction
Fallopia Japonica (F. Japonica) is a fast-spreading invasive herbaceous plant mainly known for its ecosystem disservices. F. japonica causes considerable economic and environmental damage throughout the USA, UK, and Europe [1,2]. Due to its ability to quickly spread, it reduces the diversity [3,4,5] and the activity [6] of native biota, increases soil erodibility [7], affects temporal patterns of soil nutrient availability [8], and causes significant structural and functional changes in urban and rural ecosystems [9]. As such F. japonica poses considerable economical, planning, and logistical problem in urban and rural land management [10].
To control its spread, different mechanical, chemical, and biological approaches have been applied individually or in combination. Control methods that integrate a variety of options can lead to near-effective control of F. japonica [11]. Mechanical control methods, such as hand cutting, mowing, grazing, digging, pulling, and covering have proven labor-intensive but in some cases still lackluster in limiting F. japonica’s growth. The spread of F. japonica is to some extent controllable with herbicides [12], and biological agents, such as psyllid Aphalara itadori Shinji [13] and the fungus Mycosphaerella polygoni-cuspidati [14]. The restoration of contaminated areas requires careful species selection due to the allelopathic effects of F. japonica [15]. For example, fast-growing Salix viminalis significantly reduces knotweed spread due to its rapid growth and, therefore, represents promising restoration of species in some urban environments and riparian zones [16].
F. japonica is also known for its numerous ecosystem services. The aerial or underground parts of F. japonica show insecticidal [17] and fungicidal potential [18], and have been successfully used in the production of medicine [19], biofuel [20], briquettes for heating [21], paper cellulose fibers [22], textile [23], and as carbon (C) adsorbent [24]. In some of these production processes, the aerial parts of the plant went unused and were disposed of in landfills, although these parts weigh more than half the total mass of the plants [12,19]. To avoid resource waste and develop new potential benefits, it was suggested to focus research and development on the added value of the aerial parts [19]. Existing research has not yet focused on the potential uses of the aerial parts on F. japonica in the production of organic fertilizer (OF), although the high nitrogen (N) sequestering and remobilization ability of F. japonica [1] indicates that such uses might be possible, and attempts in composting F. japonica for the production of OF have proven promising [25].
We tested the aerial parts (leaves and stems) of F. japonica as raw material for the production of OF and applied the fertilizer on a field to evaluate F. japonica’s effects on Chinese cabbage and agricultural soil. OF was, for the first time, produced by a fermentation process using a microbial inoculant known as effective microorganisms (EM). It is reported that fermented OF possesses advantages over composts, including easier and more environmentally friendly preparation from the raw material [26]. The use of fermented products rich in selected microorganisms has positive effects on soil biological activity and may improve physical and chemical soil properties, and plant growth [27,28,29].
We hypothesized that the OF produced from F. japonica would successfully replace farmyard manure, which is not easily obtainable or handled in an urban environment. Furthermore, we assumed that mineralization and nutrient availability would be faster than those from other, comparable composts. This approach could serve as an alternative method for managing the uncontrolled spread of F. japonica in urban areas.
2. Materials and Methods
The presented experiment focuses on the management problem of F. japonica in the case-study city of Ljubljana (Slovenia), where approximately 5 ha of municipality‒owned land is infected by F. japonica stands, and a further 30 ha is infested by F. × bohemica [30,31] (at least 0.13% of the total municipal area) (Figure 1a). The experiment was conducted in August, September, and October 2017. The average monthly air temperature in 2017 was 11.9 °C, and the annual precipitation was 1531 mm. The monthly average temperatures were about 1 °C above, 1 °C below, and within the long-term average (1981–2010) during all three months. The monthly precipitation in August and October was half the long-term average. September was extremely wet, with twice the amount of the long-term average precipitation. The daily maximum temperatures in months 8, 9, and 10 were 30.2 °C, 19.1 °C, and 18.7 °C, respectively. The minimum daily temperatures in months 8, 9, and 10 were 16.7 °C, 11 °C, and 6.7 °C, respectively [32]. Although the heatwave shortly after planting in early August slowed down the initial growth of the plants, the conditions in September and October were favorable for the growth of Chinese cabbage (Figure 1b).
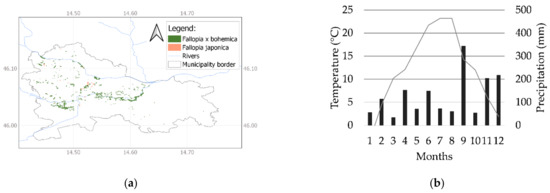
Figure 1.
The (a) stands of F. japonica and F. × bohemica, and (b) average monthly air temperature (°C) (line) and precipitation (mm) (bars) in 2017 in Ljubljana (Slovenia).
The aerial parts of F. japonica were harvested from infested grassland near a recreational pathway (46°02′48.36″ N, 14°33′51.80″ E) on 19th May 2017. Each shoot was cut approximately 5 cm above the ground to avoid harvesting roots with high vegetative reproduction potential. The plant material was collected from an unmanaged patch (29% of the harvested area) and from a one‒month‒old patch (71% of the harvested area). In total, 100 kg of fresh plant material was collected from a total area of 42 m2.
The plant material was stored in jute bags and transported to the facilities of the Biotechnical Faculty of the University of Ljubljana, where it was air dried at 40 °C for two days and then cut by machine in to pieces 1–5 cm long. The chopped plant material was transferred into two plastic 73- and 62-L barrels with screw caps, which were filled up to 57 L (i.e., three‒quarters of a barrel) and 31 L (half a barrel), respectively. The plant material was layered into the barrel in approximately 2 cm thick horizons. One fistful (30 g) of EM Bokashi—a commercial product made of lactic acid bacteria, yeast, and photosynthetic bacteria grown on a substrate of cereal bran and molasses—was spread on each horizon (30 g per 5.6 L or ca. 850 g of freshly chopped F. japonica = 35 g/kg). The mass was then firmly pressed, expelling air from the pores.
The plant material was covered with plastic foil, weighted with bricks and plastic bags filled with water, and closed with the screw caps to ensure no air entered the barrels during the process. The material was left to ferment in a dark and unheated room for two months. The average air temperature during fermentation was around 20 °C during the day and 10 °C during the night. The input plant material before fermentation and the output OF after fermentation were analyzed according to the Weender analysis—a partitioning and quantification of moisture (103 °C, 4 h), crude ash (ISO 5984:2002), crude protein (ISO 59832:2009) (or Kjeldahl protein), crude oils and fats (EC 152/2009, annex II H Procedure A), crude fiber (ISO 6865:2000), and N-free extracts (digestible carbohydrates). The fermented F. japonica to be further used in the experiment as OF was analyzed for organic fatty acids (water extraction, followed by esterification and analysis with GC-FID) (Table 1 and Table 2). A composite (bulk) sample of 15 individual samples (increments) was collected for both analyses (ISO 18400-102:2018).

Table 1.
Nutrient content of fermented F. japonica (OF) (% in fresh matter) and major nutrients application.

Table 2.
Properties of organic fertilizer (OF) made of F. japonica and Bokashi compost starter mixture before and after lactic fermentation.
The fertilization experiment was carried out in the multipurpose public urban green area, LivadaLAB (46°01′47.95″ N, 14°30′37.54″ E). The soil of the experimental field was a typical mineralized organic soil over a former lake and river sediment of lime gyttja and clay, and the texture was silt-loam. The characteristics of the plough horizon (0–20 cm) were as follows: pH (CaCl2) 6.5, plant available phosphorus (P2O5) 45 mg kg−1 dry soil (ammonium lactate method; A level according to the Slovene guidelines for professional fertilization [33]), and plant available potassium (K2O) 122 mg kg−1 soil (ammonium lactate method; B level [33]). The soil contained 12.55% of organic matter, 7.25% C, 0.64% N, and a C-to-N ratio of 11:5. In the last 10 years, the soil at the site was not utilized for agricultural production, not fertilized, and used as grassland, and the vegetation was cut once a year to prevent overgrowing with bushes and trees. The cut grass was removed from the site.
The soil was prepared by plowing with a moldboard plow 25 cm deep. Next, we crumbled the furrows and prepared the soil by harrowing, leveling, and forming raised beds. The soil was then saturated with water up to approximately 80% of the field’s capacity, which was suitable for transplanting the seedlings of Chinese cabbage.
The planting date was August 6, 2017, and the harvest date was 17 October 2017. The seedlings with four true leaves (approx. 8 cm in height) of Chinese cabbage were transplanted into the soil at a density of 14 Yuki F1 plants per 1 m2 at a distance of 30 cm in each row, with 20 cm between rows. The crop was treated with OF at a rate of 0, 1, and 2 kg m−2; the total amount of N applied in each treatment was 0 kg N ha−1 (treatment N0), 159 kg N ha−1 (treatment N159; small dose; 10 t ha−1), and 317 kg N ha−1 (treatment N317, large dose; 20 t ha−1) (Table 1). The OF was incorporated in soil (5 cm deep) manually just before planting.
The total area of the experimental site was 22.14 m2 (5.4. m × 4.1 m), organized as three slightly raised beds (ca. 10 cm high). Three experimental blocks were prepared, and one replicate of each treatment was randomly assigned in each block. Protection bands 0.5 m wide were formed between experimental blocks and bands 0.3 m wide were formed between experimental plots within each block to prevent the influence of fertilizers from the neighboring sectors. Each plot had an area of 1 m2 (1.4 m × 0.7 m).
Nearly all of the N in the OF was organically bound. Only traces were found in the form of ammonium, which is surprising, as anaerobic digestion usually leads to a greater amount of ammonia [34,35]. Nitrate and nitrite forms were measured but not detected (Table 2). The chosen fertilization rates fell both below and above the common practice of vegetable growers, who use a fertilization target value of 240 kg ha−1 mineral N to achieve 50 t ha−1 of fresh Chinese cabbage yield [33]. Since all the N was organically bound in the OF, we expected that only a small percentage would be released over the two months of our Chinese cabbage growth experiment.
Pesticides were not used in the experiment. The plants were irrigated according to visual observations every second day in August using an irrigation bucket. Then, the plants were irrigated every fourth day. We irrigated approximately 10 mm of water per irrigation to the point that the first layer of soil (up to 4 cm) was determined to be wet with a finger test.
The yield of standard and non-standard heads and the total masses of plants were then measured by weighing. Five random plants from each plot were selected; one quarter from each plant was taken for further analysis (ISO 24153:2009). Plant samples were air-dried in a fan-aerated chamber at 40 °C to a constant weight and milled with a Retsch Cutting Mill SM 100 to a final fineness smaller than 1.0 mm. The air-dried and milled bulk samples were further analyzed for their total dry matter (SIST EN 13040:2008). The analyses for total C and N were performed with a Vario MAX instrument (Elementar Analysensysteme GmbH). This instrument operates on the principle of dry combustion, by simultaneously analyzing the C and N content and capturing organic and inorganic forms (SIST ISO 10694; SIST ISO 13878).
Ammonium and nitrate content were extracted with 0.01 M CaCl2 through 2 h of shaking. Then, we filtered the extract through a 0.45 µm filter and measured ammonium and nitrate using a Thermo Scientific ™ Gallery ™ Plus Automated Photometric Analyzer (SIST ISO 14255:1999). For the elemental analyses of total P and K content, the samples were digested with multiple acids, which dissolved most of the minerals; 0.25 g of each sample was heated first in HNO3, then in HClO4, and lastly in HF until evaporation. Each sample was then dried, the residue was dissolved in HCl, and the elements were measured using an ICP-ES Inductively Coupled Plasma Emission Spectrometer [36].
Program R was used for statistical analyses, and the differences between treatments were estimated with the least significant difference test. The significance level was set at α = 0.05.
Finally, the potential impact on urban food production was estimated based on the average yield of the fresh mass of F. japonica from the vegetation canopy in July (90 t ha−1) [37]. We modeled the required application of fertilizer from F. japonica needed to cover the plant nutrient requirements of the selected vegetable crops at a given expected yield. The selection of crops was based on the research of Glavan et al. [38], who provided a detailed list of the 14 most commonly grown crops by urban allotment farmers in Ljubljana. This list represents the average allotment for a garden in Ljubljana. The calculation is based on the expected yield (t ha−1) and expected crop nutrient uptake (kg ha−1) given in the technological instructions for integrated vegetable production—plant production based on integrated plant nutrient and pest management [39].
3. Results
3.1. Organic Fertilizer Properties
The 100 kg of fresh F. japonica plant biomass harvested in May from 42 m2 equaled a fresh-matter OF substrate per ha of 23.8 t ha−1. After air drying, the 100 kg fresh matter OF substrate gave 0.088 m3 of OF, equivalent of an OF quantity of 27.1 m3 ha−1 or 0.88 t m−3.
The properties of the OF made from F. japonica and EM Bokashi before and after lactic fermentation are shown in Table 2. During the 2 months of lactic fermentation, some water was lost from the mass of OF via cell lysis and metabolic water leaching out of the cells. The produced OF contained ca. 50% moisture. Although the OF retained most of its green color (Figure 2), it smelled similar to sauerkraut. The OF contained 9% proteins, 4% carbohydrates (mostly in form of fibers), slightly less than 1% fats or oils, and a relatively large fraction of 12% non-volatile (ash) material.

Figure 2.
Fermentation-based organic fertilizer from the aboveground parts of F. japonica.
The OF was already acidic at the start of the experiment due to the addition of an acidic Bokashi compost starter. The fermentation process did not alter the acidity, which was at around pH 4.5. Lactic acid (6.6 g/kg) and acetic acid (3.5 g/kg) were prevailing organic acids in OF.
The quantity of N in fresh lactic-fermented OF was 15.9 kg t−1. The ratio of C/N was 14.5:1. In OF, practically all N was in an organic form, less than 0.1% of N was in an ammonium form, and none was in a nitrate or nitrite form. The contents of P and K were 1.9 kg P t−1, with 10.0 kg K t−1 of fresh OF. The ratio in kg t−1 of fresh OF was N:P:K = 16:2:10.
3.2. Soil Properties
After the harvest of Chinese cabbage, the soil analysis showed a significant improvement in soil-available P and especially K-levels (Table 3).

Table 3.
Soil properties before fertilization and after the Chinese cabbage harvest.
As aerial losses occur, fermented OF, in comparison to composted OF with the same origin, would probably contain a higher percentage of N. The addition of OF did not significantly change the soil pH or the soil mineral N content after the harvest despite the high application of total N in the treatment N317 (317 kg N ha−1).
3.3. Plant Yield and Quality, Nutrient Uptake
The heads at N0 treatment were loose (unsalable heads). Despite this fact, we considered marketable all the parts of the heads that were edible. The mass of the whole heads was 40–50% higher than the mass of marketable heads. The mass of the whole heads (fresh) of Chinese cabbage ranged from 49.2 t ha−1 at treatment N0 to 100.5 t ha−1 at treatment N159 (Figure 3a). The average mass of marketable single heads in our experiment ranged from 253 g with N0 to 602 g with N317. The heads of OF treatments were significantly heavier than the heads in the control, but the average weight of N159 was not significantly different from that of N317 (Figure 3b). The diameter of marketable heads of Chinese cabbage ranged from 31.3 cm at treatment N0 to 39.7 cm at treatment N159 (Figure 3c).
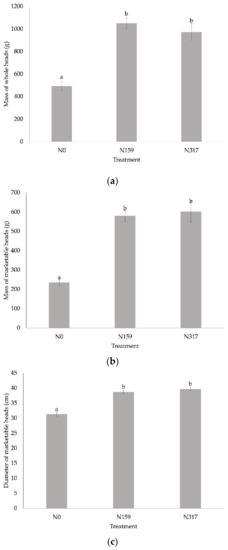
Figure 3.
The (a) mass of whole heads (g), (b) mass of marketable heads (g), and (c) diameter of marketable heads (cm) (mean ± SE); a and b lower case letters denote statistical significant differences (α < 0.05) among treatments.
The ANOVA analysis showed that there were no statistically significant differences between fertilization treatments (independent variable) in the average contents of C, N, NO3-N, P, and K (dependent variables) in Chinese cabbage plants. The K (%) achieved in our research ranged between 2.1 and 2.6% (Table 4).

Table 4.
Average contents of C, N, NO3-N, P and K in the crop of air-dried Chinese cabbage plants.
Dry matter content was 9–11%, and did not statistically differ between the fertilizations (Table 5). The difference in yield between the fertilization levels of N0, N159, and N317 decreased with increasing fertilization intensity (Table 4 and Table 5).

Table 5.
Dry matter content (DM) (%) and yield (kg DM ha−1) of Chinese cabbage plants.
The uptake of the major nutrients N, P, and K by plant biomass is listed in Table 6. In brackets, the apparent uptake from the OF used in the experiment is also provided, calculated as the difference between the N uptake at N0 variant and the N159 and N317 fertilization treatments.

Table 6.
Nutrient uptake by the yield of Chinese cabbage and apparent uptake from OF (in brackets) in kg/ha.
The relationship of nutrient plant uptake from OF by Chinese cabbage (Table 6) to the applied total nutrient (Table 1) for the main nutrients N, P, and K yielded the crop apparent nutrient recovery efficiency REN,P,K as defined by Dobermann [40] (Table 7).

Table 7.
Nutrient apparent recovery efficiency of Chinese cabbage plants from OF for N, P and K (%).
If the existing 34 ha of F. japonica and F. x bohemica in Ljubljana (Figure 1a) were harvested for the production of OF, a yield of total fresh matter between 813 and 3060 t would be expected, depending on the yield of F. japonica (between 23.9 and 90 t ha−1 of fresh plants), which would yield ca. 924–3477 m3 of OF product.
In line with Table 1, the applied nutrients with OF N159 (kg ha−1) are comprised of 159 kg N ha−1, 19 kg P ha−1, and 100 kg K ha−1, respectively. When considering an average crop uptake (kg ha−1) of the selected crops [39], the produced OF would cover the N fertilizer needs of 67 ha to 247 ha, depending on the yield of F. japonica (between 23.9 and 90 t ha−1 of fresh plants). The maximum area potentially supplied with OF considerably surpasses the urban gardening area in Ljubljana (167 ha in the year 2010). These results indicate that OF could also be used in professional urban farming adjacent the city and could significantly contribute to the local nutrient cycle and food system (Table 8). It is evident, that OF produced from F. japonica contains on average two to three times more nutrients as taken off the garden land by vegetable crops. OF is especially rich in N and K (Table 8).

Table 8.
Area (ha) potentially supplied with nutrients by F. japonica organic fertilizer produced on 34 ha when considering average crop uptake according to technological instructions for integrated vegetable production [39].
4. Discussion
F. japonica is known for its participation in numerous ecosystem services [19,20,21,22]. However, as an invasive species, it causes significant structural and functional changes in urban and rural ecosystems [1,2,3,4,5,8,9,10]. This research focused on the added value of F. japonica [19]. The rationale for this research was provided by Aguilera et al. [1], who indicated a high N uptake from the soil for F. japonica, which led us to assume the good N remobilization capacity of the produced organic fertilizer (OF) from F. japonica.
The fresh plant biomass harvested in this experiment, 23.8 t ha−1 was significantly lower than the 47 t ha−1 reported by Bernik et al. [37], who reported a harvest from an unmanaged patch. However, the plant material in this research was collected from a combination of unmanaged and managed one-month-old patches, which is why the green mass was less developed and less fresh plant biomass was harvested.
A fermentation process was used to produce an organic fertilizer, OF, from F. japonica using an innovative method of lactic fermentation by addition of EM Bokashi fermentation inoculum, complementary to some mechanical [11,16], biological [14], and combined approaches [41] already used to manage this invasive species.
Compared to classical aerobic thermophilic composting, fermentation has several advantages. The lack of aeration and mixing during the process; low energy input; further, no heat loss is produced, and no or few harmful gaseous compounds (e.g., CO2, NH3, and N2O) are released into the air, and therefore more energy and plant nutrients remain in the fermented organic matter [26]. The high level of acidity in OF, together with the inclusion of a probiotic lactic‒microorganisms consortia, was important to protect the material against further microbial degradation [42,43]. Furthermore, there is no loss of water-soluble K, as the fermentation process is conducted in a closed system where leaching does not occur [26]. Besides organic acids, secondary products are formed, such as enzymes, amino acids, and vitamins, which have a stimulating effect on both soil microorganisms and the cultivated crops [44]. Due to its production method, the C footprint of the fermented OF is lower than that of similar fertilizers (such as manure and compost), making OF an interesting alternative plant nutrient for reducing CO2 emissions from agriculture [26,45].
Pathogenic microorganisms (e.g., Escherichia coli and bacteria of the genus Salmonella) in fermented OF, moreover, do not reproduce due to acid environment (pH of OF was 4.6), making OF a safe product from a human-health perspective [26]. The disadvantage of this treatment is that during fermentation, no high temperature is generated [26], which would destroy the seeds of F. japonica or inhibit their germination. Therefore, the plant material harvested for the production of OF should not include viable seeds or propagules with high reproductive capacity (with harvesting done in the flowering phase at the latest).
The experimental results prove the hypothesis that the OF produced from F. japonica could indeed successfully replace farmyard manure, which is not easily obtainable in urban environments.
The yields of Chinese cabbage mass of the whole heads obtained in fertilization experiments in some other studies are partially comparable to ours. Staugaitis et al. [46] achieved a similar yield of 50 t ha−1 with treatment N0 and 76 t ha−1 with treatment N225, although the Chinese cabbage variety and the pedo-climatic factors were not the same as those in our study. The same statistically significant differences in the masses of marketable heads were observed between treatment groups, as reported by Staugaitis et al. [46]. Dry matter content of Chinese cabbage plants was high, 9–11%, but comparable to the figures reported by Kosson et al. [47]. The variability observed reflects a combination of abiotic factors (climate) and the soil system [48,49,50,51]. Based on previous research it would be interesting to focus further research on changes in the soil that might occur when using OF for a longer period (several years). It would be interesting to explore how the nutritive value of OF changes with an altered collection of raw F. Japonica material for its preparation (different time of year) [48,49,50,52].
The fermented OF produced by F. japonica contained 2.5-times more ash in dry matter than raw substrate from F. japonica (Table 2) and showed a well-balanced composition of main plant nutrients due to its high N sequestering and remobilization ability [1], as well as due to lactic fermentation treatment, where no/little N losses occurred. The dry matter content in the OF was comparable to that of many composts and much higher than that in digestates [53]. The quantity of N in fresh lactic-fermented OF was relatively high compared to average cattle farmyard manure but on a comparable level to solid poultry, and rabbit manure [54,55]. In comparison to compost and digestates, the relative concentration of N in OF was found to be much higher [56].
The ratio of C/N in OF was relatively narrow (14.5:1), which indicates that N would be readily mineralized after incorporating the OF into the soil. According to Lazicki et al. [57], organic matter with a C/N ratio >19:1 leads to N immobilization, whereas organic matter with C/N ratios <14:1 mineralizes N. This result is comparable to C/N ratios in well-composted plant material and higher than the ratios in many biogas digestates [35].
Our results proved that after OF being applied and incorporated into the soil, the fermented organic matter was largely mineralized in the presence of oxygen and aerobic soil microorganisms, which resulted in high apparent N, P and K recoveries in Chinese cabbage yield (Table 7). For an organic fertilizer of plant origin the contents of P and K in Chinese cabbage were relatively high. The ratio in kg t−1 of fresh OF was in line with the needs of vegetable crops, which take up 4 to 8 times more K than P [58]. However, the concentration of K (%) in the Chinese cabbage heads achieved in our research with OF (2.65% K) was a bit lower to the research of Pokluda [59], where the average K was 3.1%, but this difference can be attributed to different pedo-climatic conditions.
Increasing yields due to increasing fertilization rates by OF accompany a certain dilution of N in plant biomass, which is due to the “law of diminishing yield increments”. Consistent with the findings of Mitscherlich [60], the crop recovery efficiency decreases with an increase in fertilizer doses. The nutrient recovery efficiency is smallest for P and highest for K. Considering the large proportion of N initially organically bound in the OF, the recovery efficiency is shown here to be surprisingly high.
Studies reported that an average yield of Chinese cabbage (50 t ha−1) [47] takes up 26 kg ha−1 P and 180 kg ha−1 K. In our research, with 10 t OF ha−1, we supplied 10 kg P and 100 kg K. Here, the crop recovery efficiency REN, P, K indicates the amount of (main) nutrient taken up by the crop and the remaining share in the soil. The crop directly took up quite a substantial fraction of the applied plant nutrients from OF (apparent recoveries for N159 were 55%, 48%, and 77% of N, P, and K, respectively), which is excellent for only two months of Chinese cabbage growth and to the amount expected for mineral N and K fertilizers. This result is even better than of mineral P-fertilizers, where the percentage recovery of fertilizer P calculated by the difference method normally ranges from 10 to 25% for a given crop in a given season [61]. However, surpluses, particularly those of water-soluble nitrate, can be leached after harvest. If Chinese cabbage is the last crop during the year, a catch crop should be cultivated (e.g., winter cereals) to retain the nutrients available for the crops in the following year.
It can be assumed that OF in soil quickly mineralizes, releasing readily available N for the cabbage. In this study, the cabbage was not grown long enough to capture all N mineralized, as readily available mineral N was detected in the soil right after harvest (Table 3). However, the soil mineral N after harvest was not elevated by OF application compared to the non-fertilized control, even under the highest N application, N317. Nitrate-N, which was the predominant mineral N formed in soil after the harvest, was high in content (32 to 34 mg NO3-N/kg soil; Table 3), which indicates high mineralization capacity of the experimental soil. Despite the high N-mineralization potential of the soil, the N input from OF was able to match the dynamic needs of the Chinese cabbage for N (good apparent N efficiency; Table 8) and, consequently, achieved significantly higher yields without leaving excess mineral N in the soil after harvest compared to the Control, N0. This observation can be attributed to characteristic of OF: rather low C/N ratio, 14.5, and low content of fibers (lignocellulose), 8% (Table 3), of which all being favorable for net N mineralization.
Table 9 shows the modeled required application of F. japonica OF (1.59% N, 0.19% P, 1.00% K). We assumed garden soils with initial soil P and K supply in class C (optimal) and no plant-available mineral N readily available to meet the plant nutrient requirements of selected crops [38] at a given expected average crop production yield based on the integrated plant nutrient management guidelines [39]. Generally, N in OF was found to be the limiting factor among the main nutrients for plant growth. Moreover, the N availability for plants was significantly higher than that in comparable products [62]. Traditional organic fertilizers used in organic farming often contain a P concentration above plant needs, which leads to an accumulation of P in soils, particularly if fertilization is quantified according to the required plant needs for N [62].

Table 9.
Required application of organic fertilizer (OF) from F. japonica (1.59% N, 0.19% P, 1% K; crop recovery efficiency for N 46%) for selected crops in non-marketable urban gardening in Ljubljana, Slovenia.
The crop recovery efficiency levels of N in our short-term experiment were 55% (N159) and 37% (N317), and the average N159-N317 was 46% (Table 8). OF, therefore, is a good source of N (under the condition that the organically bound N mineralizes as rapid as in the experiment), a moderate source of K, and a low source of P. In order to achieve an average yield of 5 kg/m2 for the selected crops and to cover 100% of the N requirement of the crops, an application of 2 kg OF/m2 would be necessary (Figure 4).
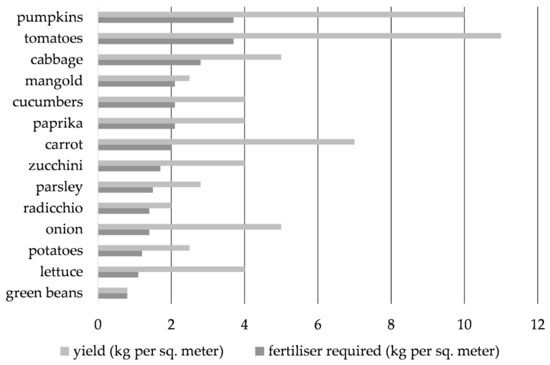
Figure 4.
Required application of organic fertilizer (OF) (kg/m2) from F. japonica (1.59% N, 0.19% P, 1% K; assumed recovery efficiency for N 46%) based on the expected yield, following the Technological instructions for integrated vegetable production [39] for selected crops in non-marketable urban gardening in Ljubljana, Slovenia, at a given expected yield (kg/m2) reported by Glavan et al. [38].
This model application would cover 66.5% of P and 88.4% K average crop requirements (Table 9). For some of the more N-demanding crops, such as cabbage, pumpkins, and tomato, higher OF application of 2–3.7 kg/m2 is needed to achieve the targeted yield. Full coverage of N requirements would result in P and K oversupply for tomato and K oversupply for radicchio and paprika but would undersupply crop P requirement for onion, green beans, and carrot (P supply < 60%) and undersupply the K crop requirements of green beans and carrot (K supply < 60%) (Table 9). Over the long run, some mineral fertilization could be applied to cover the gaps in the plant needs of P and K if mineral fertilization is permitted. Organic farming does not allow mineral N-fertilization but allows certain P- and K-fertilizers (which are not so strongly processed). In cases where P and K are oversupplied, vegetables with a longer growing season, as well as some of the plants grown as catch crops, possess the ability to access P and K resources from deeper soil layers [63].
OF fertilizer treatments (N159, N318) showed sufficient nutrient uptake to meet the needs of fast growing vegetables. However, Staugaitis et al. [46] warn that N applications greater than 180 kg ha−1 can negatively influence the inner quality of Chinese cabbage in terms of its content of vitamin C, nitrates, and soluble solids. This negative aspect of high N fertilization is more probable when soluble N fertilizers are used. In a case of OF, N is organically bound and is released gradually. Even with the application of 318 kg N/ha, the inner quality of our cabbage was not degraded.
In organic vegetable production, the nutrient recovery achieved with frequently used organic fertilizers is lower; the mineralization rate in the first year of application for organically bound N in solid manure is 10–30%, for poultry manure up to 45% [62]. Additionally, the nutrient dynamics after fertilization with OF should be further researched to explore nutrient dynamics under the repeated incorporation of OF in soil over several growing seasons and for different crops and soil conditions.
The approach adopted in this work was shown to be a viable alternative to manage the uncontrolled spread of F. japonica in urban areas. The OF production process is simple enough to be repeated by small urban farmers and has the potential to be reproduced on a larger scale, by waste management companies. In this study, plant materials for the production of OF were cut and collected by hand. A more mechanized approach could be applied for the larger condensed F. japonica patches that typically occur along the banks of highways, especially when the plants are mowed at least twice per year [64]. This would ensure a high green-mass yield and an interruption in the reproductive cycle of the plant (pollen drift and seed formation) at the stage when the plant N content is expected to be highest, although precaution is necessary as some metallic trace elements, such as Zn, can transport to aboveground parts of F. japonica [65]. The approach taken in this study seems to be able to complement frequent (weekly) management of F. japonica stands in parks, nurseries and riverbanks, where frequent cutting [11] continues to be applied as a means of suppressing F. japonica, although the long-term success of frequent mowing of F. japonica as a management method remains to be proven [66].
5. Conclusions
The use of the green parts of F. japonica to produce organic fertilizer OF not only opens up the possibility of preventing the rapid spread of the plant, but also provides new ideas for its use. There are essentially no experiments on the usefulness of organic fertilizers from F. japonica in vegetable production. The results of the present study, the first experiment in this field are of international significance as they not only demonstrate OF production by fermentation but also evaluate OF’s fertilizer value.
The disadvantages of this technically simple experiment are its low number of repetitions and use of untypical soils with a comparatively large supply of N. In the future, this experiment should be repeated with more treatments over a longer period and on different soil types. In addition, an experiment should be carried out on different crops to determine the possible negative effects of OF’s properties on some vegetable species. Instead of inoculation with EM Bokashi, it should be tested if an inoculum from previous fermentation could be used to reduce costs.
To better assess the economic potential of the OF fertilizer, it is also necessary to evaluate the possible reluctance of growers to use fertilizer from invasive plants. In this area, research involving a wide range of end-users, from hobby to market producers, should be carried out. The proposed activities are relevant to the issue of society’s attitude towards invasive species and support the advocacy for the management of invasive plant species in the light of the circular economy.
Author Contributions
Conceptualization, R.C., L.R., A.S. and R.M.; Data curation, R.C.; Formal analysis, R.C., A.S. and R.M.; Funding acquisition, R.C., M.P. and R.M.; Investigation, R.C., A.S. and R.M.; Methodology, R.C., L.R., A.S. and R.M.; Project administration, R.C.; Resources, M.P. and R.M.; Supervision, R.C. and R.M.; Validation, R.C., A.S. and R.M.; Visualization, R.C. and A.S.; Writing—original draft, R.C.; Writing—review & editing, R.C., S.K., M.P., L.R., A.S. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
The research was co-funded by the following projects and funders: the European Commission Seventh Framework Programme Green Infrastructure and Urban Biodiversity for Sustainable Urban Development and the Green Economy (GREEN SURGE), Grant number FP7-ENV.2013.6.2-5-603567; the Slovenian Research Agency Programme AGROECOSYSTEMS, Grant number P4-0085; the Slovenian Research Agency project Improved water and nutrient use efficiency in plant production to protect drinking water sources (URAVIVO), Grant number L4-8221; the Municipality of Ljubljana project Knotweed games, extended stay with a friendly enemy, Grant number C7560-17-408027; and anonymous private funder.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank Micronatura, tehnologija EM d.o.o. (Visoko, Slovenia) for providing the product EM Bokashi to the research. The idea of producing organic fertilizer from F. japonica was co-developed in cooperation with Institute BOB, a non-governmental organization for youth, and Trajna Association that produces design projects focusing on exploring the useful properties of invasive plants and their sustainable management.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguilera, A.G.; Alpert, P.; Dukes, J.S.; Harrington, R. Impacts of the Invasive Plant Fallopia Japonica (Houtt.) on Plant Communities and Ecosystem Processes. Biol. Invasions 2010, 12, 1243–1252. [Google Scholar] [CrossRef]
- Michez, A.; Piégay, H.; Jonathan, L.; Claessens, H.; Lejeune, P. Mapping of Riparian Invasive Species with Supervised Classification of Unmanned Aerial System (UAS) Imagery. Int. J. Appl. Earth Obs. Geoinf. 2016, 44, 88–94. [Google Scholar] [CrossRef]
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia Japonica Alters Soil Food Webs through Secondary Metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef]
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic Invasive Knotweeds (Fallopia Spp.) Negatively Affect Native Plant and Invertebrate Assemblages in European Riparian Habitats. Biol. Conserv. 2008, 141, 646–654. [Google Scholar] [CrossRef]
- Stoll, P.; Gatzsch, K.; Rusterholz, H.-P.; Baur, B. Response of Plant and Gastropod Species to Knotweed Invasion. Basic Appl. Ecol. 2012, 13, 232–240. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Frąc, M.; Oszust, K.; Woch, M.W.; Zubek, S. Invasive plant Reynoutria japonica produces large amounts of phenolic compounds and reduces the biomass but not activity of soil microbial communities. Sci. Total Environ. 2021, 767, 145439. [Google Scholar] [CrossRef] [PubMed]
- Colleran, B.; Lacy, S.N.; Retamal, M.R. Invasive Japanese knotweed (Reynoutria japonica Houtt.) and related knotweeds as catalysts for streambank erosion. River Res. Appl. 2020, 36, 1962–1969. [Google Scholar] [CrossRef]
- Tharayil, N.; Alpert, P.; Bhowmik, P.; Gerard, P. Phenolic Inputs by Invasive Species Could Impart Seasonal Variations in Nitrogen Pools in the Introduced Soils: A Case Study with Polygonum cuspidatum. Soil Biol. Biochem. 2013, 57, 858–867. [Google Scholar] [CrossRef]
- Bailey, J.; Wisskirchen, R. The Distribution and Origins of Faúopia × bohemica (Polygonaceae) in Europe. Nord. J. Bot. 2008, 24, 173–199. [Google Scholar] [CrossRef]
- Rouifed, S.; Cottet, M.; de Battista, M.; Le Lay, Y.-F.; Piola, F.; Rateau, P.; Rivière-Honegger, A. Landscape Perceptions and Social Representations of Fallopia Spp. in France. Sci. Nat. 2018, 105, 67. [Google Scholar] [CrossRef] [PubMed]
- Child, L.; Wade, M. The Japanese Knotweed Manual: The Management and Control of an Invasive Alien Weed; Packard Publishing Ltd.: Chichester, UK, 2000; pp. 1–152. [Google Scholar]
- Soll, J. Controlling Knotweed (Polygonum Cuspidatum, P. Sachalinense, P. Polystachyum and Hybrids) in the Pacific Northwest; The Nature Conservancy: Arlington County, VA, USA, 2004; pp. 1–15. [Google Scholar]
- Jones, I.M.; Smith, S.M.; Bourchier, R.S. Establishment of the biological control agent Aphalara itadori is limited by native predators and foliage age. J. Appl. Entomol. 2020, 144, 710–718. [Google Scholar] [CrossRef]
- Kurose, D.; Furuya, N.; Seier, M.K.; Djeddour, D.H.; Evans, H.C.; Matsushita, Y.; Tsuchiya, K.; Tsushima, S. Factors Affecting the Efficacy of the Leaf-Spot Fungus Mycosphaerella polygoni-cuspidati (Ascomycota): A Potential Classical Biological Control Agent of the Invasive Alien Weed Fallopia japonica (Polygonaceae) in the UK. Bio. Control 2015, 85, 1–11. [Google Scholar] [CrossRef]
- Dommanget, F.; Evette, A.; Spiegelberger, T.; Gallet, C.; Pacé, M.; Imbert, M.; Navas, M.-L. Differential Allelopathic Effects of Japanese Knotweed on Willow and Cottonwood Cuttings Used in Riverbank Restoration Techniques. J. Environ. Manag. 2014, 132, 71–78. [Google Scholar] [CrossRef]
- Dommanget, F.; Evette, A.; Breton, V.; Daumergue, N.; Forestier, O.; Poupart, P.; Martin, F.-M.; Navas, M.-L. Fast-Growing Willows Significantly Reduce Invasive Knotweed Spread. J. Environ. Manag. 2019, 231, 1–9. [Google Scholar] [CrossRef]
- Bohinc, T.; Horvat, A.; Ocvirk, M.; Košir, I.J.; Rutnik, K.; Trdan, S. The First Evidence of the Insecticidal Potential of Plant Powders from Invasive Alien Plants against Rice Weevil under Laboratory Conditions. Appl. Sci. 2020, 10, 7828. [Google Scholar] [CrossRef]
- Anžlovar, S.; Janeš, D.; Dolenc Koce, J. The Effect of Extracts and Essential Oil from Invasive Solidago spp. and Fallopia japonica on Crop-Borne Fungi and Wheat Germination. Food Technol. Biotechnol. 2020, 58, 273–283. [Google Scholar] [CrossRef]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, Phytochemistry, Pharmacology, and Potential Application of Polygonum cuspidatum Sieb.et Zucc.: A Review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qi, G.; Andriamanohiarisoamanana, F.J.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Anaerobic co-digestion of dairy manure and Japanese knotweed (Fallopia japonica) under thermophilic condition: Optimal ratio for biochemical methane production. Anim. Sci. J. 2021, 92, e13523. [Google Scholar] [CrossRef] [PubMed]
- Brunerová, A.; Müller, M.; Brožek, M. Potential of Wild Growing Japanese Knotweed (Reynoutria japonica) for Briquette Production. In Proceedings of the 16th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 24–26 May 2017; University of Life Sciences and Technologies, Faculty of Engineering: Jelgava, Lativa, 2017. [Google Scholar]
- Karlovits, I.; Lavrič, G.; Kavčič, U.; Zorić, V. Electrophotography toner adhesion on agro-industrial residue and invasive plant papers. J. Adhes. Sci. Technol. 2021, 1–16. [Google Scholar] [CrossRef]
- Čuk, N.; Šala, M.; Gorjanc, M. Development of antibacterial and UV protective cotton fabrics using plant food waste and alien invasive plant extracts as reducing agents for the in-situ synthesis of silver nanoparticles. Cellulose 2021, 28, 3215–3233. [Google Scholar] [CrossRef]
- Koutník, I.; Vráblová, M.; Bednárek, J. Reynoutria japonica, an invasive herb as a source of activated carbon for the removal of xenobiotics from water. Bioresour. Technol. 2020, 309, 123315. [Google Scholar] [CrossRef]
- Day, L.; Rall, J.; McIntyre, S.; Terrance, C. Japanese Knotweed Composting Feasibility Study, Delaware County (New York). Restor. Ecol. 2009, 27, 377–379. [Google Scholar] [CrossRef]
- Bosch, M.; Hitman, A.; Hoekstra, J. Fermentation (Bokashi) versus Composting of Organic Waste Materials: Consequences for Nutrient Losses and CO2-Footprint; 2017; pp. 1‒3. Available online: http://agritonsverige.se/wp-content/uploads/2019/06/Fermentation-vs-composting-of-Organic-Waste-Materials.pdf (accessed on 29 April 2020).
- Ginting, S. Promoting Bokashi as an Organic Fertilizer in Indonesia: A Mini Review. Int. J. Environ. Sci Nat. Res. 2019, 21, 556070. [Google Scholar] [CrossRef]
- Lasmini, S.A.; Nasir, B.; Hayati, N.; Edy, N. Improvement of Soil Quality Using Bokashi Composting and NPK Fertilizer to Increase Shallot Yield on Dry Land. Aust. J. Crop Sci. 2018, 12, 1743–1749. [Google Scholar] [CrossRef]
- Pandit, N.R.; Schmidt, H.P.; Mulder, J.; Hale, S.E.; Husson, O.; Cornelissen, G. Nutrient effect of various composting methods with and without biochar on soil fertility and maize growth. Arch. Agron. Soil Sci. 2020, 66, 250–265. [Google Scholar] [CrossRef]
- Fallopia japonica, Fallopia × bohemica, City Municipality of Ljubljana. Available online: https://www.ljubljana.si/sl/moja-ljubljana/varstvo-okolja/invazivne-tujerodne-vrste/invazivne-tujerodne-rastline/japonski-dresnik-ceski-dresnik/ (accessed on 29 April 2020).
- Smerdu, A.; Kanjir, U.; Kokalj, Ž. Automatic detection of Japanese knotweed in urban areas from aerial and satellite data (Special Issue: Detection and control of alien forest species in a changing world). Manag. Biol. Invasions 2020, 11, 661–676. [Google Scholar] [CrossRef]
- Meteo.si—Official Weather Forecast for Slovenia—National Meteorological Service of the Republic of Slovenia. Available online: https://meteo.arso.gov.si/ (accessed on 16 April 2021).
- Mihelič, R.; Čop, J.; Jakše, M.; Štampar, F.; Majer, D.; Tojnko, S.; Vršič, S.; Grčman, H. Smernice za Strokovno Utemeljeno Gnojenje; Ministrstvo za Kmetijstvo, Gozdarstvo in Prehrano: Ljubljana, Slovenia, 2010; pp. 1–182.
- Möller, K.; Schultheiß, U.; Wulf, S.; Schimmelpfennig, S. Düngung Mit Gärresten, Eigenschaften—Ausbringung—Kosten; Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V.: Darmstadt, Germany, 2019; pp. 1–63. [Google Scholar]
- Sänger, A.; Geisseler, D.; Ludwig, B. Effects of Rainfall Pattern on Carbon and Nitrogen Dynamics in Soil mended with Biogas Slurry and Composted Cattle Manure. J. Soil Sci. Plant. Nutr. 2010, 173, 692–698. [Google Scholar] [CrossRef]
- Bureau Veritas Metals, Minerals & Environmental Schedule of Services & Fees 2019; Bureau Veritas Commodities Canada Ltd.: Vancouver, BC, Canada, 2019; pp. 1–60.
- Bernik, R.; Tušar, R.; Zver, A. Japonski Dresnik, Plevel Kot Obnovljivi Vir Energije (OVE). In Actual Tasks on Agricultural Engineering, Proceedings of the 35th International Symposium on Agricultural Engineering, Opatija, Croatia, 19–23 February 2007; Silvio, K., Ed.; Zavod za Mehanizacijo Poljoprivrede, Agronomski Fakultet Sveučilišta: Zagreb, Croatia, 2007; pp. 347–352. [Google Scholar]
- Glavan, M.; Istenič Černič, M.; Cvejić, R.; Pintar, M. Urban Gardening: From Cost Avoidance to Profit Making—Example from Ljubljana, Slovenia. In Urban Agriculture; Mohamed, S., Ed.; IntechOpen Limited: London, UK, 2016; pp. 24–42. [Google Scholar]
- Technological Instructions for Integrated Vegetable Production; Ministry of Agriculture, Forestry and Food of the Republic of Slovenia: Ljubljana, Slovenia, 2020; pp. 1–166.
- Dobermann, A.R. Nitrogen Use Efficiency—State of the Art. Agron. Hortic. Fac. Publ. 2005, 316, 1–16. [Google Scholar]
- Martin, F.M.; Dommanget, F.; Evette, A. Improving the management of Japanese knotweed s.l.: A response to Jones and colleagues. NeoBiota 2020, 63, 147–153. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kumar, R.; Kumar, S.; Kumar, V.; Sugitha, T.; Singh, B.; Chauhan, V.; Dhaliwal, H.; Saxena, A. Beneficial Microbiomes: Biodiversity and Potential Biotechnological Applications for Sustainable Agriculture and Human Health. J. Appl. Biol. Biotech. 2017, 5, 45–57. [Google Scholar]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of Acetic Acid and Lactic Acid on the Growth of Saccharomyces Cerevisiae in a Minimal Medium. J. Ind. Microbiol. 2001, 26, 171–177. [Google Scholar] [CrossRef]
- Higa, T.; Parr, J. Beneficial and Effective Microorganisms in a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994; pp. 1–16. [Google Scholar]
- Boldrin, A.; Andersen, J.K.; Møller, J.; Christensen, T.H.; Favoino, E. Composting and Compost Utilization: Accounting of Greenhouse Gases and Global Warming Contributions. Waste Manag. 2009, 27, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Staugaitis, G.; Viškelis, P.; Venskutonis, P.R. Optimization of Application of Nitrogen Fertilizers to Increase the Yield and Improve the Quality of Chinese Cabbage Heads. Acta Agric. Scand. B Soil Plant Sci. 2008, 58, 176–181. [Google Scholar] [CrossRef]
- Kosson, R.; Felczyński, K.; Szwejda-Grzybowska, J.; Grzegorzewska, M.; Tuccio, L.; Agati, G.; Kaniszewski, S. Nutritive Value of Marketable Heads and Outer Leaves of White Head Cabbage Cultivated at Different Nitrogen Rates. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 524–533. [Google Scholar] [CrossRef]
- Vishwanath; Kumar, S.; Purakayastha, T.J.; Datta, S.P.; Rosin, K.G.; Mahapatra, P.; Subodh Kumar, S.; Surya, P.Y. Impact of forty-seven years of long-term fertilization and liming on soil health, yield of soybean and wheat in an acidic Alfisol. Arch. Agron. Soil Sci. 2020, 1–16. [Google Scholar] [CrossRef]
- Stuch, B.; Alcamo, J.; Schaldach, R. Projected climate change impacts on mean and year-to-year variability of yield of key smallholder crops in Sub-Saharan Africa. Clim. Dev. 2020, 1–15. [Google Scholar] [CrossRef]
- Safaei Khorram, M.; Zhang, G.; Fatemi, A.; Kiefer, R.; Maddah, K.; Baqar, M.; Zakaria, M.P.; Li, G. Impact of biochar and compost amendment on soil quality, growth and yield of a replanted apple orchard in a four-year field study. J. Sci. Food Agric. 2018, 99, 1862–1869. [Google Scholar] [CrossRef]
- Zhang, Q.; Xia, Q.; Liu, C.C.K.; Geng, S. Technologies for Efficient Use of Irrigation Water and Energy in China. J. Integr. Agric. 2013, 12, 1363–1370. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Guo, E.; Yan, D.; Sun, Z. The impacts of long-term and year-to-year temperature change on corn yield in China. Theor. Appl. Climatol. 2015, 119, 77–82. [Google Scholar] [CrossRef]
- Möller, K.; Schulthei, U. Organische Handelsdüngemittel im Ökologischen Landbau. Charakterisierung und Empfehlungen für die Praxis; Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V.: Darmstadt, Germany, 2014; pp. 1–392. [Google Scholar]
- Webb, J.; Sørensen, P.; Velthof, G.; Amon, B.; Pinto, M.; Rodhe, L.; Salomon, E.; Hutchings, N.; Burczyk, P.; Reid, J. Chapter Seven—An Assessment of the Variation of Manure Nitrogen Efficiency throughout Europe and an Appraisal of Means to Increase Manure-N Efficiency. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press/Elsevier: Amsterdam, The Netherlands, 2013; pp. 371–442. [Google Scholar]
- Mittlere Nährstoffgehalte organischer Dünger (Richtwerte, Stand.: Januar 2014); Landwirtschaftskammer Nordrhein-Wesefalen: North Rhine-Westphalia, Germany, 2014.
- Ebertseder, T. Humusbildung Und Nährstoffbetrachtungen von Bioabfallkompost Und Gärrückständen Im Vergleich; Witzenhausen-Institut für Abfall, Umwelt und Energie GmbH: Witzenhausen, Germany, 2007; pp. 1–16. [Google Scholar]
- Lazicki, P.; Geisseler, D.; Lloyd, M. Nitrogen Mineralization from Organic Amendments Is Variable but Predictable. J. Environ. Qual. 2020, 49, 483–495. [Google Scholar] [CrossRef]
- Baumgarten, A.; Almesberger, M.; Eschlböck, K.; Greimel, J.; Hamedinger, S.; Hoffmann, G.; Hofmair, W.; Keferböck, J.; Kovats, H.G.; Mayer, J.; et al. Richtlinien für die Sachgerechte Düngung im Garten- und Feldgemüsebau, 3rd ed.; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft: Vienna, Austria, 2008; pp. 1–64. [Google Scholar]
- Pokluda, R. Nutritional Quality of Chinese Cabbage from Integrated Culture. Hort. Sci. 2008, 35, 145–150. [Google Scholar] [CrossRef]
- Mitscherlich, E.A. Das Gesetz des Minimums und das Gesetz des Abnehmenden Bodenertrages. Landwirtsch Jahrb. 1919, 38, 537–552. [Google Scholar]
- Chien, S.H.; Sikora, F.J.; Gilkes, R.J.; McLaughlin, M.J. Comparing of the Difference and Balance Methods to Calculate Percent Recovery of Fertilizer Phosphorus Applied to Soils: A Critical Discussion. Nutr. Cycl. Agroecosyst. 2012, 92, 1–8. [Google Scholar] [CrossRef]
- Deumlich, M.; Lux, G.; Knut, S. Nährstoffmanagement im Ökologischen Landbau; Hochschule für Technik und Wirtschaft: Dresden, Germany, 2016; pp. 1–107. [Google Scholar]
- Kautz, T.; Amelung, W.; Ewert, F.; Gaiser, T.; Horn, R.; Jahn, R.; Javaux, M.; Kemna, A.; Kuzyakov, Y.; Munch, J.-C.; et al. Nutrient Acquisition from Arable Subsoils in Temperate Climates: A Review. Soil Biol. Biochem. 2013, 57, 1003–1022. [Google Scholar] [CrossRef]
- Rouifed, S.; Cottet, M.; Battista, M.; Le Lay, Y.-F.; Rateau, P.; Rivière-Honegger, A.; Florence, P. Inefficiency of cutting stems once during the vegetative growth of Fallopia spp. Manag. Biol. Invasions 2020, 11, 399–405. [Google Scholar] [CrossRef]
- Barberis, L.; Chevalier, W.; Toussaint, M.-L.; Binet, P.; Florence, P.; Michalet, S. Responses of the species complex Fallopia × bohemica to single-metal contaminations to Cd, Cr or Zn: Growth traits, metal accumulation and secondary metabolism. Environ. Monit. Assess. 2020, 192, 673. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Fowler, M.S.; Hocking, S.; Eastwood, D. Please don’t mow the Japanese knotweed! NeoBiota 2020, 60, 19–23. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).