Abstract
The paired Fluvisol and cereal samples in both the field screening and controlled experiments are reported to elucidate the soil–crop relationship for As, Cd, and Pb in relation to changing contamination levels. Significant varietal differences in plant uptake were observed for crop type (barley, triticale) and the harvested part of the crop (oat shoots and grain). When parametrizing the stepwise regression models, the inclusion of soil properties often improved the performance of soil–crop models but diverse critical soil parameters were retained in the model for individual metal(loid)s. The pH value was often a statistically significant variable for Cd uptake. For As and Pb, the more successful model fit was achieved using the indicators of quantity or quality of soil organic matter, but always with lower inherent model reliability compared to Cd. Further, a single correlation analysis was used to investigate the relationship between extractable metal concentrations in soil solution and their crop accumulation. For Cd, there were strong intercorrelations among single extractions, the NH4NO3 extraction stood out with perfect correlation with plant uptake in both experiments. For As and Pb, the CaCl2 and Na2EDTA solutions outperformed other single extractions and were the better choice for the assessment of depositional fluvial substrates.
1. Introduction
The prime conditions either for farming or various services for human society (settlement, manufacturing) have been provided by floodplains since the dawn of civilization. On the other side, flooding outlets always bring some risks either for human society or soil functions, including additional environmental and health-related hazards due to the inputs of toxic pollutants, i.e., trace elements (TEs) [1]. The changing spatial and temporal character of biogeochemical processes makes floodplains geochemically reactive pools affecting the retention/remobilization of TEs. Since intensive farming encounters vulnerable soil conditions for the mobilization of TEs, concern about their possible transfers into the human food chain may be raised [2]. Generally, the transfer of TEs into plant organs shows high element and plant-specific differences [3]. As a consequence, a soil–plant transfer seldom corresponds to stocks in soils, even in geochemically reactive soils such as floodplains soils [2,4], highly polluted mining sites [5], and industrial sites [6].
Since the fate of TEs is known to crucially depend on their sorption–desorption equilibria and dynamics in the soils, the predictive models for plant uptake for an array of TEs usually rely on the empirical multiple linear models that count for the effect of the most important soil properties i.e., pH, organic carbon, and CEC [6,7,8,9,10,11]. The element uptake depends also on the plant species, vegetation periods, and analyzed plant tissue [3,6,12,13], making them important for agronomic purposes.
Generally, understanding element uptake by various crop plants has been of general concern for food security [14], from a toxicity point of view [15] as well as remediation purposes [16], especially for the metal(loids) with established or suspected adverse effects The indirect route for entry into the food chain via bioaccumulation may considerably contribute to potential health risks [1,6], and especially cereals and grains may play a crucial role in dietary exposure [17]. Given the generally elevated load of Fluvisol, studying the behavior of trace elements within those soils and identifying controlling factors of bioaccumulation in their crops is of great concern for agronomic management purposes. Cereals are a staple crop for food production, and they are intensively cultivated within the floodplains.
Since the nation-wide or continental systematical monitoring usually reports rather the geochemical status of soils than the chemical composition of crops, and since soil quality programs rarely include data on the available pools of trace elements in soils, it is of great concerns to benefit from the extensive information on total element concentrations and key soil properties as an alternative route to derive potential crop uptake. Hence, we set the primary objective of this study to derive the relationship among various extractions of soil metal(loid)s (As, Cd, and Pb), and further investigate the relationship between those extractions and crop uptake under conditions of changing magnitude and geochemical reactivity of Fluvisol contamination. In the present study, widely used regression techniques were used to estimate the effect of different soil parameters on the concentration of three metal(loid)s (As, Cd, and Pb) in cereal crops grown in floodplains in the Czech Republic. The novelty of our study is that the relationships between soil properties and crop contents were assessed across changing reactivity gradient of soil contamination and using various field and controlled experiments. Since the atmospheric deposition can contribute to contamination of plant tissues, the indicators of atmospheric deposition for studied metal(loid)s were also included in the model building for field screening or were reduced in controlled conditions of experiments.
2. Materials and Methods
The set of experimental data was assessed to investigate the effect of various soil properties on plant uptake under various experimental settings (real terrain screening—controlled field conditions and greenhouse pot experiment), and along with the changing geochemical reactivity of soil metal(loid)s. Since the Czech legislation adopted hierarchical soil screening limits and the second level of limit values was set explicitly for As, Cd, Pb, Ni, and Hg—i.e., risky elements with elevated threats for food chain [18], the study was focused on three elements (As, Cd, and Pb) that were typically elevated in Czech floodplain soils [19,20].
2.1. The Terrain Sampling of Soils and Cereal Crops under Real Cultivation Conditions
Data for terrain screening were obtained from a nation-wide sampling of the representatives of cultivated floodplains where a high flood frequency has met a high extent of arable land and with cereal crops. For each sampling site, a set of 10 individual topsoil and plant subsamples were collected from a hectare area of arable land under real cultivation conditions in 26 randomly selected locations in the Czech Republic. For each sampling site, a set of 10 individual probes was collected from an area of 1 ha using a hand auger up to a depth of 30 cm. The soil was homogenized by quartation. The plant samples were taken in the stage of full crop maturity at the spot of the auger bores. In the case of wheat plants, spikes with ripe grains were collected and air-dried. Further, grains were threshed to yield material for chemical analyses. Similarly, the grains from corns were pulled out from the air-dried cobs. The terrain samples of soils and plants were transported to the laboratory for chemical analyses.
In an attempt to cope with differences in depositional inputs of metal(loid)s into crops under real cultivation conditions, we have used the estimated annual deposition of metal(loid)s on the sampling site calculated as a five-year moving average of deposition extracted from the spatial model that used the continuous measurement of air pollution at ground monitoring site [21]. The parameter of moving average (from period 2012–2016) of annual deposition of each metal(loid) for each sampling locality was added to the dataset and model-fitting performance.
2.2. The Experimental Setting of Greenhouse Pot Experiment with a Cereal Crop Grown in Moderately Contaminated Fluvisols
The controlled pot experiment was conducted with 6 kg Fluvisols with elevated concentrations of studied metal(loid)s. To homogenize the soil samples, the entire volume of soil was mixed in a container and sieved at a mesh size of 5 mm after the gravel had been removed and large aggregates crushed. All the samples for the pot trial were taken in moderately contaminated floodplains located at ten various sites from the middle and lower reach of the Elbe River (for localization see Figure 1) and were selected to represent moderately contaminated Fluvisols. The soils from 10 localities were prepared for the pot experiment. For each locality, the natural soil and the soil treated with liming were prepared in duplicates. The pot experiment was placed into a greenhouse to reduce the effects of atmospheric deposition. Barley and triticale were selected for the pot cultivation experiment, and were sowed after the topsoil had been finished to prepare the seedbed. During the growing phase, the pots were watered according to plant needs by adding pure water. All plants were harvested on the stage of the 5th leaf, and the shoots were separated, washed with demineralized water, and transported for chemical analysis.
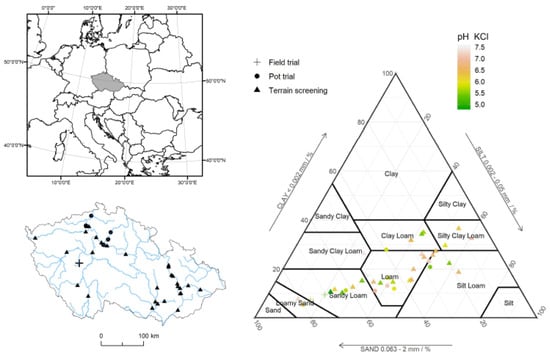
Figure 1.
The basic soil properties of Fluvisols from the terrain screening and controlled experiments.
2.3. The Experimental Setting of Small-Scale Field Experiment with a Cereal Crop Grown in Highly Contaminated Fluvisols
In order to complete the observation of soil controlling factors for As, Cd, and Pb- uptake by cereal across metal(loid) contamination gradient, we have used the results of our field experiment conducted at well-known heritage burden of highly contaminated overbank sediments deposited after several flood-induced failures of tailing ponds in the Příbram Ore District [22,23]. The experimental area was divided into 1 × 1 m square fields, where a set of various soil treating was tested in four replicates to simulate various common management practices—four fields under untreated, natural soil conditions, four fields with the addition of dolomitic limestone (pH 8.2), four fields with the application of the compost (pH 7.2, 35.5% Cox), four fields with an acid peat addition (pH 3.8, 30.1% Cox), and finally with a muck-material of sapric histosol (pH 7.4, 7.2% Cox) ploughed under as described by [24]. The fields were left to incubate after the addition of amendments for a season, and the next season sowed by oat plants. During the growing phase, the fields were watered according to plant needs by adding pure water. The first half of the oat yield was harvested on the stage of the 5th leaf to analyze the oat stalks, the second one in the stage of full oat maturity to separate the oat grains from ears.
2.4. Joint Determination of Basic Soil Properties and Metal(loid)s Contents in Soils and Plants
For the soils from all experimental designs (terrain screening, controlled pot, and field experiments), a stable set of basic soil properties was recorded with the common methodical framework—a grain size distribution according to ISO 11277; soil pH potentiometrically using the 1 mol/L KCl solution according to ISO 10390; the content of organic carbon by the oxidimetric method using potassium dichromate according to ISO 14235, and qualitative humus indicator using the color quotient Q4/6 measured in 0.1M pyrophosphate mixture (after filtration and appropriate dilution) within the range 300–600 nm.
The soils from both terrain screening and controlled experiments were air-dried and sieved according to ISO 11464, and then extracted using chemical extraction media of different strengths. The soils from terrain screening were extracted using the aqua regia [25] and ammonium nitrate [26]. The soils from controlled experiments were extracted using the ammonium nitrate [26] and acid mixture (acid digestion with HNO3, HClO4, and HF) [27]. In both controlled (pot, field) experiments an extended series of partial weak extractions (Table 1) was conducted to test suitable extraction of bioavailable pools for best-fit of plant uptake prediction. Trace elements in all extracts were measured by the atomic absorption spectrometry in flame (VARIAN FAAS 240 FS; Pb), atomic absorption spectrometry with electrothermal atomization (VARIAN ETA 240 Z; Cd), and with the hydride generation atomic absorption spectrometry (As) [28]. The quality assurance of analytical data is guaranteed by the control process using certified reference materials (soils and sediments, CRM 7001–7004, Prague, Czech Republic) and certified controls of the laboratory according to CSN EN ISO/IEC 17043:2010. The relative standard deviation tolerance for analytical replicates was determined lower than 5%.

Table 1.
Overview of the used extraction methods for studied soil metal(loid)s (As, Cd, and Pb).
The metal(loid) contents in plants were determined shortly after the harvest by AAS (VARIAN ETA 240 Z and FAAS 240) after the plant samples had been mineralized using the acid mixture (HNO3, HClO4). All the chemical analyses were performed in the accredited laboratory of the Research Institute for Soil and Water Conservation, Prague.
2.5. Data Preparation and Statistical Analyses
All the plant and soil contents were transformed using the natural logarithm of the concentration to ensure homogeneity of variances before the statistical analysis. Pearson correlation coefficients were calculated to determine the relationship between plant contents and soil contents using various extracting agents. Then, stepwise multiple linear regression analysis was built to identify the significant soil variables, which may be used to fit a model of estimating the plant contents of metal(loid)s in various cereals (straw or grains). A total of four soil variables (pH, soil organic carbon, color quotient Q4/6, and clay content) were used for regression modeling in all stages of experimental design. The set of variables was further extended by atmospheric deposition for terrain screening and broader series of partial weak extractions of soil metal(loid)s for the controlled pot and field experiments. Stepwise regression analysis was performed to identify that set of variables, which gave its calculation best-fit models measured by percentage variance explained—i.e., adjusted R2. Given the use of the ratio of mean-squares instead of the sums of squares, computing of the adjusted value allows a comparison of regression models containing different numbers of variables. The significance level for deciding if the predictor enters into the stepwise model was set p < 0.05, and the significance level for deciding when to remove a predictor from the stepwise model was set p > 0.15. Data analyses were performed using the R program version 3.6.1.
3. Results
The basic descriptive statistics of HM contents in the soil samples from field screening and for soils used for controlled experiments are listed in Table 2 and the main soil properties are summarized in Figure 1. As shown in Figure 1, the experimental soils also differed in basic soil properties—i.e., pH, particle size distribution, and organic carbon content.

Table 2.
The basic statistics of soil properties and raw concentrations of As, Cd, and Pb in soils from three experimental stages of the Fluvisol testing.
The mean concentrations of metal(loid)s in soils followed the magnitude order Pb > As > Cd, and slightly changed in crop plants Pb > As ~ Cd across all experimental designs. The mean concentration of both total and bioavailable pools of studied metal(loid)s increased in the sequence of terrain screening < pot experiment < field experiment, and hence representing the condition of Fluvisols with background contamination, moderately contaminated Fluvisols with local incidence, and the highly contaminated Fluvisols as exceptional hotspots. Additionally, when the pollution status of experimental soils was assessed according to the current Czech limit thresholds, the terrain soils were on average below the first level of soil threshold for preventing soil from being polluted. The soils used for the pot experiment exceeded the preventive values, and the soils of the field experiment exceeded the second level of limit values for evaluating hazards (for health hazards in case of As and Pb, and for food safety threats in case of Cd).
3.1. The Results from Background Fluvisol-Terrain Screening of Soils and Cereal Crops
As shown in Table 2, Fluvisols randomly selected in areas, where the risk of extensive inundations met a high cropland extent, showed low metal(loid) contents (As, Cd, and Pb), and were close to their background levels in Czech soils [18]. Further, the established metal(loid) extractability using the weak extraction by NH4NO3 detected a very low portion of mobile forms of studied elements during the screening of fluvial croplands. The field screening showed that despite being more vulnerable to soil contamination and potential mobility, it is usually an issue of site-specific spots that may be successfully traced by various geochemical tools adapted for fluvial records [31,32]. Lower metal(loid) extractability was also reflected in their low translocation into grains of cereals cultivated in the floodplain areas. Indirect effects of soil properties on risky elements in cereal crops were determined from the product of the simple correlation coefficient between soils and plants after the soils had been grouped according to the various soil properties. The results were grouped according to the median values of pH, organic carbon content, the color quotient of humic substances Q4/6, and estimated annual deposition of the studied metal(loid)s at the site of soil sampling derived from the spatial model of the Czech Hydrometeorological Institute. For both groups (higher and lower than median values of selected parameters), Pearson’s correlation coefficients between total soil contents and grain contents are summarized in Table 3.

Table 3.
Pearson’s correlation coefficients between log-transformed total soil contents and grain contents after the screening soils had been grouped according to the median values of basic soil/site characteristics.
Between the pH groups, the grain contents were better correlated with soil contents for Cd in soils with lower pH, showing the well-known fact of pH-dependency of Cd mobility in various soils [13,33], not excluding Fluvisols [34,35]. The opposite relationship, but with lower statistical confidence, hinted at the better correlation of As contents between soils and grains within the group of soils with higher pH. These findings corresponded to the conclusion that liming-induced changes of pH did not influence the plant uptake of those elements with very low transfer factors, like As or Pb [36]. When focusing on the soil constituent influencing the binding capacities, the correlation between Cd content in soil and cereals differs between the soil’s groupings. Generally, a higher correlation was observed in soils with lower content of clay and lower quality of humus (higher values for the color quotient of humic substances Q4/6). The results indicated that high quality of soil organic matter or higher clay content may affect the soil’s ability to retain risky elements in an exchangeable form, and hence prevent potential translocation of metal(loid) into aboveground biomass. Eriksson [37] found that for a given total Cd concentration, Cd appeared higher soluble and bioavailable in sandy soil than clay soil. Moreover, the experiment with ryegrass showed that the transfer-preventing effect of organic matter was higher in sandy than clay loam soils [38]. After the organic carbon grouping, As showed significant correlations only for the low carbon group (Cox < 1.35). Trace elements may complex directly with solid-phase components such as humic substances, and hence the content of exchangeable forms in Fluvisol may be both reduced or enhanced, depending on the distribution between organic fractions [4,39]. There was not proved a statistically significant effect of the soil constituent for Pb correlations in our soil screening results. It corresponds to the published results of less satisfactory prediction of the Pb-solubility from measured soil properties in comparison to Cd, Cu, and Zn [33]. It should be noted that Cd proved an obvious difference in correlation patterns after the soils had been divided using the estimated annual Cd deposition on the area of the sampling. A statistically significant correlation between soils and plants was only proved for soils with lower atmospheric deposition of Cd.
The stepwise regression analysis as a function of both available soil pools (NH4NO3 extraction), total content and soil properties (pH, organic carbon, clay content, color quotient of humic substances Q4/6, estimated annual deposition of the given elements on the area of the sampling) were built for As, Cd, and Pb using the soil screening data. The empirical models using the soil characteristics test were able to explain the very low portion (8%, 21%, and 16%) of the variability in levels of As, Cd, and Pb in cereal grains. The results of the multiple stepwise regression analysis showed that only the predictors of estimated atmospheric deposition remained in an attempted model fit for As and Pb in grains of cereal crops grown in low-contaminated Fluvisols (Table 4). But it should be noted that the depositional parameters alone have not allowed reliable fit for As and Pb. The soil parameters pH and color quotient Q4/6 together with atmospheric deposition of Cd contributed significantly to the model fit for Cd with the better yet not acceptable portion of explained variability (Table 4).

Table 4.
Terrain screening-the results of stepwise regression using the log-transformed soil contents (As, Cd, and Pb) and basic soil/site characteristic.
The results confirmed that the interaction between plant and soil is very complicated and of multifactorial nature. The weak soil–plant correlation was usually found in floodplains soils [2,39], even in those with elevated soil stocks [40,41]. Given the multifactorial nature of soil–plant interaction and the relatively low number of sampled cereal plants, the result should be interpreted with appropriate caution, but the regression results hinted at the effect of the depositional parameters. Differences in depositional atmospheric load can contribute to variation in correlation levels between soils and aboveground biomass of plants. Given the difficulty in identifying significant relationships of soil–plant interaction using field data, we researched the possible contributory factors to metal(loid) uptake under controlled experimental conditions.
3.2. The Results from Moderately Contaminated Fluvisols-a Greenhouse Pot Experiment with a Cereal Crop
Since there was not possible to build reliable regression models for crop production using the results from low-contaminated Fluvisols, we have used the result from the greenhouse pot experiment with a cereal crop and soils from moderately contaminated Fluvisols from the Elbe floodplain. The median contents of all HMs in soil used for the pot experiment were approximately four times higher than their background levels in Fluvisols from terrain screening soils (Table 2), and did not meet the Czech thresholds preventing cultivated soils from excessive inputs of risky elements and substances.
The low levels of mobile portion of risky elements observed in the Fluvisol screening area led us to investigate further various weak extractions and their correlations with plant uptake. As shown in Figure 2 negligible differences were observed in correlation patterns between soil total contents and weak extraction results for studied elements As, Cd, and Pb. Their total contents generally showed the tightest correlations with the contents after the HNO3 and Na2EDTA extraction. Noticeably, a less tight relationship proved the total contents with the results of the weaker CaCl2 and NH4NO3 extractions. Even in moderately contaminated soils, there was still a very low portion of Pb extractable by NH4NO3 (with a significant amount of left-censored values).
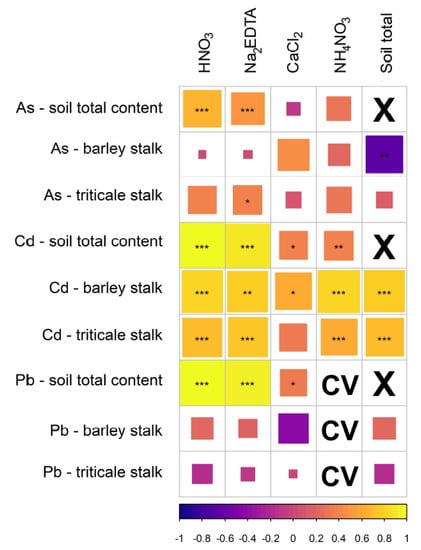
Figure 2.
Pot experiment results—the correlogram of the log-transformed concentrations of As, Cd, and Pb after their weak extractions with their total soil and plant content (the cells are sized and asterisked according to significance test of correlation p-value (* p < 0.05, ** p < 0.01, *** p < 0.001); the color gradient represent the direction and strength of the correlation; CV—the correlation was affected by the high portion of left-censored values below the detection limit, X—diagonal elements of the correlation matrix—the correlation of a column with itself).
Considering the correlation between various extraction of soil risky elements and plant contents, significant differences were found among the risky elements, and between crop types (barley vs. triticale shoots). For As, we found a very low correlation for the total contents of soil As and shoot contents for both cereal crops (barley and triticale). Whilst As in barley shoots showed the acceptable correlation with soil As extracted with neutral CaCl2 salt, As in triticale better correlated with soil As in the Na2EDTA extractions. Cd in both studied crop plants significantly correlated with soil Cd pools in the case of all extracting agents. Whereas, negligible correlations were observed Pb in plants and soils regardless of the extraction method.
Further, stepwise multiple linear regression was used to derive empirical models capable of predicting risky elements content in cereal shoots based on soil properties and various extraction of soil risky elements. In contrast to field screening models, we have not used the depositional parameters due to reducing the atmospheric effects in a greenhouse experiment. Nevertheless, the extended series of partial weak extractions were used for the stepwise model build. The regression analysis considered the same critical soil properties such as soil pH, quantity, and quality of soil organic matter content (Cox and color quotient Q4/6), and clay content. Comparing the results of stepwise regression modeling, the effect of the dummy variable for crop type manifested the differences for As and Cd in their plant uptake (Table 5). The results corresponded to the published results for cereal crops [42] as well as wild-growing plants [43] and probably referred to physiological controls of metal(loid) accumulation by different plant species [44]. These effects justify rather preferential use of the crop-specific prediction models against the universal plant uptake models [45].

Table 5.
Pot experiment-the results of stepwise regression using the log-transformed soil contents (As, Cd, and Pb) and basic soil/site characteristic.
In most cases, the inclusion of soil properties improves the model performance between metal concentration in plant and soil but diverse critical soil parameters were retained in the stepwise modeling for individual metal(loid)s. Soil pH was the critical soil variable that controls the Cd uptake by crops through the dissolution of Cd soil species that is also manifested by the significant effect of NH4NO3-extracted Cd in the regression fit. This relationship is well-known from both field and experimental conditions of crop uptake [36,46,47].
Whilst many studies suggest a link between soil organic matter content and fixation of metals in the soil solid phase in terms of ion exchange other authors described solubilization of some risky elements due to the formation of unstable complexes with organic matter in fluvial soils [34,35]. Our experimental result showed that under the controlled conditions of pot experiment the organic fraction in Fluvisols had rather a fixation effect on metal binding. Therefore, in the present study, the plant uptake of As obviously decreased with the increasing content of soil organic carbon in Fluvisol. The fractionation of soil organic matter is of great importance for the mobility of trace elements in soils. Concerning the fractionation of soil organic matter, the regression results from the pot experiment showed that the decreasing color quotient (Q4/6) as an indicator of increasing humus quality had a significant effect on the plant uptake of soil Pb. The results showed that Pb can be trapped more effectively by Fluvisols with higher humus quality, preventing plant uptake.
But it should be noted that these findings are valid under the stable soil conditions of Fluvisol during the controlled pot experiment, and the field conditions of Fluvisols may significantly vary during the episodic changing of flooding–drying cycles with serious impacts on soil conditions affecting the mobility of trace elements [35,48]. The experimental settings rather simulated the field conditions during an equilibration period of a dry cycle. Whilst many authors discussed that a drop of redox potential due to temporary waterlogging may push the metals in the soil solid phase to be dissolved ([33,34], and references therein), using the results from selective extractions, X-ray diffraction, and Mössbauer spectroscopy, Tack et al. [49] concluded that such changes were not detectable during short temporarily changes (drying and waterlogging) of experimental soils. Moreover, soil solution concentrations of trace elements were significantly higher in dried sandy and acidic or poorly buffered soils, and even the saturated soils had lower concentrations of metals in the soil solution.
3.3. The Results from Highly Contaminated Fluvisol-a Small-Scale Field Experiment with a Cereal Crop
In order to complete the observation of soil controlling factors for As, Cd, and Pb- uptake by cereal across metal(loid) contamination gradient, we have used a series of extracting agent with a changing strength of reactivity (Table 1) to test their efficiency in the prediction of plant uptake in highly contaminated Fluvisol, similarly to the controlled pot experiment. As shown in Figure 3, the total contents generally proved tight correlations with partial extractions using various agents which can be attributed to the higher reactivity of soil metal(loid)s in tailing-impacted Fluvisol. The increased pool of bioavailable forms of metal(loid) was also manifested in an increase of correlations between their soil contents and contents in the oat straw, especially for Cd, As, and less for Pb. The correlation between the oat grains and bioavailable pools of risky elements was lower and varied significantly among the studied metal(loid)s as well as among the partial extractions being used to assess their bioavailable pools. The experimental results showed that the neutral salt solution CaCl2 was quite effective in indicating the As and Cd contents in both oat straws and grains. For Pb, various weak extractions were comparable in correlation with Pb content in straws, but negatively correlated with contents in oat grains, in both cases the linear relationship in the sample data was not strong enough (p > 0.05). The results indicated the order of mobility and plant uptake Cd > As > Pb, and also the existence of functional defense mechanisms preventing the excessive translocation of metal(loid)s into cereal grains [12].
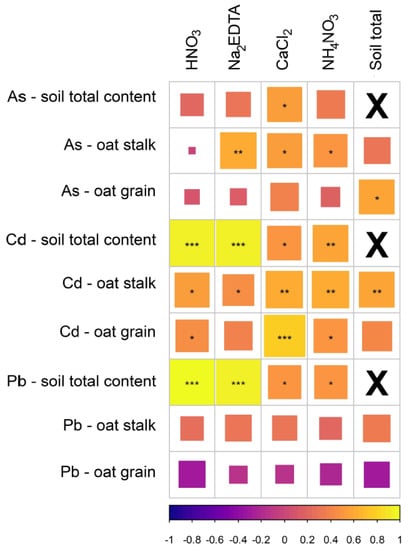
Figure 3.
Controlled field experiment—the correlogram of the log-transformed concentrations of As, Cd, and Pb after their weak extractions with their total soil and plant content (the cells are sized and asterisked according to significance test of correlation p-value (* p < 0.05, ** p < 0.01, *** p < 0.001); the color gradient represents the direction and strength of the correlation; X—diagonal elements of the correlation matrix—the correlation of a column with itself).
In addition, stepwise multiple linear regression was used to derive empirical models capable of predicting risky elements content in oat shoots and grains based on soil properties and various single extractions of soil metal(loid)s. Again, the extended series of partial weak extractions were used for the stepwise model build. The regression analysis considered the same critical soil properties such as soil pH, quantity and quality of soil organic matter content (Cox and the color quotient Q4/6), and clay content.
To see if the models for grains and straw differ in the critical soil parameters, we also compared the differences between the regression coefficients for two individual models. The study showed a considerable value of the regression models in accounting for the variation in metal(loid)s contents between oat straw (Table 6) and seed (Table 7). Amounts of As, Cd, and Pb were generally significantly higher in straw than in respective seed samples, and also the critical soil properties with strong relationship within regression models little varied. Generally, the inclusion of basic soil properties (pH, Cox, Q4/6, and clay content) improved the model performance rather for straw contents than for grain contests. The models for grain content generally showed lower values of adjusted R2, and relied mainly on the various pools of soil metal(loid)s (Table 7).

Table 6.
Controlled field experiment—the results of stepwise regression for metal(loid) content in oat stalks using the log-transformed soil contents (As, Cd, and Pb) and basic soil characteristic.

Table 7.
Controlled field experiment—the results of stepwise regression for metal(loid) content in oat grain using the log-transformed soil contents (As, Cd, and Pb) and basic soil characteristic.
4. Discussion
4.1. Comparison of Regression Models of Crop Uptake under Various Experimental Conditions
Whilst the regression results for soil–crop relationship for cereals grown in Fluvisols with background metal(loid) concentration showed that it was harder to obtain reliable model fit under field conditions, the results from controlled experiments with higher contamination levels showed significantly better model fit. Although the observed poor linear relationship between soil and plant seeds in terrain screening corresponded to the general crop behavior under natural soil conditions [6,42,45,50,51] as well as the uptake by wild-growing plants in heavy-impacted soils [43,52], it is important to note that the poor model-fit in the survey may be caused by underlying factors not studied. Very weak soil–crop relationships for various metal(loid)s were also observed within the terrain screening of moderately contaminated fluvial soils [2,39]. It may be the effects of uptake of metals via aerial plant organs, differences in the development stage of plants, as well as the low number of samples, and their spatial heterogeneity [2]. The results of metal(loid) content may also significantly differ among both crop types and their cultivars [42,53]. Accounting for the variation of soil–crop relationship in our terrain screening, the addition of mean annual deposition of metal(loid)s into linear modeling indicated the appreciable role of atmospheric deposition. The plant uptake may be significant if the soil metal(loid)s are elevated to excessive amounts or occurred in highly reactive forms, and hence for low-contaminated soils, the model-fit is hampered by a low variation of plant contents near the detection limit, and usually a large number of samples below detection limits. The effect may be seriously inflated for those elements with very low transfer factors, like As, Be, Cr, V, or Pb [35,54]. On the contrary, for the elements with higher mobility, the linear relationships are usually achieved and exploited for prediction—like for Cd in our study but were also published for Cu, Ni, and Zn [55,56]. Summarizing the extensive nationwide monitoring data, the Pb concentration in various crops (wheat, barley, potato, hop, and rapeseed) were often close to the detection limit, even as their respective soil concentrations were elevated, and hence the reliable model fit was excluded [45]. The issue of left-censored data was familiar for Pb in both low contaminated set of Fluvisol (excessive amounts of censored values for both plant contents and soil bioavailable pools after the NH4NO3 extraction) and moderately contaminated set of Fluvisol (higher number of samples below the detection limit for soil bioavailable pools after the NH4NO3 extraction). When studying the linear relationship between cereal crops and Fluvisols with elevated metal(loid) pools (controlled pot and field experiments), the reliability of models increased, and allow to account for the effect of soil physicochemical properties. When parametrizing these models, the inclusion of soil properties improves the model performance between metal concentration in plant and soil but diverse critical soil parameters were retained in the stepwise modeling for individual metal(loid)s. On the side of crop type, there were proved significant differences between barley and triticale (pot experiment) and between vegetative and reproductive parts of oat (field experiment). Similarly, Adams et al. [42] observed the differences in the regression fit for Cd uptake between barley and wheat. The physiological controls that can explain variability in the accumulation of trace elements by different plant species are rather unknown but are probably connected to various strategies adopted by various species to cope with the harmful consequences of heavy metal toxicity [43,44]. These effects justify rather preferential use of the crop-specific prediction models against the universal plant uptake models [45].
Since pH has been widely found as a reliable predictor for bioavailable contents of Cd [33], it was not surprising that pH retained a significant predictor for Cd-content in barley, triticale, and oat stalks under controlled experimental conditions. Nevertheless, the tight relationship between Cd-uptake and pH was also observed in correlation and regression results from terrain screening of cereal crops (Table 3 and Table 4). It showed that Cd uptake with its pH-sensitivity allowed to build quite universal uptake models. Adopting a set of regression models available from the scientific literature on results from the Czech soil–crop monitoring, only Cd-models proved acceptable prediction ability [45]. It should be noted, that the prediction potential of pH on plant uptake turned out insignificant as soon as the Cd content in oat grains was modeled, yet the pH-dependent bioavailable pools of Cd (extracted using CaCl2 and Na2EDTA) remained significant regressors. In contrary to Cd, for As and Pb, the more successful model-fit was generally achieved using the indicators of quantity or quality of organic matter, but always with lower inherent model reliability compared with Cd.
4.2. Evaluation of Single-Extraction Methods to Estimate Crop Uptake in Fluvisols
Since Vácha et al. [57] discussed that NH4NO3 solution may not be the most suitable for the assessment of depositional substrates thanks to continual leaching by water in a semi-aquatic environment, we intercorrelated the results of different single extraction methods for soil As, Cd, and Pb in relation to their cereal crop uptake from the controlled pot and field experiment. From Table 1, it can be concluded that the NH4NO3 extraction became inadequate for studied metal(loid)s in low-contaminated fluvial records, and remained problematic for low-mobile elements (As, Pb) even in a fluvial environment with elevated contamination. A decent solution may be achieved by the replacement of the simple NH4NO3 extraction according to the ISO protocol [22] by the first extraction step of Zeien and Brümmer’s sequential extraction procedure [58] with a longer extraction duration and higher proportion of extracting agent to sample [59]. But during the controlled experiments, the soil contents extracted by both the CaCl2 and Na2EDTA solutions showed decent correlations with plant contents in cereal stalks. In the case of Cd, the choice of suitable weak extraction seemed simpler, since there were strong correlations among them, and the NH4NO3 extraction stood out with a near-perfect positive correlation with plant uptake in both experiments. In contrast to Cd, reliable choice of candidate indicators for Pb plant uptake was not allowed by our correlation analysis. The result showed that even within the controlled experiment of crops grown in highly reactive Fluvisol, it was hard to obtain reliable uptake models that would allow a greater understanding of the factors that lead to high metal(loid) content in grain, especially for Pb and As. It is important to note that the poor model fit in the survey may be caused by underlying factors not studied—i.e., the plant physiology of metal(loid) uptake, the different response of plant growth, and contents of individual elements in plant biomass. The results of metal(loid) content may be significantly different among various cereal cultivars [42,53]. A proper description of these physiologically-based differences of plant uptake mechanism may bring some possibilities to develop cereal crops with the lower intake or translocation of metal(loid)s [60]. The comparison of various cereals in their strategies to cope with the adverse effects of soil contamination may be used to search the genotypes with tolerance resp. vulnerability to metal contamination [61,62].
Author Contributions
Conceptualization, R.V. and J.Č.; methodology, R.V.; formal analysis, J.S.; data curation, J.Č.; writing—original draft preparation, J.S.; writing—review and editing, R.V. and J.Č.; visualization, J.S.; supervision, R.V.; project administration, J.Č.; funding acquisition, R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Agriculture of the Czech Republic [Institutional support MZE-RO0218] and the Czech Science Foundation [project No. GA17-00859S].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health Risk Assessment of Potentially Toxic Elements in Soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Overesch, M.; Rinklebe, J.; Broll, G.; Neue, H.U. Metals and Arsenic in Soils and Corresponding Vegetation at Central Elbe River Floodplains (Germany). Environ. Pollut. 2007, 145, 800–812. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 432. [Google Scholar]
- Krüger, F.; Gröngröft, A. The Difficult Assessment of Heavy Metal Contamination of Soils and Plants in Elbe River Floodplains. Acta Hydrochim. Hydrobiol. 2004, 31, 436–443. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Sun, Q.; Zhang, H. Trace Elements in Soils and Selected Agricultural Plants in the Tongling Mining Area of China. Int. J. Environ. Res. Public Health 2018, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Golia, E.E.; Liu, Y.T.; Wang, S.L.; Shaheen, S.M.; Rinklebe, J. Soil and Maize Contamination by Trace Elements and Associated Health Risk Assessment in the Industrial Area of Volos, Greece. Environ. Int. 2019, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Efroymson, R.A.; Sample, B.E.; Suter, G.W. Uptake of Inorganic Chemicals from Soil by Plant Leaves: Regressions of Field Data. Environ. Toxicol. Chem. 2001, 20, 256–262. [Google Scholar] [CrossRef]
- Elzinga, E.J.; Van Grinsven, J.J.M.; Swartjes, F.A. General Purpose Freundlich Isotherms for Cadmium, Copper and Zinc in Soils. Eur. J. Soil Sci. 1999, 50, 139–149. [Google Scholar] [CrossRef]
- Römkens, P.F.A.M.; Guo, H.Y.; Chu, C.L.; Liu, T.S.; Chiang, C.F.; Koopmans, G.F. Prediction of Cadmium Uptake by Brown Rice and Derivation of Soil-Plant Transfer Models to Improve Soil Protection Guidelines. Environ. Pollut. 2009, 157, 2435–2444. [Google Scholar] [CrossRef]
- Němeček, J.; Podlešáková, E.; Vácha, R. Prediction of the Transfer of Trace Elements from Soils into Plants. Rostl. Vyroba 2001, 47, 425–432. [Google Scholar]
- Podlešáková, E.; Němeček, J. Impact of Soil Factors on the Transfer of Trace Elements into Plants. Rostl. Vyroba 2001, 47, 104–110. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Khan, I.; Tanveer, M.; Ali, M.; Hussain, I.; Wang, L.C. Chromium and Aluminum Phytotoxicity in Maize: Morpho-Physiological Responses and Metal Uptake. Clean-Soil Air Water 2016, 44, 1075–1084. [Google Scholar] [CrossRef]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The Contamination of Plants and Soils with Heavy Metals and the Transport of Metals in Terrestrial Food Chains. In Changing Metal Cycles and Human Health; Nriagu, J.O., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 113–141. [Google Scholar] [CrossRef]
- Jackson, A.P.; Alloway, B.J. The Transfer of Cadmium from Agricultural Soils to the Human Food Chain. In Biogeochemistry of Trace Metals; Adriano, D.C., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1992; pp. 109–152. [Google Scholar]
- Cuypers, A.; Smeets, K.; Vangronsveld, J. Plant Stress Biology: From Genomics to Systems Biology. In Heavy Metal Stress in Plants; Hirt, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; pp. 161–178. [Google Scholar] [CrossRef]
- Nanda Kumar, P.B.A.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The Use of Plants To Remove Heavy Metals from Soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.M.; Bensryd, I.; Lundh, T.; Ottosson, H.; Skerfving, S.; Oskarsson, A. Cadmium in Blood and Urine—Impact of Sex, Age, Dietary Intake, Iron Status, and Former Smoking—Association of Renal Effects. Environ. Health Perspect. 2002, 110, 1185–1190. [Google Scholar] [CrossRef]
- Vácha, R.; Sáňka, M.; Skála, J.; Čechmánková, J.; Horváthová, V. Soil Contamination Health Risks in Czech Proposal of Soil Protection Legislation. In Environmental Health Risk, 1st ed.; Larramendy, M., Ed.; InTechOpen: London, UK, 2016; pp. 57–75. [Google Scholar]
- Podlešáková, E.; Němeček, J.; Hálová, G. The Load of Fluvisols of Labe by Risk Compounds. Rostl. Vyroba 1994, 40, 69–80. [Google Scholar]
- Skála, J.; Vácha, R.; Hofman, J.; Horváthová, V.; Sáňka, M.; Čechmánková, J. Spatial Differentiation of Ecosystem Risks of Soil Pollution in Floodplain Areas of the Czech Republic. Soil Water Res. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Czech Hydrometeorological Institute. Five-Year Averages in 2012–2016. Available online: http://portal.chmi.cz/files/portal/docs/uoco/isko/ozko/ozko_CZ.html (accessed on 12 April 2021).
- Borůvka, L.; Huan-Wei, C.; Kozák, J.; Krištoufková, S. Heavy Contamination of Soil with Cadmium, Lead and Zinc in the Alluvium of the Litavka River. Rostl. Vyroba 1996, 42, 543–550. [Google Scholar]
- Vaněk, A.; Borůvka, L.; Drábek, O.; Mihaljevič, M.; Komárek, M. Mobility of Lead, Zinc and Cadmium in Alluvial Soils Heavily Polluted by Smelting Industry. Plant Soil Environ. 2005, 51, 316–321. [Google Scholar] [CrossRef]
- Vácha, R.; Podlešáková, E.; Němeček, J.; Poláček, O. Immobilisation of As, Cd, Pb and Zn in Agricultural Soils by the Use of Organic and Inorganic Additives. Rostl. Vyroba 2002, 48, 335–342. [Google Scholar] [CrossRef]
- ISO 11466:1995 Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia; International Organization for Standardization: Geneva, Switzerland, 1995.
- ISO/DIS 19730:2008 Soil Quality—Extraction of Trace Elements Using Ammonium Nitrate Solution; International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 14869-1:2001 Soil Quality—Dissolution for the Determination of Total Element Content—Part 1: Dissolution with Hydrofluoric and Perchloric Acids; International Organization for Standardization: Geneva, Switzerland, 2001.
- Dědina, J.; Tsalev, D.L. Hydride Generation Atomic Absorption Spectrometry, 3rd ed.; John and Wiley and Sons: Chichester, UK, 1995; p. 544. [Google Scholar]
- Zbíral, J.; Honsa, I.; Malý, S.; Čižmár, D. Soil Analysis III; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2004; p. 199. (In Czech) [Google Scholar]
- Podlešáková, E.; Němeček, J.; Vácha, R. Mobility and Bioavailability of Trace Elements in Soils. In Trace Elements in Soil: Bioavailability, Flux, and Transfer; Iskandar, I.K., Kirkham, M.B., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 21–42. [Google Scholar]
- Matys Grygar, T.; Nováková, T.; Bábek, O.; Elznicová, J.; Vadinová, N. Robust Assessment of Moderate Heavy Metal Contamination Levels in Floodplain Sediments: A Case Study on the Jizera River, Czech Republic. Sci. Total Environ. 2013, 452–453, 233–245. [Google Scholar] [CrossRef]
- Matys Grygar, T.; Popelka, J. Revisiting Geochemical Methods of Distinguishing Natural Concentrations and Pollution by Risk Elements in Fluvial Sediments. J. Geochem. Explor. 2016, 170, 39–57. [Google Scholar] [CrossRef]
- McBride, M.; Sauvé, S.; Hendershot, W. Solubility Control of Cu, Zn, Cd and Pb in Contaminated Soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- Du Laing, G.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M.G. Trace Metal Behaviour in Estuarine and Riverine Floodplain Soils and Sediments: A Review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar] [CrossRef]
- Schulz-Zunkel, C.; Krueger, F. Trace Metal Dynamics in Floodplain Soils of the River Elbe: A Review. J. Environ. Qual. 2009, 38, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Němeček, J.; Podlešáková, E. The Main Features of the Transfer of Trace Elements into Plants. Rostl. Vyroba 2001, 47, 7–13. [Google Scholar]
- Eriksson, J.E. The Influence of pH, Soil Type and Time on Adsorbtion and Uptake by Plants of Cd Added to the Soil. Water Air Soil Pollut. 1989, 48, 317–335. [Google Scholar] [CrossRef]
- Rieuwerts, J.S.; Thornton, I.; Farago, M.E.; Ashmore, M.R. Factors Influencing Metal Bioavailability in Soils: Preliminary Investigations for the Development of a Critical Loads Approach for Metals. Chem. Speciat. Bioavailab. 1998, 10, 61–75. [Google Scholar] [CrossRef]
- Borůvka, L.; Drábek, O. Heavy Metal Distribution between Fractions of Humic Substances in Heavily Polluted Soils. Plant Soil Environ. 2004, 50, 339–345. [Google Scholar] [CrossRef]
- Notten, M.J.M.; Oosthoek, A.J.P.; Rozema, J.; Aerts, R. Heavy Metal Concentrations in a Soil-Plant-Snail Food Chain along a Terrestrial Soil Pollution Gradient. Environ. Pollut. 2005, 138, 78–90. [Google Scholar] [CrossRef]
- Wijnhoven, S.; Van Der Velde, G.; Leuven, R.S.E.W.; Eijsackers, H.J.P.; Smits, A.J.M. Metal Accumulation Risks in Regularly Flooded and Non-Flooded Parts of Floodplains of the River Rhine: Extractability and Exposure through the Food Chain. Chem. Ecol. 2006, 22, 463–477. [Google Scholar] [CrossRef]
- Adams, M.L.; Zhao, F.J.; McGrath, S.P.; Nicholson, F.A.; Chambers, B.J. Predicting Cadmium Concentrations in Wheat and Barley Grain Using Soil Properties. J. Environ. Qual. 2004, 33, 532–541. [Google Scholar] [CrossRef]
- Dahmani-Muller, H.; Van Oort, F.; Gélie, B.; Balabane, M. Strategies of Heavy Metal Uptake by Three Plant Species Growing near a Metal Smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Novotná, M.; Mikeš, O.; Komprdová, K. Development and Comparison of Regression Models for the Uptake of Metals into Various Field Crops. Environ. Pollut. 2015, 207, 357–364. [Google Scholar] [CrossRef]
- Hooda, P.S.; McNulty, D.; Alloway, B.J.; Aitken, M.N. Plant Availability of Heavy Metal in Soils Previously Amended with Heavy Applications of Sewage Sludge. J. Sci. Food Agric. 1997, 73, 446–454. [Google Scholar] [CrossRef]
- Tlustoš, P.; Száková, J.; Kořínek, K.; Pavlíková, D.; Hanč, A.; Balík, J. The Effect of Liming on Cadmium, Lead, and Zinc Uptake Reduction by Spring Wheat Grown in Contaminated Soil. Plant Soil Environ. 2006, 52, 16–24. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Rupp, H.; Meissner, R. Lysimeter Trials to Assess the Impact of Different Flood-Dry-Cycles on the Dynamics of Pore Water Concentrations of As, Cr, Mo and V in a Contaminated Floodplain Soil. Geoderma 2014, 228–229, 5–13. [Google Scholar] [CrossRef]
- Tack, F.M.G.; Van Ranst, E.; Lievens, C.; Vandenberghe, R.E. Soil Solution Cd, Cu and Zn Concentrations as Affected by Short-Time Drying or Wetting: The Role of Hydrous Oxides of Fe and Mn. Geoderma 2006, 137, 83–89. [Google Scholar] [CrossRef]
- Dudas, M.J.; Pawluk, S. Heavy Metals in Cultivated Soils and in Cereal Crops in Alberta. Can. J. Soil Sci. 1977, 57, 329–339. [Google Scholar] [CrossRef]
- Cai, K.; Zhang, M.; Yu, Y.; Kim, K. Pollution, Source, and Relationship of Trace Metal(Loid)s in Soil-Wheat System in Hebei Plain, Northern China. Agronomy 2019, 9, 391. [Google Scholar] [CrossRef]
- Králová, L.; Száková, J.; Kubík, Š.; Tlustoš, P.; Balík, J. The Variability of Arsenic and Other Risk Element Uptake by Individual Plant Species Growing on Contaminated Soil. Soil Sediment Contam. 2010, 19, 617–634. [Google Scholar] [CrossRef]
- Corguinha, A.P.B.; Souza, G.A.d.; Gonçalves, V.C.; Carvalho, C.d.A.; Lima, W.E.A.d.; Martins, F.A.D.; Yamanaka, C.H.; Francisco, E.A.B.; Guilherme, L.R.G. Assessing Arsenic, Cadmium, and Lead Contents in Major Crops in Brazil for Food Safety Purposes. J. Food Compos. Anal. 2015, 37, 143–150. [Google Scholar] [CrossRef]
- Němeček, J.; Podlešáková, E.; Vácha, R. Transfer of Trace Elements with Low Soil Mobility into Plants. Rostl. Vyroba 2002, 48, 45–50. [Google Scholar] [CrossRef]
- Podlešáková, E.; Němeček, J.; Vácha, R. The Transfer of Less Hazardous Trace Elements with High Mobility from Soils into Plants. Rostl. Vyroba 2001, 47, 433–439. [Google Scholar]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Proposal of New Convenient Extractant for Assessing Phytoavailability of Heavy Metals in Contaminated Sandy Soil. Environ. Sci. Pollut. Res. Int. 2017, 17, 14857–14866. [Google Scholar] [CrossRef] [PubMed]
- Vácha, R.; Sáňka, M.; Sáňka, O.; Skála, J.; Čechmánková, J. The Fluvisol and Sediment Trace Element Contamination Level as Related to their Geogenic and Anthropogenic Source. Plant Soil Environ. 2013, 59, 136–142. [Google Scholar] [CrossRef]
- Zeien, H.; Brümmer, G.W. Chemische Extraktion Zur Bestimmung von Schwermetallbindungsformen in Böden. Mitt. Dtsch. Bodenkd.Ges. 1989, 59, 505–510. [Google Scholar]
- Vácha, R.; Macurová, H.; Skála, J.; Havelková, M.; Čechmánková, J.; Horváthová, V. Possibilities of Some Methods for Risk Assessment of Arsenic Load in Soils. Plant Soil Environ. 2008, 54, 279–287. [Google Scholar] [CrossRef]
- Deng, F.; Yu, M.; Martinoia, E.; Song, W.-Y. Ideal Cereals with Lower Arsenic and Cadmium by Accurately Enhancing Vacuolar Sequestration Capacity. Front. Genet. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, L.; Panozzo, A.; Radhouane, L.; Dal Cortivo, C.; Barion, G.; Vamerali, T. Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination. Agronomy 2021, 11, 178. [Google Scholar] [CrossRef]
- Puschenreiter, M.; Horak, O.; Friesl, W.; Hartl, W. Low-Cost Agricultural Measures to Reduce Heavy Metal Transfer into the Food Chain—A Review. Plant Soil Environ. 2005, 51, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).