One Step Bioremediation of Olive-Oil-Mill Waste by Organoinorganic Catalyst for Humics-Rich Soil Conditioner Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocatalyst Preparation
2.3. Bioremediation Process
2.4. Physicochemical Analysis
2.5. Microbial Populations
2.6. Plant Germination/Cultivation Experiments
- 50 plants without any soil improvers (control).
- 50 plants with 250 g OMW compost added per plant.
- 50 plants with 250 g GW compost added per plant.
- 50 plants with 500 g OMW compost added per plant.
- 50 plants with 500 g GW compost added per plant.
- 70% OMW compost: 30% redhead (v/v) (Substrate OMW).
- 70% peat: 30% redhead (v/v) (Substrate Peat).
2.7. Statistical Analysis
3. Results and Discussion
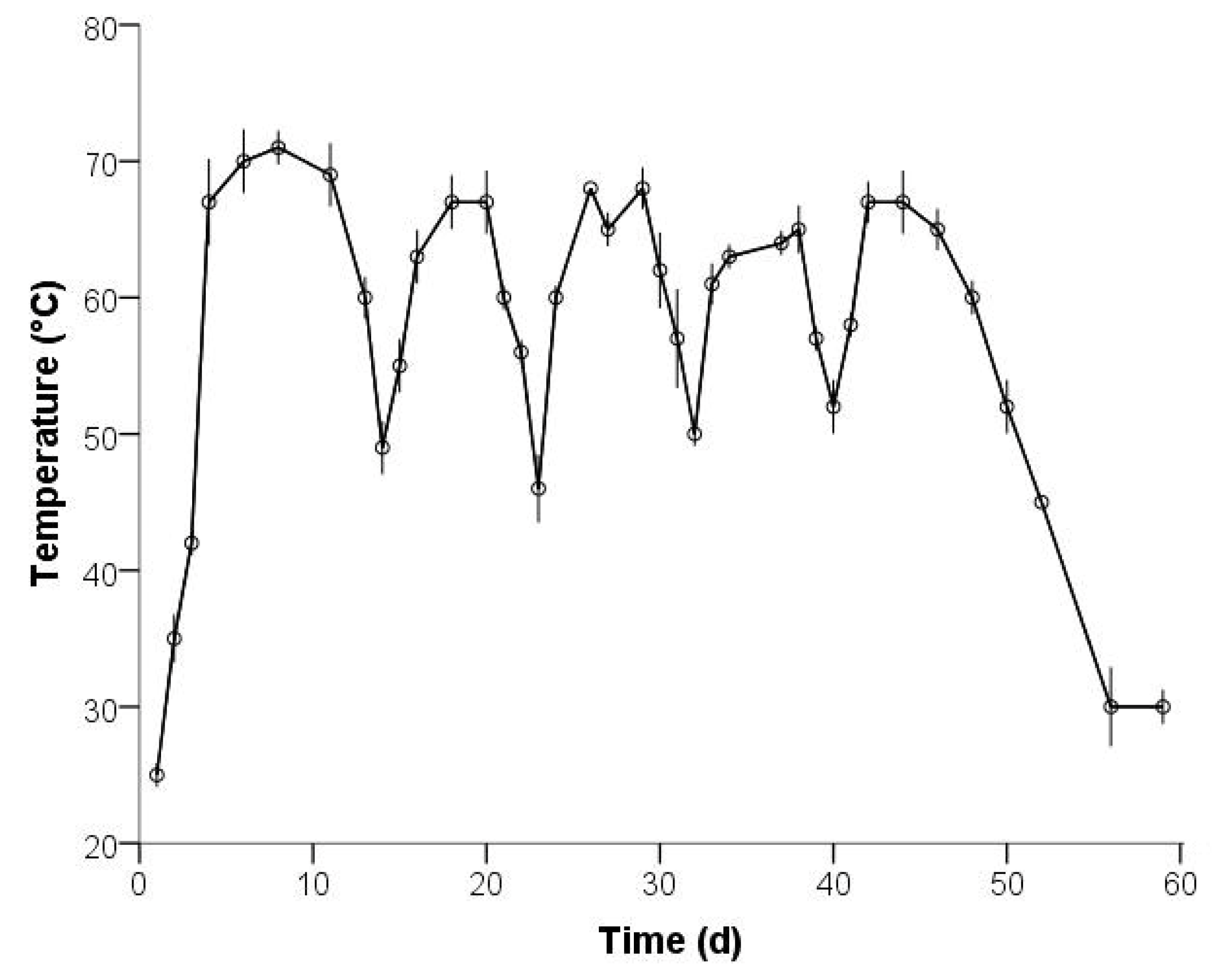
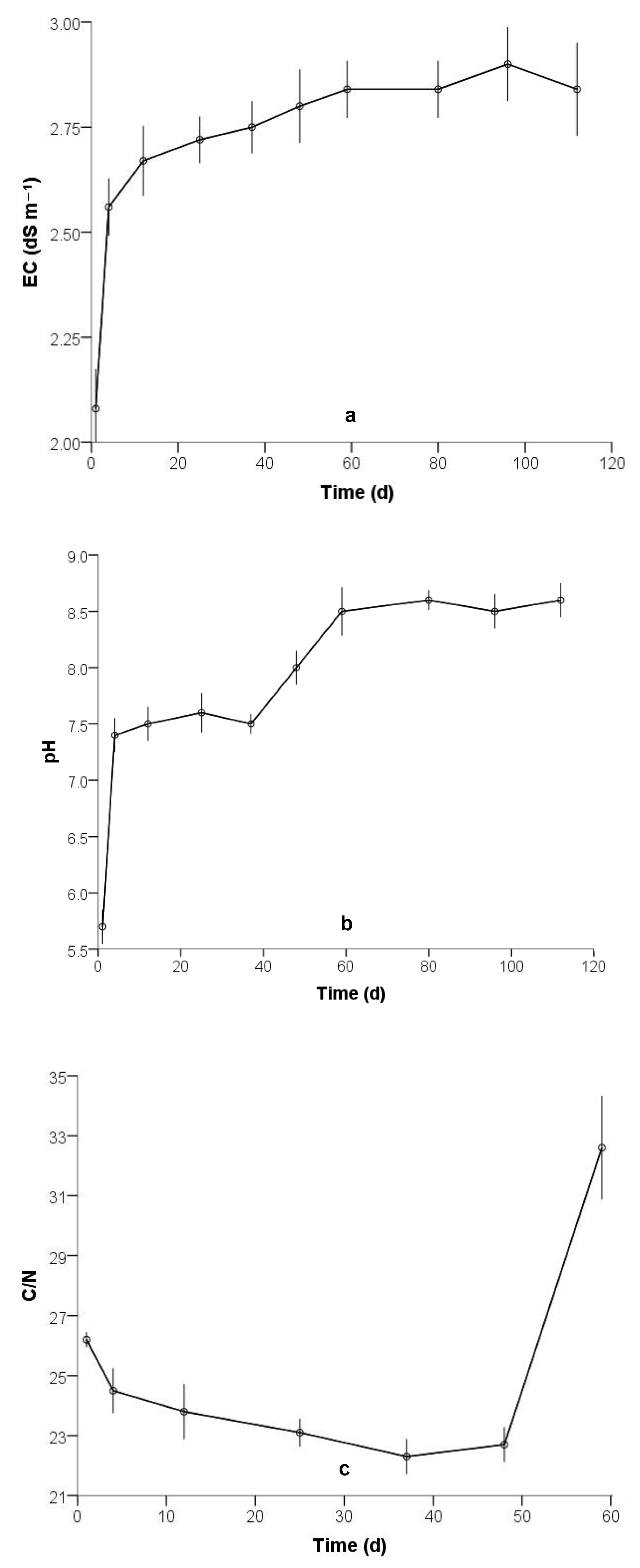
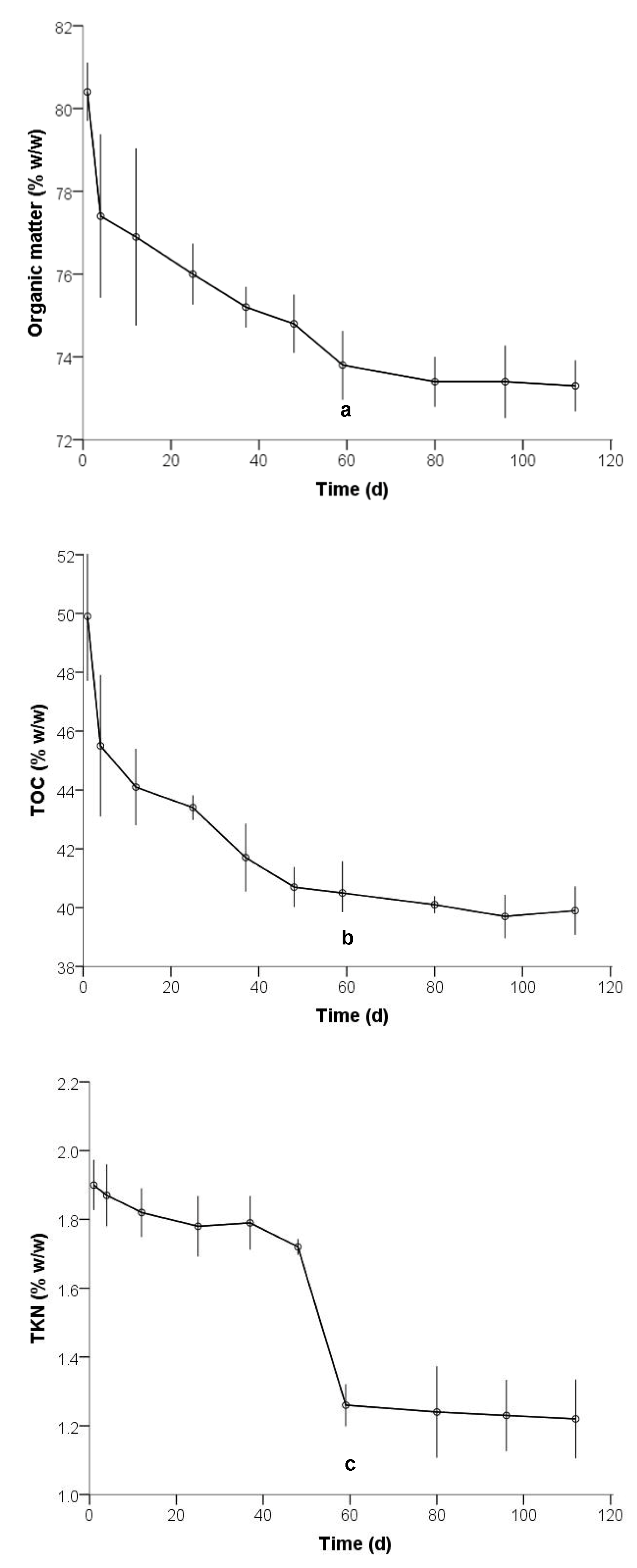
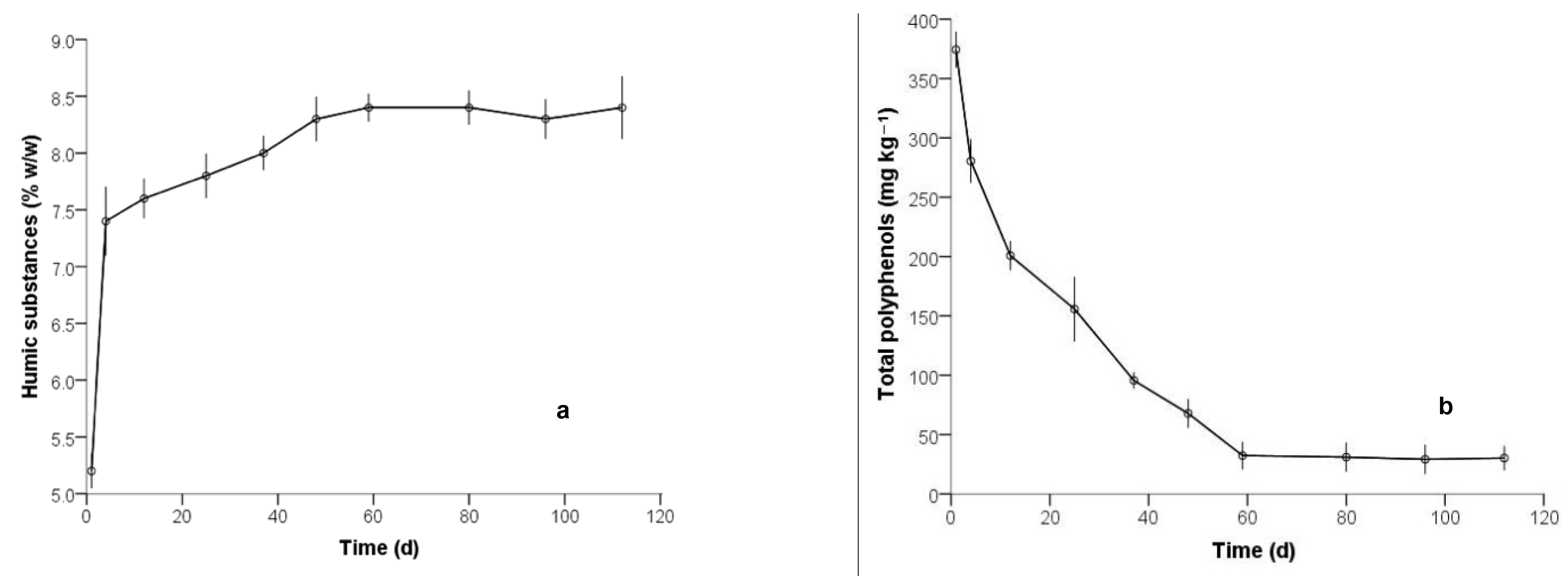
3.1. Physicochemical Characterization
3.2. Microbial Populations
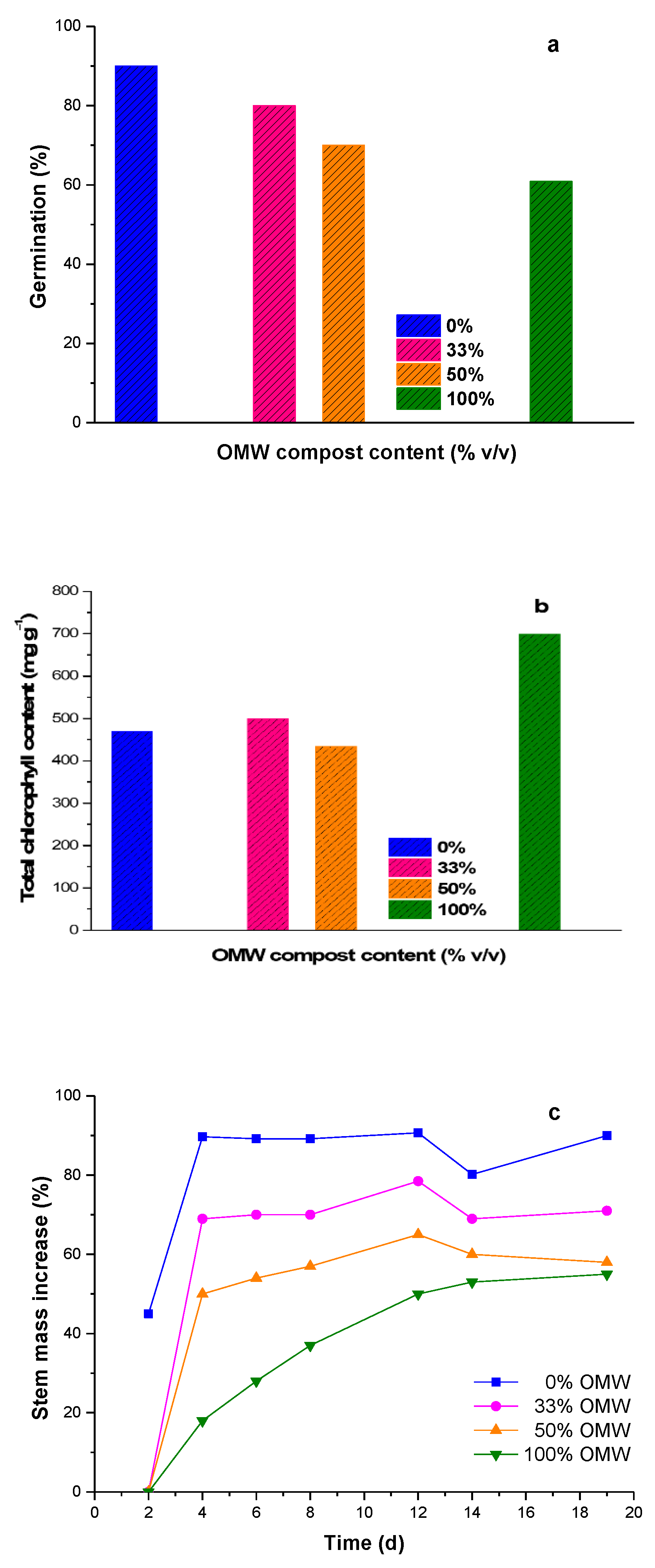
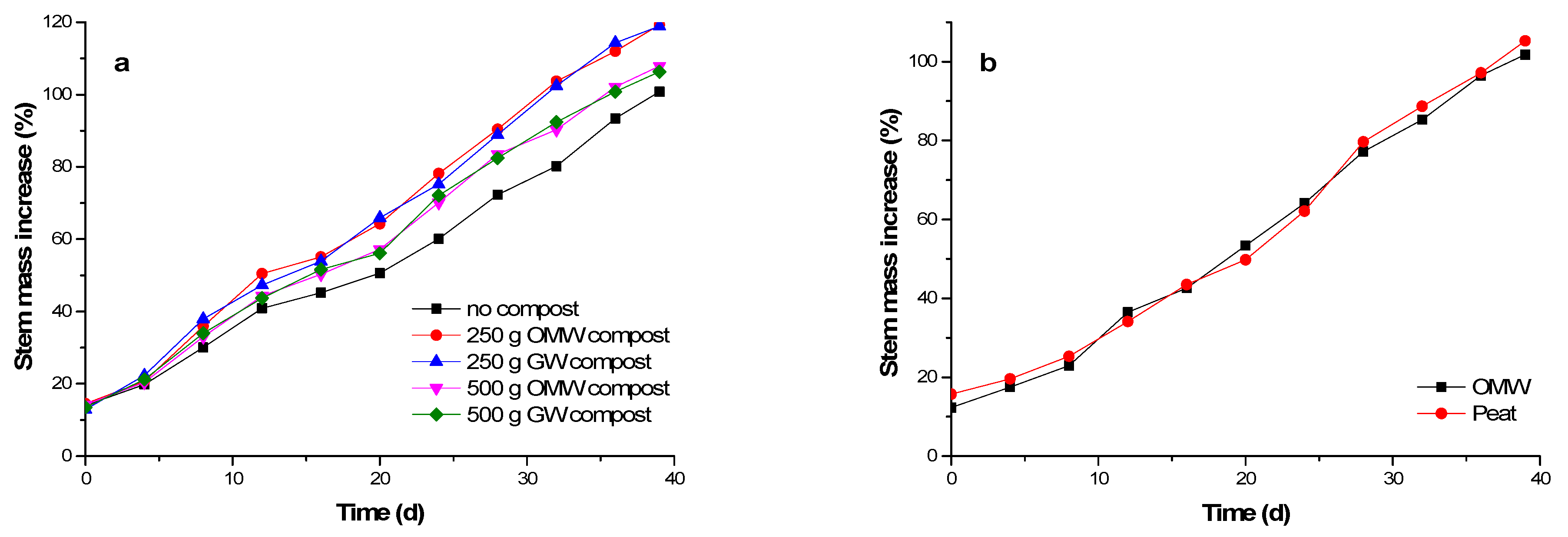
3.3. Germination/Cultivation Experimentes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buckland, G.; Gonzales, A.C. Trends in olive oil production, supply and consumption in Mediterranean countries from 1961 to the present day. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier Publications Inc.: New York, NY, USA, 2010; pp. 689–698. [Google Scholar]
- La Cara, F.; Ionata, E.; Del Monaco, G.; Marcolongo, L.; Gonçalves, M.R.; Marques, I.P. Olive Mill Wastewater Anaerobically Digested: Phenolic Compounds with Antiradical Activity. Chem. Eng. Trans. 2012, 27, 325–330. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management Literature Review and Patent Survey, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 5. [Google Scholar]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Davies, L.C.; Vilhena, A.M.; Novais, J.M.; Martins-Dias, S. Olive mill wastewater characteristics: Modelling and statistical analysis. Grasas Aceites 2004, 55, 233–241. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Recovery and preservation of phenols from olive waste in ethanolic extracts. J. Chem. Technol. Biotechnol. 2010, 85, 1148–1155. [Google Scholar] [CrossRef]
- Souilem, S.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; Sayadi, S.; Galanakis, C.M. Olive oil production sector: Environmental effects and sustainability challenges. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2017; pp. 12–19. [Google Scholar] [CrossRef]
- Cereti, C.F.; Rossini, F.; Federici, F.; Quaratino, D.; Vassilev, N.; Fenice, M. Reuse of microbially treated olive mill wastewater as fertiliser for wheat (Triticum durum Desf.). Bioresour. Technol. 2004, 91, 135–140. [Google Scholar] [CrossRef]
- Komilis, D.F.; Karatzas, E.; Halvadakis, C.P. The effect of olive mill wastewater on seed germination after various pre-treatment techniques. J. Environ. Manag. 2005, 74, 339–348. [Google Scholar] [CrossRef]
- Grioui, N.; Halouani, K.; Agblevor, F.A. Assessment of upgrading ability and limitations of slow co-pyrolysis: Case of olive mill wastewater sludge/waste tires slow co-pyrolysis. Waste Manag. 2019, 92, 75–88. [Google Scholar] [CrossRef]
- Lee, Z.S.; Chin, S.Y.; Lim, J.W.; Witoon, T.; Cheng, C.K. Treatment technologies of palm oil mill effluent (POME) and olive mill wastewater (OMW): A brief review. Environ. Technol. Innov. 2019, 15, 100377. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Arvaniti, E.C.; Papadakis, V.G.; Paraskeva, C.A. Sustainability analysis and benchmarking of olive mill wastewater treatment methods. J. Chem. Technol. Biotechnol. 2013, 88, 742–750. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents Part I. Organic matter degradation by chemical and biological processes—An overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jeguirim, M.; Kinigopoulou, V.; Doulgeris, C.; Goddard, M.-L.; Jellali, S.; Matei Ghimbeu, C. Olive mill wastewater: From a pollutant to green fuels, agricultural and water source and bio-fertilizer—Hydrothermal carbonization. Sci. Total Environ. 2020, 733, 139314. [Google Scholar] [CrossRef] [PubMed]
- Milanović, V.; Osimani, A.; Cardinali, F.; Taccari, M.; Garofalo, C.; Clementi, F.S.; Mozzon, M.; Foligni, R.; Canonico, L.; Ciani, M.; et al. Effect of inoculated azotobacteria and Phanerochaete chrysosporium on the composting of olive pomace: Microbial community dynamics and phenols evolution. Sci. Rep. 2019, 9, 16966. [Google Scholar] [CrossRef]
- Aviani, I.; Laor, Y.; Medina, S.; Krassnovsky, A.; Raviv, M. Co-composting of solid and liquid olive mill wastes: Management aspects and the horticultural value of the resulting composts. Bioresour. Technol. 2010, 101, 699–6706. [Google Scholar] [CrossRef]
- Federici, E.; Pepi, M.; Esposito, A.; Scargetta, S.; Fidati, L.; Gasperini, S.; Cenci, G.; Altieri, R. Two-phase olive mill waste composting: Community dynamics and functional role of the resident microbiota. Bioresour. Technol. 2011, 102, 10965–10972. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A. Two-phase olive mill waste composting: Enhancement of the composting rate and compost quality by grape stalks addition. Biodegradation 2010, 21, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, S.; Sallemi, F.; Medhioub, K.; Hachicha, R.; Ammar, E. Quality assessment of composts prepared with olive mill wastewater and agricultural wastes. Waste Manag. 2008, 28, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.; Bonanomi, G.; Scelza, R.; Zoina, A.; Rao, M.A. Organic amendments as sustainable tool to recovery fertility in intensive agricultural systems. J. Soil Sci. Plant Nutr. 2015, 15, 333–352. [Google Scholar] [CrossRef]
- Chassapis, K.; Roulia, M. Evaluation of low-rank coals as raw material for Fe and Ca organomineral fertilizer using a new EDXRF method. Int. J. Coal Geol. 2008, 75, 185–188. [Google Scholar] [CrossRef]
- Chassapis, K.; Roulia, M.; Tsirigoti, D. Chemistry of metal–humic complexes contained in Megalopolis lignite and potential application in modern organomineral fertilization. Int. J. Coal Geol. 2009, 78, 288–295. [Google Scholar] [CrossRef]
- Chassapis, K.; Roulia, M.; Nika, G. Fe(III)–humate complexes from Megalopolis peaty lignite: A novel eco-friendly fertilizer. Fuel 2010, 89, 1480–1484. [Google Scholar] [CrossRef]
- Kazamias, G.; Roulia, M.; Kapsimali, I.; Chassapis, K. Innovative biocatalytic production of soil substrate from green waste compost as a sustainable peat substitute. J. Environ. Manag. 2017, 203, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Zorpas, A.A.; Loizidou, M. Sawdust and natural zeolite as a bulking agent for improving quality of a composting product from anaerobically stabilized sewage sludge. Bioresour. Technol. 2008, 99, 7545–7552. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Khosla, C.; Curtis, J.; Bydalek, P.; Swartz, J.R.; Bailey, J.E. Expression of Recombinant Proteins in Escherichia coli Using an Oxygen-Responsive Promoter. Nat. Biotechnol. 1990, 8, 554–558. [Google Scholar] [CrossRef]
- EN 13040:1999. Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density; European Committee for Standardization, Technical Committee CEN/TC 223; iTeh, Inc.: Newark, NJ, USA, 1999. [Google Scholar]
- EN 13038:1999. Soil Improvers and Growing Media—Determination of Electrical Conductivity; European Committee for Standardization, Technical Committee CEN/TC 223; iTeh, Inc.: Newark, NJ, USA, 1999. [Google Scholar]
- EN 13037:1999. Soil Improvers and Growing Media—Determination of pH; European Committee for Standardization, Technical Committee CEN/TC 223; iTeh, Inc.: Newark, NJ, USA, 1999. [Google Scholar]
- Agnew, J.M.; Leonard, J.J.; Feddes, J.; Feng, Y. A modified air pycnometer for compost air volume and density determination. Can. Biosyst. Eng. 2003, 45, 27–35. [Google Scholar]
- ASTM (American Society for Testing and Materials) (D3174-82). Standard test method for ash in the analysis sample of coal and coke from coal. In Annual Book of ASTM Standards. Gaseous Fuels: Coal and Coke; ASTM: Philadelphia, PA, USA, 1983; Volume 5, pp. 398–401. [Google Scholar]
- EN 13039:1999. Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash; European Committee for Standardization, Technical Committee CEN/TC 223; iTeh, Inc.: Newark, NJ, USA, 1999. [Google Scholar]
- Navarro, A.E.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Mebius, L.J. A rapid method for the determination of organic carbon in soil. Anal. Chim. Acta 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Jiménez, E.I.; Garcia, V.P. Relationships between Organic Carbon and Total Organic Matter in Municipal Solid Wastes and City Refuse Composts. Bioresour. Technol. 1992, 41, 265–272. [Google Scholar] [CrossRef]
- EN 13654-1:2001. Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method; European Committee for Standardization, Technical Committee CEN/TC 223; iTeh, Inc.: Newark, NJ, USA, 2001. [Google Scholar]
- Vermerris, W.; Nicholson, R. Phenolic Compound Biochemistry; Springer Science + Business Media B.V.: Berlin, Germany, 2008. [Google Scholar]
- Meng, Q.; Yang, W.; Men, M.; Bello, A.; Xu, X.; Xu, B.; Deng, L.; Jiang, X.; Sheng, S.; Wu, X.; et al. Microbial Community Succession and Response to Environmental Variables During Cow Manure and Corn Straw Composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dhouib, A.; Sayadi, S. Changes in microbial and soil properties following amendment with treated and untreated olive mill wastewater. Microbiol. Res. 2006, 161, 93–101. [Google Scholar] [CrossRef]
- Hassen, A.; Belguith, K.; Jedidi, N.; Cherif, A.; Cherif, M.; Boudabous, A. Microbial characterization during composting of municipal solid waste. Bioresour. Technol. 2001, 80, 217–225. [Google Scholar] [CrossRef]
- Chroni, C.; Kyriacou, A.; Georgaki, I.; Manios, T.; Kotsou, M.; Lasaridi, K. Microbial characterization during composting of biowaste. Waste Manag. 2009, 29, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Ottow, J.C.G. Rose Bengal as a Selective Aid in the Isolation of Fungi and Actinomycetes from Natural Sources. Mycologia 1972, 64, 304–315. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S.R. Co-composting of invasive Acacia longifolia with pine bark for horticultural use. Environ. Technol. 2014, 36, 1632–1642. [Google Scholar] [CrossRef]
- VanderGheynst, J.S.; Pettygrove, S.; Dooley, T.M.; Arnold, K.A. Estimating Electrical Conductivity of Compost Extracts at Different Extraction Ratios. Compost Sci. Util. 2004, 12, 202–207. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Larney, F.J.; Olson, A.F.; Carcamo, A.A.; Chang, C. Physical changes during active and passive composting of beef feedlot manure in winter and summer. Bioresour. Technol. 2000, 75, 139–148. [Google Scholar] [CrossRef]
- Droussi, Z.; D’Orazio, V.; Provenzano, M.R.; Hafidi, M.; Ouatmane, A. Study of the biodegradation and transformation of olive-mill residues during composting using FTIR spectroscopy and differential scanning calorimetry. J. Hazard. Mater. 2009, 164, 1281–1285. [Google Scholar] [CrossRef]
- Francioso, O.; Ferrari, E.; Saladini, M.; Montecchio, D.; Gioacchini, P.; Ciavatta, C. TG–DTA, DRIFT and NMR characterisation of humic-like fractions from olive wastes and amended soil. J. Hazard. Mater. 2007, 149, 408–417. [Google Scholar] [CrossRef]
- De Nobili, M.; Petrussi, F. Humification Index (HI) as Evaluation of the Stabilization Degree during Composting. J. Ferment. Technol. 1988, 66, 577–583. [Google Scholar] [CrossRef]
- Boguta, P.; D’ Orazio, V.; Senesi, N.; Sokołowska, Z.; Szewczuk-Karpisza, K. Insight into the interaction mechanism of iron ions with soil humic acids. The effect of the pH and chemical properties of humic acids. J. Environ. Manag. 2019, 245, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Perminova, I.V. Humic Substances-Assisted Synthesis of Nanoparticles in the Nature and in the Lab. In Functions of Natural Organic Matter in Changing Environment; Xu, J., Wu, J., He, Y., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Olive mill waste: Recent advances for the sustainable development of olive oil industry. In Olive Mill Waste: Recent Advances for Sustainable Management; Galanakis, C.M., Ed.; Academic Press, Elsevier Inc.: Cambridge, MA, USA, 2017; p. 44. [Google Scholar]
- Chassapis, K.; Roulia, M.; Vrettou, E.; Fili, D.; Zervaki, M. Biofunctional Characteristics of Lignite Fly Ash Modified by Humates: A New Soil Conditioner. Bioinorg. Chem. Appl. 2010, 457964. [Google Scholar] [CrossRef] [PubMed]



| Microbial Populations (c.f.u. g–1) | |||
|---|---|---|---|
| Fraction No. | Bacteria | Fungi and Yeasts | Actinomycets |
| 1 | 2 × 103 | 5 × 102 | 2 × 102 |
| 2 | 6 × 104 | 2 × 104 | 4 × 103 |
| 3 | 6 × 106 | 3 × 106 | 2 × 104 |
| 4 | 8 × 106 | 5 × 106 | 3 × 106 |
| 5 | 2 × 106 | 4 × 106 | 3 × 106 |
| 6 | 2 × 106 | 4 × 106 | 3 × 106 |
| 7 | 2 × 106 | 4 × 106 | 3 × 106 |
| 8 | 2 × 106 | 2 × 106 | 2 × 106 |
| 9 | 2 × 106 | 2 × 106 | 2 × 106 |
| 10 | 2 × 106 | 2 × 106 | 2 × 106 |
| Compost | ||||
|---|---|---|---|---|
| Parameter | OMW | Green Wastes (GW) | Initial | 60-d Treatment |
| Moisture (%) | 90.3 | 58.1 | 68.1 | 48.9 |
| Electrical conductivity (dS m–1) | 11 | 0.99 | 1.92 | 2.8 |
| pH | 5.48 | 6.85 | 5.7 | 7.3 |
| Bulk density (kg L–1) | 0.98 | 0.12 | 0.33 | 0.4 |
| Ash (% w/w) | 19.8 | 7.3 | 14.0 | 21.9 |
| Organic matter (% w/w) | 80.2 | 92.7 | 86.0 | 78.1 |
| Total organic carbon (% w/w) | 45.7 | 53.8 | 49.9 | 40.3 |
| Total Kjeldahl nitrogen (% w/w) | 1.7 | 2.0 | 1.9 | 1.3 |
| Humic substances (% w/w) | - | 1.8 | 2.8 | 8.5 |
| Total phenols (mg kg–1) | 374.3 | 93.2 | 280.3 | 32.3 |
| Parameter (Dry Basis) | OMW Soil Conditioner | Greek Regulations | ECOLABEL Regulations |
|---|---|---|---|
| Pb (mg kg–1) | 0.05 | 300 | 100 |
| Cd (mg kg–1) | 0.18 | 3 | 1 |
| Cr (mg kg–1) | 0.10 | 250 | 100 |
| Cu (mg kg–1) | 40 | 400 | 100 |
| Ni (mg kg–1) | 28 | 100 | 50 |
| Zn (mg kg–1) | 123 | 1200 | 300 |
| Hg (mg kg–1) | - | 2.5 | 1 |
| As (mg kg–1) | - | 10 | |
| PCBs (mg kg–1) | - | 0.4 | |
| PAHs (mg kg–1) | - | 3 | 6 |
| Salmonella spp. (c.f.u. g–1) | 0 | 0 | 0 |
| Admixtures > 2 mm (%) | 1.8 | 3 | |
| Moisture (% w/w) | 36 | 40 |
| Parameter | t 1 | df 2 | Sig. (2-Tailed) | Mean Difference | 95% Confidence Interval of the Difference | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Temperature (°C) | 66.233 | 189 | 0.000 | 57.053 | 55.35 | 58.75 |
| pH | 64.224 | 49 | 0.000 | 7.7900 | 7.546 | 8.034 |
| Bulk density (kg L−1) | 57.598 | 49 | 0.000 | 0.33200 | 0.3204 | 0.3436 |
| Ash (% w/w) | 51.065 | 49 | 0.000 | 21.5200 | 20.673 | 22.367 |
| Organic matter (% w/w) | 228.59 | 49 | 0.000 | 75.4600 | 74.797 | 76.123 |
| TOC (% w/w) | 92.345 | 49 | 0.000 | 42.5500 | 41.624 | 43.476 |
| TKN (% w/w) | 37.821 | 49 | 0.000 | 1.58300 | 1.4989 | 1.6671 |
| C/N | 43.208 | 34 | 0.000 | 25.02857 | 23.8514 | 26.2058 |
| Humic substances (% w/w) | 58.121 | 49 | 0.000 | 7.7800 | 7.511 | 8.049 |
| Total polyphenols (mg kg–1) | 7.855 | 49 | 0.000 | 129.7290 | 96.540 | 162.918 |
| EC (dS m–1) | 80.590 | 49 | 0.000 | 2.70000 | 2.6327 | 2.7673 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roulia, M.; Kontezaki, E.; Kalogeropoulos, N.; Chassapis, K. One Step Bioremediation of Olive-Oil-Mill Waste by Organoinorganic Catalyst for Humics-Rich Soil Conditioner Production. Agronomy 2021, 11, 1114. https://doi.org/10.3390/agronomy11061114
Roulia M, Kontezaki E, Kalogeropoulos N, Chassapis K. One Step Bioremediation of Olive-Oil-Mill Waste by Organoinorganic Catalyst for Humics-Rich Soil Conditioner Production. Agronomy. 2021; 11(6):1114. https://doi.org/10.3390/agronomy11061114
Chicago/Turabian StyleRoulia, Maria, Evangelia Kontezaki, Nikolaos Kalogeropoulos, and Konstantinos Chassapis. 2021. "One Step Bioremediation of Olive-Oil-Mill Waste by Organoinorganic Catalyst for Humics-Rich Soil Conditioner Production" Agronomy 11, no. 6: 1114. https://doi.org/10.3390/agronomy11061114
APA StyleRoulia, M., Kontezaki, E., Kalogeropoulos, N., & Chassapis, K. (2021). One Step Bioremediation of Olive-Oil-Mill Waste by Organoinorganic Catalyst for Humics-Rich Soil Conditioner Production. Agronomy, 11(6), 1114. https://doi.org/10.3390/agronomy11061114






