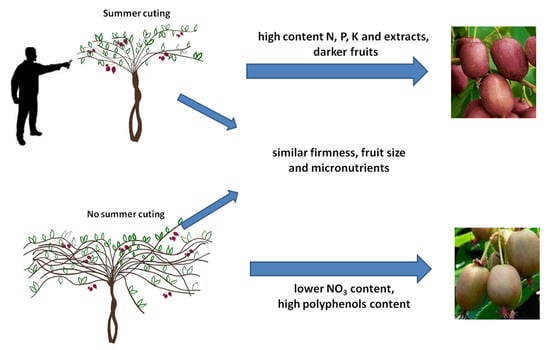
Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Area of Research and Plant Material
2.2. Weather Conditions during the Experiment
2.3. Cutting Plants
- One cut-control (control) On all plants a clear-cutting cut was made annually at the end of May and the beginning of June, according to a standard procedure. Shoots were cut a few leaves above the fruit, shoots without flowers and shoots growing too low were removed.
- Two cuts-A second (additional) cut was carried out between July and August. It was performed on half of the tested plants. Again, all shoots growing too low and shoots that grew too strong into the interrows or neighbouring plants, strong growing and thick shoots >20 mm, thickening the bush in the central part, were shortened. Pruning was to prevent the plants from becoming too dense.
2.4. Elemental Analysis
2.5. General Fruits Parameters
2.6. Determination of Colour
2.7. Extraction Procedure and Identification of Phenolic Compounds, Antioxidant Activity
2.8. Reagents
2.9. Statistical Analysis
3. Results and Discussion
3.1. Mineral Compounds in Actinidia Fruits
3.2. Fruit Color and Quality in Actinidia Fruits
3.3. Basic Physical and Chemical Properties of Actinidia Fruits
3.4. Polyphenolic Compounds of Actinidia Fruits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishiyama, I.; Fukuda, T.; Oota, T. Varietal differences in actinidin concentration and protease activity in the fruit juice of Actinidia arguta and Actinidia rufa. J. Jpn. Soc. Hortic. Sci. 2004, 73, 157–162. [Google Scholar] [CrossRef]
- Chesoniene, L.; Daubaras, R.; Viskelis, P. Biochemical composition of berries of some kolomikta kiwi (Actinidia kolomikta) cultivars and detection of harvest maturity. In XI Eucarpia Symposium on Fruit Breeding and Genetics; International Society for Horticultural Science: Angers, France, 2003; Volume 663, pp. 305–308. [Google Scholar]
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH•, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Drummond, L. The composition and nutritional value of kiwi fruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar]
- Nishiyama, I.; Fukuda, T.; Shimohashi, A.; Oota, T. Sugar and organic acid composition in the fruit juice of different Actinidia varieties. Food. Sci. Technol. Res. 2008, 14, 67–73. [Google Scholar] [CrossRef]
- Zuo, L.L.; Wang, Z.Y.; Fan, Z.L.; Tian, S.Q.; Liu, J.R. Evaluation of antioxidant and antiproliferative properties of three Actinidia (Actinidia kolomikta, Actinidia arguta, Actinidia chinensis) extracts in vitro. Int. J. Mol. Sci. 2012, 13, 5506–5518. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal difference in vitamin C content in the fruit of kiwi fruit and other Actinidia species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, I.; Fukuda, T.; Oota, T. Genotypic differences in chlorophyll, lutein, and β-carotene contents in the fruits of actinidia species. J. Agric. Food. Chem. 2005, 53, 6403–6407. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Li, M.; Ma, F.; Liang, D. Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chem. 2009, 113, 557–562. [Google Scholar] [CrossRef]
- Muto, Y.; Fujii, J.; Shidoji, Y.; Moriwaki, H.; Kawaguchi, T.; Noda, T. Growth retardation in human cervical dysplasia-derived cell lines by beta-carotene through down-regulation of epidermal growth factor receptor. Am. J. Clin. Nutr. 1995, 62, 1535S–1540S. [Google Scholar] [CrossRef] [PubMed]
- Karas, M.; Amir, H.; Fishman, D.; Danilenko, M.; Segal, S.; Nahum, A.; Koifmann, A.; Giat, Y.; Levy, J.; Sharoni, Y. Lycopene interferes with cell cycle progression and insulin-like growth factor i signaling in mammary cancer cells. Nutr. Cancer 2000, 36, 101–111. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef]
- Strik, B.C.; Cahn, H. Growing Kiwi Fruit; Oregon State University Extension Service Publication PNW: Corvallis, OR, USA, 1998; Volume 507, pp. 1–6. [Google Scholar]
- Tiyayon, C.; Strik, B. Influence of time ofoverhead shading on yield, fruit quality, and subsequent flowering of hardy kiwi fruit, Actinidia arguta. N. Z. J. Crop Hortic. Sci. 2004, 32, 235–241. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmiański, J.; Lachowicz, S.; Krupa-Małkiewicz, M. Rootstock effect on physico-chemical properties and content of bioactive compounds of four cultivars Cornelian cherry fruits. Sci. Hortic. 2019, 256, 108588. [Google Scholar] [CrossRef]
- IUNG Institute of Soil Science and Plant Cultivation. Fertiliser Recommendations Part I—Limits for Estimating Soil Macro- and Microelement Content; Series P. Państwowy Instytut Badawczy w Puławach: Puławy, Poland, 1990; pp. 26–28. [Google Scholar]
- Ochmian, I.; Oszmiański, J.; Jaśkiewicz, B.; Szczepanek, M. Soil and high bush blueberry responses to fertilization with urea phosphate. Folia Hortic. 2018, 30, 295–305. [Google Scholar] [CrossRef]
- Polish Committee for Standardisation. Fruit and Vegetable Preparations—Sample preparation and Physicochemical Test Methods; PN-EN 12145; Polish Committee for Standardisation: Warszawa, Poland, 2001. [Google Scholar]
- Mijowska, K.; Ochmian, I.; Oszmiański, J. Impact of cluster zone leaf removal on grapes cv. regent polyphenol content by the UPLC-PDA/MS method. Molecules 2016, 21, 1688. [Google Scholar] [CrossRef] [PubMed]
- Pijanowski, E.; Mrożewski, S.; Horubała, A.; Jarczyk, A. Technology of Fruit and Vegetable Products; PWRiL: Warsaw, Poland, 1973; pp. 137–155. [Google Scholar]
- Kruczek, A.; Ochmian, I.; Krupa-Małkiewicz, M.; Lachowicz, S. Comparison of morphological, antidiabetic and antioxidant properties of goji fruits. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 1–14. [Google Scholar] [CrossRef]
- Ochmian, I.; Grajkowski, J.; Smolik, M. Comparison of some morphological features, quality and chemical content of four cultivars of chokeberry fruits (Aronia melanocarpa). Not. Bot. Horti Agrobot. Cluj Napoca 2012, 40, 253–260. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of photochemical composition and antioxidant capacity of 22 old apple cultivars grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Akar, Z.; Küçük, M.; Doğan, H. A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem. 2017, 32, 640–647. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar]
- Bieniek, A. Mineral Composition of Fruits of Actinidia Arguta and Actinidia Purpurea and Some of Their Hybrid Cultivars Grown in Northeastern Poland. Pol. J. Environ. Stud. 2012, 21, 1543–1550. [Google Scholar]
- Ochmian, I.; Kozos, K. Influence of foliar fertilization with calcium fertilizers on the firmness and chemical composition of two high bush blueberry cultivars. J. Elementol. 2015, 20, 185–201. [Google Scholar]
- Nascimento, A.N.; Silvestre, D.M.; de Oliveira Leme, F.; Nomura, C.S.; Naozuka, J. Elemental analysis of goji berries using axially and radially viewed inductively coupled plasma-opical emissin spectometry. Spectometry 2015, 30, 36–41. [Google Scholar]
- Regulation EU. No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers; No 1169/2011; Official Journal of the European Union: Luxembourg, 2011; Volume 304, pp. 18–63.
- Sunde, R.A.; Raines, A.M. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv. Nutr. 2011, 2, 138–150. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Calomme, M.; Vanden Berghe, D. Selenium deficiency triggering intractable seizures. Neuropediatrics 1994, 25, 217–223. [Google Scholar] [CrossRef]
- Falandysz, J.; Lipka, K. Selenium in mushrooms. Roczn. Państw. Zakł. Hig. 2006, 52, 217–233. [Google Scholar]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef]
- Dougnon, T.V.; Bankolé, H.S.; Johnson, R.C.; Klotoé, J.R.; Dougnon, G.; Gbaguidi, F.; Rhin, B.H. Phytochemical screening, nutritional and toxicological analyses of leaves and fruits of Solanum macrocarpon Linn (Solanaceae) in Cotonou (Benin). Food Nutri. Sci. 2012, 3, 1595–1603. [Google Scholar] [CrossRef]
- Reports of the Scientific Committee for Food (SCF). Nutrient and energy intakes for the European Community; Thirty-First Series; Office for Official Publications of the European Communities: Brussels, Belgium; Luxembourg, 1993. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods. 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Montefiori, M.; Comeskey, D.J.; Wohlers, M.; McGhie, T.K. Characterization and quantification of anthocyanins in red kiwi fruit (Actinidia spp.). J. Agric. Food Chem. 2009, 57, 6856–6861. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Jáuregui, P.; Carbonell-Barrachina, A.A.; Oszmiański, J.; Golis, T. Variability of phytochemical properties and content of bioactive compounds in Lonicera caerulea L. var. kamtschatica berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Kubus, M.; Dobrowolska, A. Description of plants and assessment of chemical properties of three species from the Amelanchier genus. Dendrobiology 2013, 70, 59–64. [Google Scholar] [CrossRef]
| Month | ||||||||
|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X | Mean | |
| Year | average temperature (°C) | |||||||
| 2019 | 10.1 | 12.1 | 21.5 | 18.8 | 20.1 | 14.5 | 10.7 | 15.4 |
| 2018 | 12.3 | 16.6 | 18.5 | 20.2 | 20.1 | 15.4 | 10.3 | 16.2 |
| 2017 | 6.8 | 13.5 | 16.8 | 17.2 | 17.9 | 13.3 | 11.0 | 13.8 |
| 2016 | 8.8 | 15.7 | 18.5 | 19.0 | 17.8 | 16.8 | 8.6 | 15.0 |
| 1951–2012 | 8.0 | 13.0 | 16.4 | 18.2 | 17.6 | 13.8 | 9.2 | 13.7 |
| rainfall (mm) | Total | |||||||
| 2019 | 10.7 | 68.7 | 70.8 | 23.5 | 41.8 | 39.4 | 46.1 | 301 |
| 2018 | 26.8 | 22.5 | 15.0 | 92.8 | 21.4 | 16.3 | 20.2 | 215.0 |
| 2017 | 42.3 | 99.2 | 118.1 | 182.4 | 145.4 | 31.6 | 95.1 | 714.1 |
| 2016 | 20.2 | 18.9 | 69.0 | 50.1 | 47.8 | 18.3 | 55.3 | 279.6 |
| 1951–2012 | 39.7 | 62.9 | 48.2 | 69.6 | 74.2 | 58.7 | 37.3 | 390.6 |
| Compounds (g/kg) | Cultivar | |||||
|---|---|---|---|---|---|---|
| Summer Cuts | Sientiabrskaja | Genewa | Issai | Ken’s Red | Mean | |
| N | 1 cut-control | 9.88f * | 9.04d | 8.15c | 7.06a | 8.53A |
| 2 cuts | 10.23g | 9.41e | 8.37c | 7.45b | 8.87B | |
| Mean | 10.06D | 9.23C | 8.26B | 7.26A | ||
| P | 1 cut-control | 4.95e | 3.89b | 4.34cd | 3.11a | 4.07A |
| 2 cuts | 5.11e | 4.20c | 4.42d | 3.64b | 4.34B | |
| Mean | 5.03D | 4.04B | 4.38C | 3.37A | ||
| K | 1 cut-control | 14.21a | 17.23d | 15.06b | 17.60e | 16.03A |
| 2 cuts | 15.18b | 18.02f | 15.87c | 17.99f | 16.77B | |
| Mean | 14.70A | 17.63C | 15.47B | 17.80C | ||
| Ca | 1 cut-control | 2.79a | 4.58e | 4.11d | 3.45c | 3.73A |
| 2 cuts | 2.83a | 4.22d | 4.87f | 3.03b | 3.74A | |
| mean | 2.81A | 4.40C | 4.49C | 3.24B | ||
| Mg | 1 cut-control | 0.94d | 0.57b | 1.04e | 0.83c | 0.85A |
| 2 cuts | 0.88cd | 0.49a | 1.23f | 0.81c | 0.85A | |
| mean | 0.91BC | 0.53A | 1.13C | 0.82B | ||
| Compounds (mg/kg) | Cultivar | |||||
|---|---|---|---|---|---|---|
| Summer Cuts | Sientiabrskaja | Genewa | Issai | Ken’s Red | Mean | |
| Fe | 1 cut-control | 14.07b * | 12.37a | 15.71c | 21.44e | 15.90A |
| 2 cuts | 14.22b | 12.52a | 15.88c | 21.06d | 15.92A | |
| mean | 14.15B | 12.45A | 15.80C | 21.25D | ||
| Zn | 1 cut-control | 11.89h | 5.44a | 6.12d | 6.88f | 7.58A |
| 2 cuts | 11.05g | 5.63b | 5.91c | 6.36e | 7.24A | |
| mean | 11.47D | 5.54A | 6.02B | 6.62C | ||
| Mn | 1 cut-control | 2.55e | 1.57a | 1.73b | 2.11c | 1.99A |
| 2 cuts | 2.34d | 1.88b | 1.52a | 2.40d | 2.04A | |
| mean | 2.45C | 1.73A | 1.63A | 2.26B | ||
| Cu | 1 cut-control | 3.84b | 4.50e | 4.02c | 1.62a | 3.50A |
| 2 cuts | 3.79b | 4.28de | 4.19d | 1.72a | 3.50A | |
| Mean | 3.82B | 4.39D | 4.11C | 1.67A | ||
| Se | 1 cut-control | 0.13c | 0.23d | 0.29e | 0.09a | 0.19A |
| 2 cuts | 0.11b | 0.19c | 0.31f | 0.08a | 0.17A | |
| mean | 0.12B | 0.21C | 0.30D | 0.09A | ||
| Compounds | Cultivar | ||||||
|---|---|---|---|---|---|---|---|
| Summer Cuts | Sientiabrskaja | Genewa | Issai | Ken’s Red | Mean | ||
| Colour parameters CIE fruit | L* | 1 cut-control | 50.6f * | 42.5d | 43.8d | 29.1b | 41.5A |
| 2 cuts | 48.5e | 42.0d | 39.5c | 25.9a | 39.0A | ||
| mean | 49.5C | 42.2B | 41.6B | 27.5A | |||
| a* | 1 cut-control | −29.4b | 18.4c | −7.8a | 47.5e | 7.2A | |
| 2 cuts | −30.4b | 24.6d | −7.0a | 51.8f | 9.7B | ||
| mean | −29.9A | 21.5C | −7.4B | 49.6D | |||
| b* | 1 cut-control | 37.2f | 24.8a | 28.8b | 31.6d | 30.6A | |
| 2 cuts | 34.6e | 28.9b | 29.7b | 30.8cd | 31.0A | ||
| mean | 35.9C | 26.8A | 29.2B | 31.2B | |||
| Firmness (G/mm) | 1 cut-control | 112b | 125b | 144cd | 155d | 134A | |
| 2 cuts | 98a | 119b | 140c | 148cd | 126A | ||
| mean | 105A | 122B | 142C | 151C | |||
| Weight of 100 fruits (g) | 1 cut-control | 616c | 792d | 519a | 531ab | 615A | |
| 2 cuts | 631c | 788d | 548ab | 557b | 631A | ||
| mean | 624B | 790C | 534A | 544A | |||
| Compounds | Cultivar | |||||
|---|---|---|---|---|---|---|
| Summer Cuts | Sientiabrskaja | Genewa | Issai | Ken’s Red | Mean | |
| Soluble Solid Content-SSC (°Bx) | 1 cut-control | 12.8a * | 13.0ab | 14.2cd | 13.5b | 13.4A |
| 2 cuts | 13.3ab | 13.2ab | 14.4d | 13.9c | 13.7B | |
| mean | 13.1A | 13.1A | 14.3C | 13.7B | ||
| Dry weight (g/100 g) | 1 cut-control | 17.4a | 17.7bc | 18.6d | 19.3e | 18.3A |
| 2 cuts | 17.5ab | 17.9c | 18.8d | 19.6f | 18.5A | |
| mean | 17.5A | 17.8A | 18.7B | 19.5C | ||
| Pectines (g/100 g) | 1 cut-control | 2.44d | 2.22bc | 2.25bc | 1.98a | 2.22A |
| 2 cuts | 2.52e | 2.28c | 2.17b | 1.91a | 2.22A | |
| mean | 2.48C | 2.25B | 2.21B | 1.95A | ||
| Total acidity (g/100) | 1 cut-control | 1.42d | 1.17b | 1.33c | 1.21b | 1.28A |
| 2 cuts | 1.37cd | 1.04a | 1.38cd | 1.18b | 1.24A | |
| mean | 1.40C | 1.11A | 1.36BC | 1.20AB | ||
| L-ascorbic acid (mg/1000) | 1 cut-control | 70d | 64cd | 58bc | 41a | 58A |
| 2 cuts | 62bc | 56bc | 51b | 43a | 53A | |
| mean | 66C | 60BC | 54B | 42A | ||
| β-carotene - provitamin A (mg/100 g) | 1 cut-control | 0.17a | 0.32c | 0.20b | 0.37d | 0.27A |
| 2 cuts | 0.16a | 0.34c | 0.22b | 0.41e | 0.28A | |
| mean | 0.17A | 0.33C | 0.21B | 0.39D | ||
| DPPH• (μmol T/g) | 1 cut-control | 17.7c | 14.5b | 21.3 | 12.5a | 16.5A |
| 2 cuts | 17.5c | 12.8a | 21.6dd | 11.3a | 15.8A | |
| mean | 17.6B | 13.7A | 21.5C | 11.9A | ||
| ABTS•+ (μmol T/g) | 1 cut-control | 32.5b | 42.5 | 35.7c | 28.9a | 34.9A |
| 2 cuts | 33.9b | 38.6de | 32.4b | 28.0a | 33.2A | |
| mean | 33.2B | 40.6C | 34.1B | 28.5A | ||
| FRAP (μmol/T g) | 1 cut-control | 23.6d | 17.4b | 21.5c | 13.6a | 19.0A |
| 2 cuts | 21.1c | 14.6a | 21.3c | 14.4a | 17.9A | |
| mean | 22.4B | 16.0A | 21.4B | 14.0A | ||
| NO3 (mg/1000 g) | 1 cut-control | 45.4e | 31.7c | 27.5bc | 22.6a | 31.8A |
| 2 cuts | 57.7f | 40.8d | 20.9a | 24.2ab | 35.9B | |
| mean | 51.6C | 36.3B | 24.2A | 23.4A | ||
| Compounds (mg/100 g) | Cultivar | |||||
|---|---|---|---|---|---|---|
| Summer Cuts | Sientiabrskaja | Genewa | Issai | Ken’s Red | Mean | |
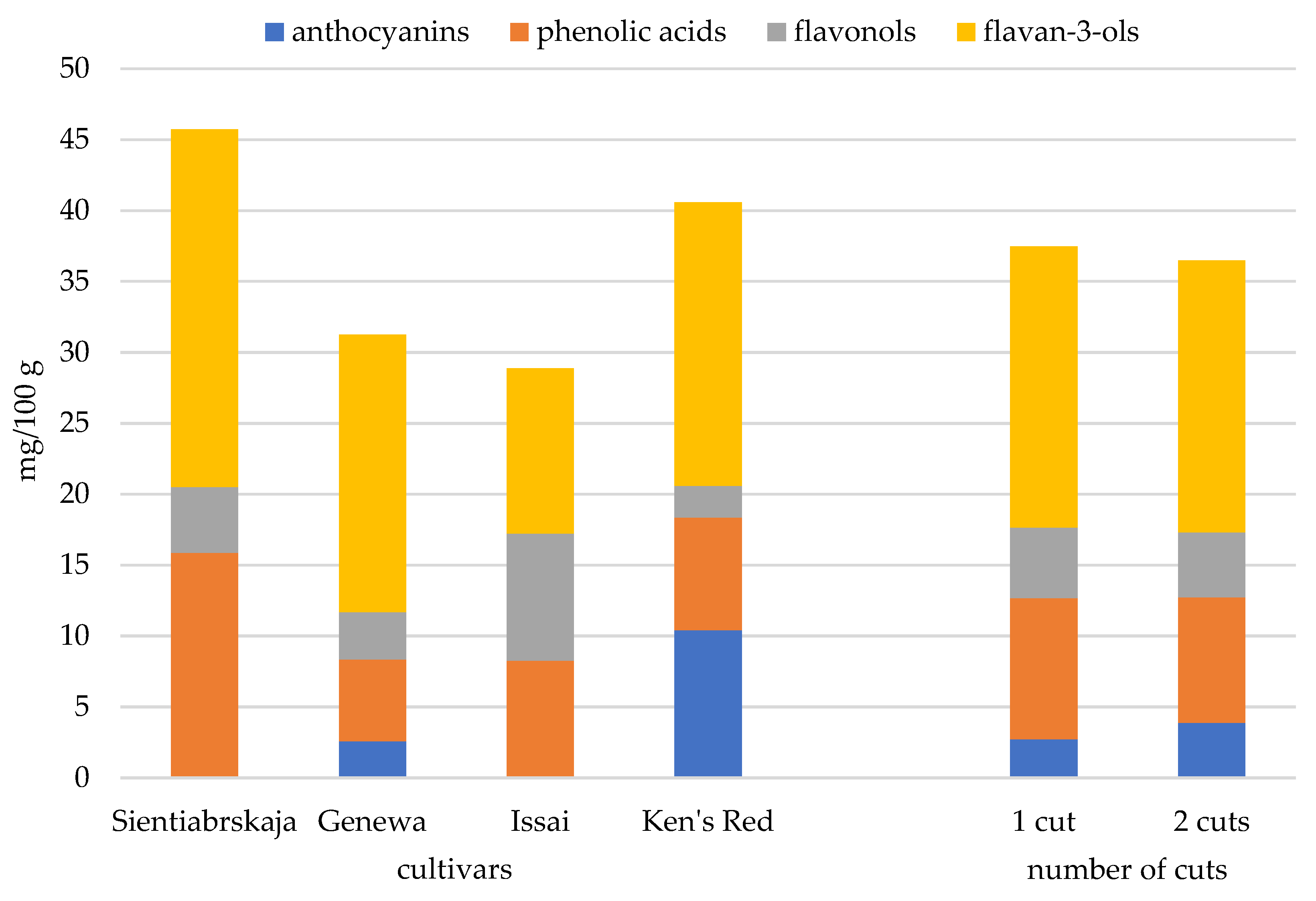
| Cyanidin 3-O-sambubioside | 1 cut-control | n.d. | 1.93a * | n.d. | 7.81c | 2.44A |
| 2 cuts | n.d. | 2.44b | n.d. | 11.78d | 3.56B | |
| mean | n.d. | 2.19A | n.d. | 9.80B | ||
| Cyanidin 3-O-hexoside | 1 cut-control | n.d. | 0.14b | 0.05a | 0.19c | 0.10A |
| 2 cuts | n.d. | 0.17bc | 0.02a | 0.20c | 0.10A | |
| mean | n.d. | 0.16B | 0.04A | 0.20C | ||
| Cyanidin 3-O-pentoside | 1 cut-control | 0.09a | 0.22b | n.d. | 0.35d | 0.17A |
| 2 cuts | 0.11a | 0.25c | n.d. | 0.48e | 0.21A | |
| mean | 0.10A | 0.24B | n.d. | 0.42C | ||
| Caffeoyl-O-hexoside | 1 cut-control | 4.44e | 0.41b | 0.05a | 2.62c | 1.88A |
| 2 cuts | 3.58d | 0.31b | 0.08a | 3.85d | 1.96A | |
| mean | 4.01D | 0.36B | 0.07A | 3.24C | ||
| Caffeoyl-O-hexoside | 1 cut-control | 1.70b | 1.06a | 5.25e | 2.62c | 2.66B |
| 2 cuts | 1.42b | 0.89a | 4.93d | 2.36c | 2.40A | |
| mean | 1.56B | 0.98A | 5.09D | 2.49C | ||
| Caffeoyl-O-hexoside | 1 cut-control | 2.47de | 1.81c | 2.56e | 1.73bc | 2.14B |
| 2 cuts | 2.30de | 1.06a | 2.22d | 1.58b | 1.79A | |
| mean | 2.39B | 1.44A | 2.39B | 1.66A | ||
| Caffeoyl-malic acid | 1 cut-control | 7.47f | 2.18d | 0.31b | 0.10a | 2.52B |
| 2 cuts | 5.82e | 1.69c | 0.28b | 0.12a | 1.98A | |
| mean | 6.65D | 1.94C | 0.30B | 0.11A | ||
| Chlorogenic acid | 1 cut-control | 0.42e | 0.36d | 0.17a | 0.27c | 0.31B |
| 2 cuts | 0.35d | 0.22b | 0.14a | 0.30c | 0.25A | |
| mean | 0.39C | 0.29B | 0.16A | 0.29B | ||
| Dimethyl caffeic acid hexoside | 1 cut-control | 0.22c | 0.08b | 0.06ab | 0.06ab | 0.11A |
| 2 cuts | 0.25d | 0.06ab | 0.05a | 0.08b | 0.11A | |
| mean | 0.24B | 0.07A | 0.06A | 0.07A | ||
| Feruoylglucoside | 1 cut-control | 0.11c | 0.04ab | 0.02a | 0.05b | 0.06A |
| 2 cuts | 0.13c | 0.06b | 0.03a | 0.04ab | 0.07A | |
| mean | 0.12B | 0.05A | 0.03A | 0.05A | ||
| Cryptochlorogenic acid | 1 cut-control | 0.39d | 0.60c | 0.10b | 0.03a | 0.28A |
| 2 cuts | 0.42d | 0.63c | 0.12b | 0.02a | 0.30A | |
| mean | 0.41C | 0.62D | 0.11B | 0.03A | ||
| Rutin | 1 cut-control | 0.39c | 0.15b | 0.71d | 0.01a | 0.32A |
| 2 cuts | 0.42c | 0.18b | 1.55e | 0.03a | 0.55B | |
| mean | 0.41C | 0.17B | 1.13D | 0.02A | ||
| Quercetin 3-O-hexoso-rhamnoside | 1 cut-control | 1.83c | 1.84c | 2.16d | 0.98b | 1.70A |
| 2 cuts | 2.04d | 1.78c | 2.55e | 0.77a | 1.79A | |
| mean | 1.94B | 1.81B | 2.36C | 0.88A | ||
| Quecetin 3-O-galactoside | 1 cut-control | 0.59d | 0.53cd | 3.22f | 0.04a | 1.10B |
| 2 cuts | 0.45c | 0.28b | 1.89e | 0.03a | 0.66A | |
| mean | 0.52B | 0.41B | 2.56C | 0.04A | ||
| Quecetin 3-O-glucoside | 1 cut-control | 0.06ab | 0.03a | 1.68e | 0.05a | 0.46A |
| 2 cuts | 0.05a | 0.12b | 1.23d | 0.38c | 0.45A | |
| mean | 0.06A | 0.08A | 1.46C | 0.22B | ||
| Kaempferol 3-O-rutinoside | 1 cut-control | 0.14d | 0.09bc | 0.08b | 0.04a | 0.09A |
| 2 cuts | 0.07ab | 0.05a | 0.25e | 0.12cd | 0.12B | |
| mean | 0.11B | 0.07A | 0.17C | 0.08AB | ||
| Kaempferol 3-O-glucorhamnoside | 1 cut-control | 1.23e | 0.70c | 1.03d | 0.35a | 0.83B |
| 2 cuts | 0.58b | 0.32a | 0.47b | 0.51b | 0.47A | |
| mean | 0.91C | 0.51A | 0.75B | 0.43A | ||
| Kaempferol 3-O-galactoside | 1 cut-control | 0.52e | 0.15bc | 0.34d | 0.10a | 0.28A |
| 2 cuts | 0.37d | 0.11ab | 0.19c | 0.38d | 0.26A | |
| mean | 0.45C | 0.13A | 0.27B | 0.24AB | ||
| Kaempferol 3-O-glucoside | 1 cut-control | 0.17bc | 0.18bc | 0.19c | 0.16b | 0.18A |
| 2 cuts | 0.10a | 0.12a | 0.34d | 0.48e | 0.26B | |
| mean | 0.14A | 0.15A | 0.27B | 0.32B | ||
| Kaempferol 3,7-dirhamnoside | 1 cut-control | 0.06b | n.d. | 0.01a | n.d. | 0.02A |
| 2 cuts | 0.12c | n.d. | n.d. | n.d. | 0.03A | |
| mean | 0.09B | n.d. | 0.01A | n.d. | ||
| Proanthocyanidin dimer B2 | 1 cut-control | 4.13d | 2.77b | 3.05b | 5.12e | 3.77B |
| 2 cuts | 3.67c | 1.89a | 2.28a | 5.89f | 3.43A | |
| mean | 2.33A | 2.67B | 2.33A | 5.51C | ||
| Proanthocyanidin dimer B2 | 1 cut-control | 0.83b | 2.45e | 1.22d | 0.58a | 1.27A |
| 2 cuts | 1.16d | 3.89f | 0.87bc | 0.99cd | 1.73B | |
| mean | 1.00B | 3.17C | 1.05B | 0.79A | ||
| (+)-Catechin | 1 cut-control | 18.20g | 10.55d | 5.86b | 8.64c | 10.81A |
| 2 cuts | 15.33f | 11.57e | 4.25a | 10.35d | 10.38A | |
| mean | 16.77D | 11.06C | 5.06A | 9.50B | ||
| (−)-Epicatechin | 1 cut-control | 5.38f | 2.78b | 3.92c | 3.83c | 3.98B |
| 2 cuts | 4.89e | 2.55ab | 2.47a | 4.52d | 3.61A | |
| mean | 5.14D | 2.67A | 3.20B | 4.18C | ||
| Total | 1 cut-control | 45.46d | 28.27b | 28.12b | 31.90b | 33.44A |
| 2 cuts | 38.74c | 28.09b | 23.74a | 40.74c | 32.83A | |
| mean | 42.10D | 28.18B | 25.93A | 36.32C | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figiel-Kroczyńska, M.; Ochmian, I.; Lachowicz, S.; Krupa-Małkiewicz, M.; Wróbel, J.; Gamrat, R. Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting. Agronomy 2021, 11, 964. https://doi.org/10.3390/agronomy11050964
Figiel-Kroczyńska M, Ochmian I, Lachowicz S, Krupa-Małkiewicz M, Wróbel J, Gamrat R. Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting. Agronomy. 2021; 11(5):964. https://doi.org/10.3390/agronomy11050964
Chicago/Turabian StyleFigiel-Kroczyńska, Monika, Ireneusz Ochmian, Sabina Lachowicz, Marcelina Krupa-Małkiewicz, Jacek Wróbel, and Renata Gamrat. 2021. "Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting" Agronomy 11, no. 5: 964. https://doi.org/10.3390/agronomy11050964
APA StyleFigiel-Kroczyńska, M., Ochmian, I., Lachowicz, S., Krupa-Małkiewicz, M., Wróbel, J., & Gamrat, R. (2021). Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting. Agronomy, 11(5), 964. https://doi.org/10.3390/agronomy11050964