The Response of Wheat with Different Allele Statuses of the Gpc-B1 Gene under Zinc Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Conditions
2.3. Biometric Measurements
2.4. Chemical Analysis
2.5. Statistical Analyses
3. Results
3.1. Effect of Zn Deficiency on Plant Growth
3.2. Effect of Zn Deficiency on Grain Yield Components of Plants
3.3. Effect of Zn Deficiency on Grain Zn Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinclair, S.A.; Krämer, U. The zinc homeostasis network of land plants. Biochem. Biophys. Acta 2012, 1823, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gladyshev, V.N. Comparative genomics of trace element dependence in biology. J. Biol. Chem. 2011, 286, 23623–23629. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Parveen, N.; Ansari, M.O.; Ahmad, M.F.; Jameel, S.; Shadab, G.G.H.A. Zinc: An element of extensive medical importance. Cur. Med. Res. Prac. 2017, 7, 90–98. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; IZA: Brussels, Belgium; IFA: Paris, France, 2008; Volume 137. [Google Scholar]
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A.; Fett, J.P. Got to hide your Zn away: Molecular control of Zn accumulation and biotechnological applications. Plant Sci. 2015, 236, 1–17. [Google Scholar] [CrossRef]
- Maret, W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition—A review. Am. J. Exp. Agricul. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutri. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Pazos, R. Interplay between autophagy and zinc. J. Trace Elem. Med. Biol. 2020, 62, 126636. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Wuehler, S.E.; Peerson, J.M. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nuti. Bull. 2001, 22, 113–125. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.M.; Broadley, M.R. Approaches to reduce zinc and iron deficits in food systems. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, T.; Watanabe, M.; Hatakeyama, S.; Kimura, S.; Nakazono, A.; Honma, A.; Nakamaru, Y.; Vreugde, S.; Homma, A. Role of intracellular zinc in molecular and cellular function in allergic inflammatory diseases. Allergol. Intern. 2020, 70, 190–200. [Google Scholar] [CrossRef]
- Nriagu, J. Zinc Deficiency in Human Health. Encyclopedia of Environmental Health, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med.Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of zinc for human health and biomarkers of zinc deficiency. In Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals; Collins, J.F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 241–260. [Google Scholar]
- FAO. 2013. The State of Food and Agriculture. Food Systems for Better Nutrition. 2013. Rome. Available online: www.fao.org/3/i3300e/i3300e.pdf (accessed on 15 April 2021).
- FAO. Crop Prospects and Food Situation—Quarterly Global Report No. 1; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Rawat, N.; Tiwari, V.K.; Singh, N.; Randhawa, G.S.; Singh, K.; Chhuneja, P.; Dhaliwal, H.S. Evalution and utilization of Aegilops and wild Triticum species for enhancing iron and zinc content in wheat. Gen. Resour. Crop Evol. 2009, 56, 53–64. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, Ecology, In Situ and Ex Situ Conversation and Expected Actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, Y.-F.; Chen, X.-P.; Zhang, F.-S.; Zou, C.-Q. Zinc nutrition for high productivity and human health in intensive production of wheat. Adv. Agron. 2020, 163, 179–217. [Google Scholar]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.P. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J.A. NAC gene regulating senescence improves grain protein, zinc and irons content in wheat. Science 2006, 314, 1133649. [Google Scholar] [CrossRef]
- Avni, R.; Zhao, R.; Pearce, S.; Jun, Y.; Uauy, C.; Tabbita, F.; Fahima, T.; Slade, A.; Dubcovsky, J.; Distelfeld, A. Functional characterization of GPC-1 genes in hexaploid wheat. Planta 2014, 239, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Brevis, J.C.; Dubcovsky, J. Effects of the chromosome region including the Gpc-B1 locus on wheat grain and protein yield. Crop Sci. 2010, 50, 93–104. [Google Scholar] [CrossRef]
- Pokhylko, S.Y.; Schwartau, V.V.; Mykhalska, L.M. ICP-MS analysis of bread wheat carrying the GPC-B1 gene of Triticumturgidum ssp. Dicoccoides. Biotech. Acta 2016, 9, 64–69. [Google Scholar] [CrossRef][Green Version]
- Vishwakarma, M.K.; Mishra, V.K.; Gupta, P.K.; Yadav, P.S. Integression of the high grain protein gene Gpc-B1 in a elite wheat variety of Indo-Gangetic Plains through marker assisted backcross breeding. Cur. Plant Biol. 2014, 1, 60–67. [Google Scholar] [CrossRef]
- Anikiev, V.V.; Kutuzov, F.F. A new method for determining the leaf surface of cereals. Rus. J. Plant Phys. 1961, 8, 375–378. (In Russian) [Google Scholar]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 97–103. [Google Scholar] [CrossRef]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Shao, H.; Wang, H.; Tang, X. NAC transcription factors in plant multiple abiotic stress responses: Progress and prospects. Fron. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Tabbita, F.; Pearce, S.; Barneix, A.J. Breeding for increased grain protein and micronutrient content in wheat: Ten years of the GPC-B1 gene. J. Cereal Sci. 2017, 73, 183–191. [Google Scholar] [CrossRef]
- Distelfeld, A.; Cakmak, I.; Peleg, Z.; Öztürk, L.; Yazici, A.M.; Budak, H.; Saranga, Y.; Fahima, T. Multiple QTL-effects of wheat Gpc-B1 locus on grain protein and micronutrient concentrations. Physiol. Plant. 2007, 129, 635–643. [Google Scholar] [CrossRef]
- Cakmak, I.; Torun, B.; Erenoğlu, B.; Öztürk, L.; Marschner, H.; Kalayci, M.; Ekiz, H.; Yilmaz, A. Morphological and physiological differences in the response of cereals to zinc deficiency. Euphytica 1998, 100, 349–357. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ismail, A.M.; Yanagihara, S. Effects of zinc deficiency on rise growth and genetic factors contributing to tolerance. Plant Physiol. 2006, 142, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Hossain, M.M.; Khatun, M.A.; Sarcar, M.R.; Haider, S.A. Biochemical and molecular mechanisms associated with Zn deficiency tolerance and signaling in rice (Oryza sativa L.). J. Plant. Interac. 2017, 12, 447–456. [Google Scholar] [CrossRef]
- Ehsanullah; Tariq, A.; Randhawa, M.A.; Anjum, S.A.; Nadeem, M.; Naeem, M. Exploring the role of zinc in maize (Zea mays L.) through soil and foliar application. Univers. J. Agricul. Res. 2015, 3, 69–75. [Google Scholar] [CrossRef]
- Liu, H.; Gan, W.; Rengel, Z. Zn fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea (Cicer arientinum L.). J. Soil Sci. Plant Nutr. 2016, 16, 550–662. [Google Scholar]
- Khattak, S.G.; Dominy, P.J.; Ahmad, W. Effect of Zn as soil addition and foliar application on yield and protein content of wheat in alkaline soil. J. Nation. Sci. Found. Sri Lanka 2015, 43, 303–312. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 1995; pp. 184–200. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.; Sharma, C.P. Zinc is critically required for pollen function and fertilization in lentil. J. Trace Elem. Med. Biol. 2006, 20, 89–96. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Singh, A.K. Differential sensitivity of maize to zinc and high light intensity. Plant Stress 2010, 4, 18–24. [Google Scholar]
- Mousavi, S.R. Zinc in crop production and interaction with phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Mesfin, A.; Frohberg, R.; Anderson, J.A. RFLP markers associated with high grain protein from Triticum turgidum L. var. dicoccoides introgressed into hard red spring wheat. Crop Sci. 1999, 39, 508–513. [Google Scholar] [CrossRef]
- Brevis, J.C.; Morris, C.F.; Manthey, F.; Dubcovsky, J. Effect of grain protein content locus Gpc-B1 on bread and pasta quality. J. Cereal Sci. 2010, 51, 357–365. [Google Scholar] [CrossRef]
- Carter, A.H.; Santra, D.K.; Kidwell, K.K. Assessment of the effects of the Gpc-B1 allele on senescence rate, grain protein concentration and mineral content in hard red spring wheat (Triticum aestivum L.) from the Pacific Northwest Region of the USA. Plant Breed. 2012, 131, 62–68. [Google Scholar] [CrossRef]
- Nadolska-Orczyk, A.; Rajchel, I.K.; Orczyk, W.; Gasparis, S. Major genes determining yield-related traits in wheat and barley. Global Food Sec. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
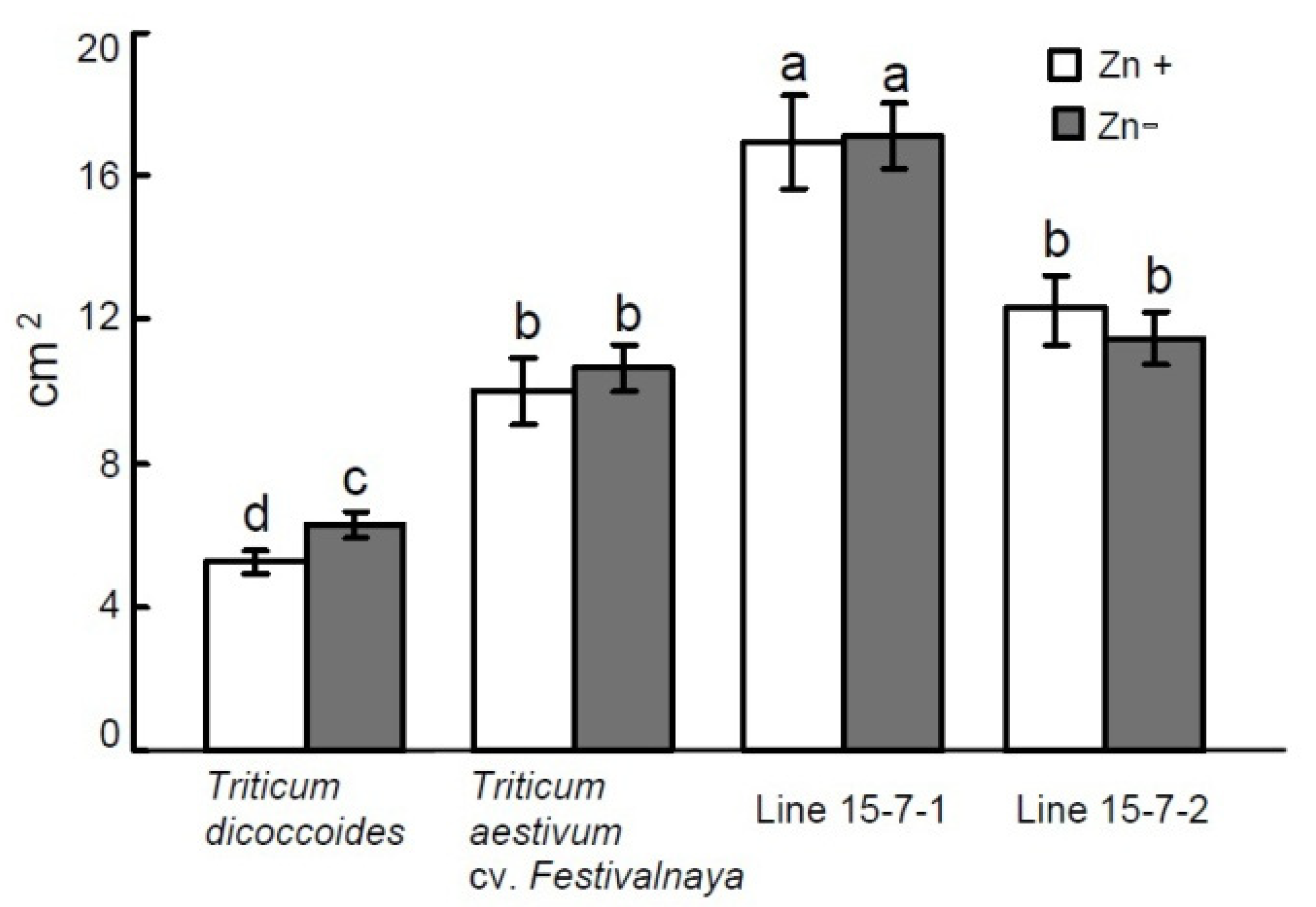
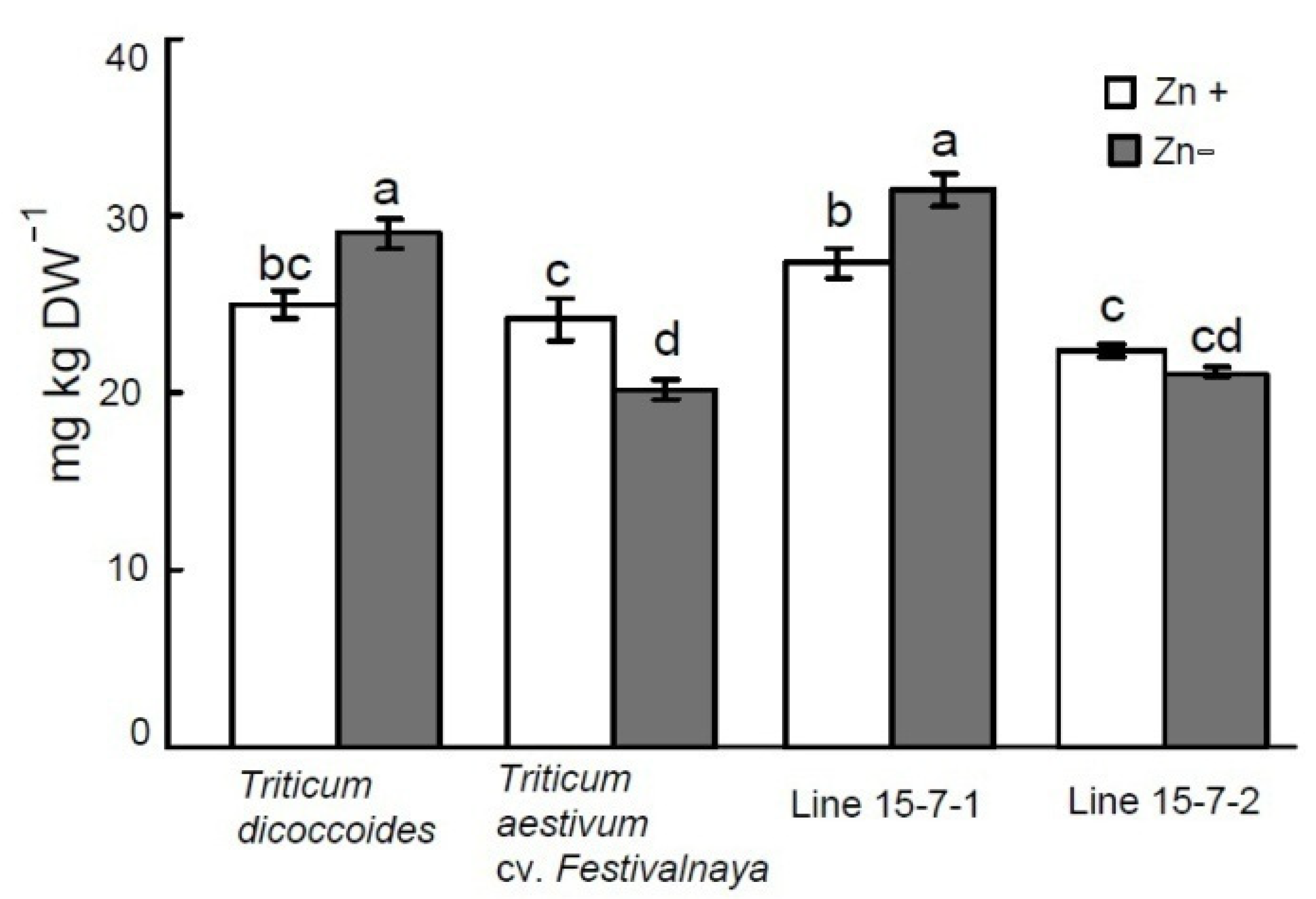
| Variant | Shoot Height (cm) | Shoot FW (g) | ||
|---|---|---|---|---|
| Zn+ | Zn− | Zn+ | Zn− | |
| Triticum dicoccoides | 101.5 ± 1.98 b | 95.55 ± 1.77 c | 2.38 ± 0.17 c | 2.60 ± 0.17 c |
| Triticum aestivum cv. Festivalnaya | 87.42 ± 2.58 de | 81.85 ± 2.31 e | 5.36 ± 0.27 ab | 5.06 ± 0.18 b |
| Line 15-7-1 | 99.85 ± 3.78 bc | 94.05 ± 3.59 cd | 6.07 ± 0.46 a | 5.87 ± 0.41 ab |
| Line 15-7-2 | 114.26 ± 3.33 a | 114.45 ± 2.54 a | 4.97 ± 0.27 b | 4.84 ± 0.26 b |
| Variant | Spike Length (cm) | Spike DW (g) | ||
|---|---|---|---|---|
| Zn+ | Zn− | Zn+ | Zn− | |
| Triticum dicoccoides | 4.38 ± 0.14 c | 4.59 ± 0.13 c | 0.45 ± 0.02 c | 0.53 ± 0.02 b |
| Triticum aestivum cv. Festivalnaya | 7.08 ± 0.22 b | 6.67 ± 0.11 b | 1.01 ± 0.10 a | 1.20 ± 0.10 a |
| Line 15-7-1 | 8.20 ± 0.32 a | 8.41 ± 0.29 a | 1.01 ± 0.10 a | 1.18 ± 0.11 a |
| Line 15-7-2 | 8.21 ± 0.19 a | 7.99 ± 0.18 a | 1.33 ± 0.08 a | 1.42 ± 0.08 a |
| Variant | Grain Number, Pieces per Spike | Grain Yield, g per Spike | ||
|---|---|---|---|---|
| Zn+ | Zn– | Zn+ | Zn– | |
| Triticum dicoccoides | 12.84 ± 0.87 d | 14.30 ± 0.65 c | 0.29 ± 0.02 e | 0.37 ± 0.02 d |
| Triticum aestivum cv. Festivalnaya | 20.63 ± 2.47 b | 20.75 ±1.92 b | 0.68 ± 0.09 c | 0.91 ± 0.09 abc |
| Line 15-7-1 | 21.20 ± 2.23 b | 21.10 ± 2.08 b | 0.76 ± 0.09 bc | 1.01 ± 0.09 ab |
| Line 15-7-2 | 27.79 ± 1.23 a | 26.20 ± 1.28 a | 0.96 ± 0.06 ab | 1.07 ± 0.06 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaznina, N.; Dubovets, N.; Batova, Y.; Ignatenko, A.; Orlovskaya, O.; Repkina, N. The Response of Wheat with Different Allele Statuses of the Gpc-B1 Gene under Zinc Deficiency. Agronomy 2021, 11, 1057. https://doi.org/10.3390/agronomy11061057
Kaznina N, Dubovets N, Batova Y, Ignatenko A, Orlovskaya O, Repkina N. The Response of Wheat with Different Allele Statuses of the Gpc-B1 Gene under Zinc Deficiency. Agronomy. 2021; 11(6):1057. https://doi.org/10.3390/agronomy11061057
Chicago/Turabian StyleKaznina, Natalia, Nadezhda Dubovets, Yuliya Batova, Anna Ignatenko, Olga Orlovskaya, and Natalia Repkina. 2021. "The Response of Wheat with Different Allele Statuses of the Gpc-B1 Gene under Zinc Deficiency" Agronomy 11, no. 6: 1057. https://doi.org/10.3390/agronomy11061057
APA StyleKaznina, N., Dubovets, N., Batova, Y., Ignatenko, A., Orlovskaya, O., & Repkina, N. (2021). The Response of Wheat with Different Allele Statuses of the Gpc-B1 Gene under Zinc Deficiency. Agronomy, 11(6), 1057. https://doi.org/10.3390/agronomy11061057






