Abstract
Microalgae produce a variety of high-value products. Enhancing product contents in microalgal cells is one of the efficient ways to decrease production costs. Improved germplasm and heterotrophic cultivation may enhance microalgae biomass and lipid content. In this study, we investigated the effect of three types of laser irradiation and heterotrophic cultivation on lipid productivity, lipid content, and biomass of two Chlorella strains (i.e., FACHB 9 and FACHB 31). Results showed that the highest biomasses of 4.81 g/L (15.03-fold) and 4.66 g/L (7.32-fold) were obtained in the third generation of FACHB 9 and FACHB 31 induced by a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser for 8 min and 12 min, respectively. The highest lipid contents were 525.6 mg/g (1.67-fold) dry weight (DW) and 780.0 mg/g DW (2.20-fold) in the third and the first generations of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and by a helium–neon (He–Ne) laser for 4 min, respectively. The highest lipid productivities of 69.82 ± 3.29 mg/L/d (19.7-fold) and 30.71 ± 3.77 mg/L/d (3.1-fold) were obtained in FACHB 9 and FACHB 31 treated by a semiconductor (SC) laser for 4 min and by a He–Ne laser for 12 min, respectively. Our study suggested that laser mutagenesis is a potential method for screening economically important oleaginous microalgae strains.
1. Introduction
Human development and societal progress have promoted a rapidly growing reliance on fossil fuel reserves or imported fuel to meet energy demands, creating socio-economic burdens for industrial growth worldwide. Biofuel products have attracted increasing attention due to the gradually depleting fossil reserves in addition to the global environmental pollution and political conflicts. Microalgae have been identified as potential candidates for biodiesel industries due to their high biomass production and fast growth [1]. To date, the microalgal biotechnology for biodiesel production has been advanced rapidly, showing significant prospects in the growing market [2].
Microalgae have been exploited for the production of high-value compounds such as lipids (including omega-3 fatty acids), enzymes, polymers, toxins, antioxidants, and pigments (i.e., carotenoids) that can be used directly for numerous purposes in the fields of food, feed nutrition, cosmetics, and pharmaceuticals. Furthermore, microalgae biomass can be a source of different types of bioenergy components, including biodiesel, bioethanol, methane, and hydrogen [3]. Microalgae have attracted considerable interest among scientists and policymakers due to the increasing demand for an alternative source of renewable energy to replace fossil fuels. Moreover, the high cost of producing algal oil has led to the generation of the concept of “microalgae biorefinery,” where high-value compounds and biofuels could be produced to make industrial businesses economically feasible [4].
Many countries have already begun to seriously develop various forms of renewable energy. Compared to fossil fuels, biodiesel is more environmentally friendly and sustainable, becoming an appropriate alternative to replace conventional petroleum [5]. As one of the third generation biofuels, microalgal biodiesel may be an ideal choice to investigate among the popular areas in the current global research fields of bioenergy [6]. Microalgae are photosynthetic microorganisms with a simple cellular structure, converting sunlight, water, and carbon dioxide into biomass [7] and producing large quantities of lipids (accounting for ~20–70% of dry cell weight) and secondary metabolites. Therefore, microalgae are considered to be the most promising feedstocks for bioenergy production, predicted to possibly completely replace fossil energy [3,8,9,10,11].
One of the ultimate goals of mutation breeding is to make organisms produce more products that people need. In general, three types of microbial mutagenesis are currently available based on the type of methods used, including biological mutagenesis, chemical mutagenesis, and physical mutagenesis. As a type of physical mutagenesis, laser mutagenesis, widely used in the field of microbiology, applies physical effects of electromagnetic fields (i.e., heat, pressure, and light) on the organisms to induce a series of genetic mutations, as confirmed at both cellular and molecular levels [12]. Compared with genetic engineering and chemical mutagenesis, laser mutagenesis shows more advantages, i.e., a large amount of biochemical and genetic information of the organisms is not necessarily required, the laser mutations are more flexibly controlled to avoid secondary pollution, and a large number of random mutations are generated for further convenient and rapid screening based on desired phenotypes [13].
Studies have shown that low intensity laser irradiation can improve the stimulating effects on organisms of different complexities. For example, accelerated cell division and enhanced protein synthesis are achieved in various microorganisms treated with low intensity laser irradiation with wavelengths of 400–500 nm and 600 nm [14], while both the quantity and the quality of lipids produced in microalgal cells vary depending on the algal species as well as site-specific growing conditions [15,16]. Among the factors inducing microalgae to accumulate lipids, light is known to have an evident influence on lipid production, and it is easy to control and inexpensive compared with many other factors. Therefore, it is practically appropriate to enhance the lipid content by light. In addition, a successful heterotrophic algal culture requires the maintenance of various culture conditions, such as temperature, aeration, and pH value in the medium. Autotrophic algal cultures usually induce alkalinization through carbon dioxide uptake and nitrate/H+ symport, while pH variation in heterotrophic algal cultures depends on the types of organic substrates and their concentrations [17,18]. For example, glucose at high concentrations can increase pH values with the activation of hexose/H+ symport but decreases pH values at low concentrations [19].
Heterotrophic cultivation of microalgae has shown several advantages and the potential to overcome or minimize the challenges associated with autotrophic cultivation: (i) heterotrophic culture systems do not rely heavily on light and can be quickly scaled up; (ii) contamination from other microorganisms can be effectively prevented under the closed systems of heterotrophic cultivation, which can be properly controlled to optimize cell yield; (iii) organic carbon can rapidly increase cell density, consequently achieving a high biomass productivity [20]; and (iv) high-cell-density cultures can reduce the cost of down-stream processing and help decrease production costs. Studies have shown that the protein content of heterotrophic cells (10.3–25.8%) is much lower than that of autotrophic cells (up to 52.6%) [21].
Compared to phototrophic growth, heterotrophic algal cultures are more advantageous due to their fast growth, high production rate, and convenient harvesting procedures. A series of heterotrophic microalgal species have been successfully used in industry-scale production of polyunsaturated fatty acids (PUFAs) [22]. Recently, a heterotrophic microalgal culture was reported to produce biodiesel, showing promising prospects, although the high cost of organic carbon was noted as one of the limiting factors in this process [23]. Furthermore, medium composition was optimized using different carbon and nitrogen sources through response surface methodology (RSM) for heterotrophic cultivation of Tetraselmis suecica to obtain increased algal biomass [24], while Raman spectroscopy applications were utilized to screen oil-rich algal strains for the potential mass production of commercial biofuels and utilization in food industry [25]. Therefore, it is expected that in industrial settings, the characteristics of laser mutations could be used to screen microalgae strains with enhanced heterotrophic capability to increase biomass and reduce production costs through laser mutagenesis and heterotrophic cultivation.
In this study, our goals were to investigate the potential enhancing effects of laser mutagenesis and heterotrophic cultivation on lipid productivity, biomass, and contents of PUFAs in two strains of Chlorella, i.e., C. vulgaris strain FACHB 9 and C. pyrenoidesa strain FACHB 31. Our operational objectives were to identify mutant strains with increased biomass, lipid content, and lipid productivity derived from the treatment of strains FACHB 9 and FACHB 31 by three types of laser irradiation, including neodymium-doped yttrium aluminum garnet (Nd:YAG), helium–neon (He–Ne), and semiconductor (SC) lasers. Our study provides experimental evidence to support the application of laser irradiation as a potential mutagenesis method for screening economically important oleaginous microalgae strains.
2. Materials and Methods
2.1. Microalgae Culture
Most of the chemicals used in the experiments were of analytical grade except for Hexane which was of chromatographic purity. Two microalgae strains, i.e., Chlorella vulgaris strain FACHB 9 and Chlorella pyrenoidesa strain FACHB 31, were obtained from the Institute of Hydrobiology, Chinese Academy of Sciences and preserved in the Microalgae Biotechnology Lab of the Shandong University of Technology, China. The microalgae were maintained in the original BG11 growth medium (Table 1) at 22 ± 2 °C, under light illumination of 40 µmol photons/m2s with a photoperiod cycle of 12 h light and 12 h dark. The cultures were hand-shaken 3–5 times daily or as needed to avoid clumping. The heterotrophic algal cultivations were initiated from a single colony taken from the stock agar plate and cultured in modified BG11 growth medium (Table 1). Both the culture medium and the Erlenmeyer flasks were sterilized at 121 °C for 30 min. The microalgal cultures were illuminated with cool-white fluorescent light under 36 µmol photons/m2s, with a photoperiod cycle of 12 h light and 12 h dark at 24 ± 2 °C and were shaken manually 3–5 times each day, with nutrients of the concentration two times (v/v) that of BG11 added once a week (each generation) for three weeks.

Table 1.
The chemical components of the original and modified BG11 growth media used in this study.
2.2. Laser Irradiation
The algal samples (20 mL) at the exponential growth phase were irradiated by three types of laser irradiation, including a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser (WGL-3) with a transition wavelength of 1064 nm (GangDong, Tianjin, China) at 40 mW, a helium–neon (He–Ne) laser (LOS-BLD-0808-12 W-C) with a transition wavelength of 808 nm at 6 W (HTOE, Beijing, China), and a semiconductor (SC) JD-2 laser with a transition wavelength of 632.8 nm at 40 mW (Peking University, Department of Physics, Beijing, China) for 0.5, 1, 2, 4, 8, and 12 min with pulse radiation, respectively, with a treatment diameter of ~12 mm and a beam diameter of 100 μm. Then, the algal samples were kept in the dark for 24 h and incubated in 250 mL Erlenmeyer flasks. The control samples were treated as described above without exposure to the laser irradiation.
Results of preliminary experiments on the heterotrophic cultivation of microalgal laser mutants using glucose as a carbon source showed that glucose was suitable for cell growth and lipid accumulation. The medium containing glucose as a carbon source was sterilized in an autoclave sterilizer at 115 °C for 20 min, with the final pH value adjusted to 7. The algae were kept in the dark at 25 °C in 300 mL Erlenmeyer flasks containing 200 mL of culture medium with 3 mL glucose. Glucose was used as the carbon source at a concentration of 200 g/L in the stock solution and 3 g/L as the work concentration. The autotrophic microalgal cultures were illuminated with cool-white fluorescent light under 36 µmol photons/m2s with a light/dark cycle of 12 h/12 h at 25 °C.
2.3. Determination of Mortality Content and Biomass
The microalgal cell mortality was determined using the fluorescein diacetate (FDA) fluorescence method. FDA was dissolved in acetone to generate a stock solution of 5 mg/mL and stored at 4 °C. The final concentration of FDA was 100 ug/mL in the culture of microalgae treated with laser irradiation in their exponential growth period. The mixture of FDA and algal culture was shaken well and kept in the dark at room temperature for 5 min. Then, 0.1 mL algal liquid culture was loaded into the phytoplankton counting slide and observed at 400× under a professional fluorescent refrigeration microscope (TP905200A, Nanjing Xinfeida OETECH Co., Ltd., Nanjing, China). The living cells were stained as bright green by FDA, while the dead cells were stained as dark red. Microalgal biomass was determined by either the OD680 value or the dry weight of the microalgal culture. The microalgal solution was first shaken well, then 2 mL algal solution was taken and loaded into the quartz cuvette for the spectrophotometer (T6, Beijing Purkinje General Instrument Co., Ltd., Beijing, China) to measure the OD680 value. A total of 50 mL of algal liquid culture was first centrifuged (TG16-W, XiangYi Centrifuge Instrument Co., Ltd., Changsha, China) at 7500 rpm for 5 min, then the precipitate was dried in a drying oven (WGL-125B, Tianjin City TAISITE Instrument Co., Ltd., Tianjin, China) at 80 °C to measure the dry weight of the algae.
2.4. Determination of Total Lipid Content and Lipid Productivity
The measurement of total lipid contents in the dried biomass was conducted as previously reported [26] with minor modifications. Specifically, 50 mg of dry biomass was added to a 5 mL chloroform–methanol (2:1) extraction solvent and placed in an ultrasonic crusher (VC605, SONICS, Newtown, CT, USA) in an ice bath operated with an effective power of 240 W, a cycle of 2 min, work of 15 s, and interval of 5 s. The mixture was centrifuged at 11,100× g for 10 min at 4 °C, and the supernatant was collected and added to chloroform. An equal volume of water was added to the mixture to allow the separation of the organic layers for a few minutes. The clear layer on the top was collected and dried at 61 °C. The total lipid contents were calculated based on the equation of total lipid (mg/g) (dry weight, DW) = C/M, with C representing the weight of the lipid layer (mg) and M representing the biomass (g, DW). The lipid productivity of the microalgal culture was defined as the algae lipid production rate per unit volume within per unit time and was calculated using the equation of lipid productivity (mg/L/d) = C/VD, with C representing the weight of the lipid layer (mg), V representing the volume (L) of the culture medium, and D representing the time of cultivation (day). Each measurement was repeated three times.
2.5. Analysis of High-Value Polyunsaturated Fatty Acids
The fatty acids in the algal samples were extracted and modified into fatty acid methyl esters (FAMEs) following the method as previously described [27] with some minor modifications. Dried algae (20 mg), dried by a vacuum dryer, were used for fatty acid analysis. Lipids presented in dried algal pellets were hydrolyzed and methyl-esterified with 3 mL 0.4 mol/L KOH/methanol solution for 1 h at 60 °C, while 20 µL methyl nonadecanoate (Sigma, Ronkonkoma, NY, USA) was added to the pellets as an internal standard prior to the reactions. A total of 1 mL saturated NaCl and 1 mL of hexane were then added and mixed for 20 s. To separate the phases, anhydrous Na2SO4 was added to the sample, and the mixture was then centrifuged at 16,000× g for 3 min. A total of 1 µL of the hexane layer was injected into a GC-2010 gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a HP-FFAP capillary column (0.25 mm inner diameter × 30 cm in length) and a flame ionization detector. Nitrogen was used as a carrier gas with a constant flow rate of 30 mL/min. Split injections at 1:18 were performed at 300 °C, with the oven temperature initially set to 120 °C for 1 min and then increased to 210 °C at a rate of 6 °C/min, and held for 10 min. Identification of fatty acids was determined by comparing the peaks and retention times of the FAMEs to those of the standards in the Supelco 37 Component FAME Mix (Sigma Aldrich, Shanghai, China). Each measurement was repeated three times.
2.6. Statistical Analysis
Data were statistically analyzed by SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and presented as mean ± standard error (SE). Fisher’s least significant difference (LSD) multiple comparison test was used to evaluate the differences among groups of different trials, with the p-values set at 0.05 and 0.01, respectively.
3. Results
Results showed that the laser irradiation and heterotrophic cultivation enhanced the microalgae growth, accumulation of lipid contents, and lipid productivity in two Chlorella strains (i.e., FACHB 9 and FACHB 31). Specifically, the highest biomasses of 4.81 g/L (15.03-fold) and 4.66 g/L (7.32-fold) were obtained in the third generation of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and 12 min, respectively. The highest lipid contents of 525.6 mg/g (1.67-fold) DW and 780.0 mg/g DW (2.20-fold) were achieved in the third and the first generations of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and by He–Ne for 4 min, respectively. The highest lipid productivities of 69.82 ± 3.29 mg/L/d (19.7-fold) and 30.71 ± 3.77 mg/L/d (3.1-fold) were obtained in FACHB 9 and FACHB 31 treated by SC for 4 min and by He–Ne for 12 min, respectively.
3.1. Screening Laser Mutants of FACHB 9 and FACHB 31
Figure 1 shows the microalgae cell mortality of laser mutants induced by three types of laser irradiation, i.e., neodymium-doped yttrium aluminum garnet (Nd:YAG), helium–neon (He–Ne), and semiconductor (SC), with different lengths of treatment time. A total of seven strains of monoclonal mutants with higher growth rates than that of a wild type were obtained based on Chlorella vulgaris strain FACHB 9 (i.e., 1, 4, and 12 min irradiation of He–Ne, 8 and 12 min of Nd:YAG, and 0.5 and 4 min of SC), and six strains of monoclonal mutants were derived from Chlorella pyrenoidesa strain FACHB 31 (i.e., 4 and 12 min treatment of He–Ne, 2 and 12 min of Nd:YAG, and 0.5 and 8 min of SC).
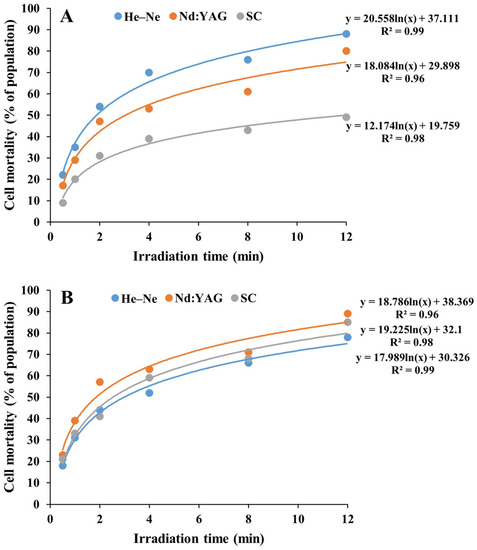
Figure 1.
Cell mortality of laser mutants derived from Chlorella vulgaris strain FACHB 9 (A) and C. pyrenoidesa strain FACHB 31 (B) treated with three types of laser irradiation under different lengths of treatment time.
3.2. Heterotrophic Growth of FACHB 9 and FACHB 31 Laser Mutants
Using glucose as the carbon source for heterotrophic cultivation, a total of seven laser mutants of FACHB 9 (1, 4, and 12 min irradiation of He–Ne, 8 and 12 min of Nd:YAG, and 0.5 and 4 min of SC) and six laser mutants of FACHB 31 (4 and 12 min of He–Ne, 2 and 12 min of Nd:YAG, and 0.5 and 8 min of SC) showed normal growth (i.e., with no decreased growth rate), while three out of the seven FACHB 9 mutants (1 min of He–Ne, 8 min of Nd:YAG, and 0.5 min of SC) and three out of the six FACHB 31 mutants (4 min of He–Ne, 12 min of Nd:YAG, and 0.5 min of SC) cultured for seven days (one generation) in a heterotrophic condition showed significantly higher growth rates than those in an autotrophic condition (Figure 2).
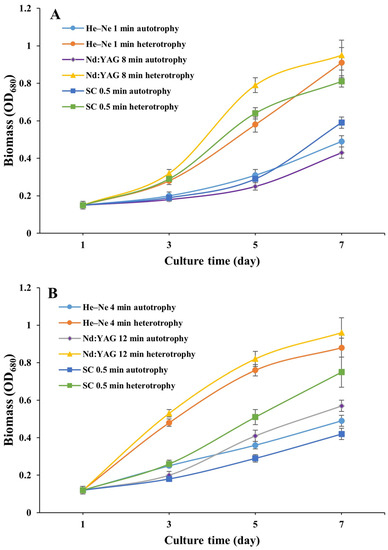
Figure 2.
Growth curves of laser mutants of Chlorella vulgaris strain FACHB 9 (A) and C. pyrenoidesa strain FACHB 31 (B) cultured under autotrophic and heterotrophic conditions for 7 days (one generation).
3.3. Measurement of Biomass of Laser Mutants of FACHB 9 and FACHB 31
Results of biomass showed that laser irradiations significantly promoted cell growth, as demonstrated by the significantly increased growth rates in some of the mutants of strain FACHB 9 cultured under heterotrophic conditions (Figure 3). The highest biomass of 4.81 g/L was obtained in the third generation of the laser mutant irradiated by Nd:YAG for 8 min. In the first generation, biomass increased by ~1 g/L (0.78-fold) compared with the controls and was achieved in mutants irradiated with He–Ne for 4 and 12 min, Nd:YAG for 12 min, and SC for 0.5 min, respectively. In the second generation, a significantly increased biomass of 2.16 g/L (3.41-fold), 1.37 g/L (1.80-fold), and 2.02 g/L (3.12-fold) was obtained in mutants treated with He–Ne for 1, 4, and 12 min respectively, and of 3.48 g/L (6.10-fold) with Nd:YAG for 8 min. In the third generation, a significantly increased biomass of 3.83 g/L (11.77-fold) and 2.79 g/L (8.30-fold) were observed in mutants treated with He–Ne for 1 and 4 min, respectively, of 4.81 g/L (15.03-fold with Nd:YAG for 8 min, and of 4.78 g/L (14.93-fold) and 2.35 g/L (6.83-fold) with SC for 0.5 and 4 min, respectively.
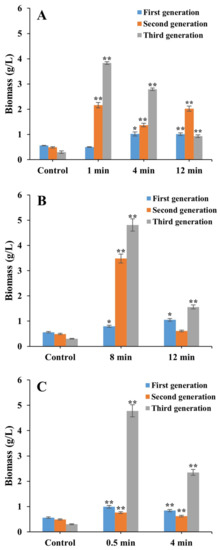
Figure 3.
Biomass of laser mutants of Chlorella vulgaris strain FACHB 9 derived from the treatment of He–Ne (A), Nd:YAG (B), and SC (C) cultured under a heterotrophic condition for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbols “*” and “**” indicate statistically significant differences compared to controls at p < 0.05 and p < 0.01, respectively.
Results of the heterotrophic growth of laser mutants of FACHB 31 showed that the highest biomass of 4.66 g/L was obtained in the third generation of the algae treated with Nd:YAG for 12 min (Figure 4). In the first generation, a significantly increased biomass was observed in the laser mutants treated with He–Ne for 12 min (increasing by 0.85 g/L (1.74-fold)), Nd:YAG for 2 min (increasing by 0.7 g/L (1.26-fold)), and SC for 0.5 and 8 min (increasing by 0.75 g/L (1.42-fold) and 1.0 g/L (2.22-fold), respectively). In the second generation, biomass was significantly increased in laser mutants treated with He–Ne for 12 min (increasing by 1.16 g/L (1.58-fold)), Nd:YAG for 2 min (increasing by 1.37 g/L (2.04-fold)), and SC for 8 min (increasing by 0.93 g/L (1.07-fold), respectively). In the third generation, significantly increased biomass was obtained in laser mutants treated with He–Ne for 4 and 12 min (increasing by 2.89 g/L (4.16-fold) and 2.86 g/L (4.11-fold), respectively), Nd:YAG for 12 min (increasing by 4.66 g/L (7.32-fold)), and SC for 0.5 min (increasing by 2.16 g/L (2.86-fold)).
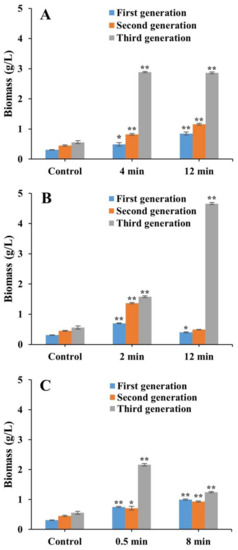
Figure 4.
Biomass of laser mutants of Chlorella pyrenoidesa strain FACHB 31 treated with He–Ne (A), Nd:YAG (B), and SC (C) cultured under a heterotrophic condition for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbols “*” and “**” indicate statistically significant differences compared to controls at p < 0.05 and p < 0.01, respectively.
3.4. Determination of Lipid Content in Laser Mutants of FACHB 9 and FACHB 31
In the third generation, no significant difference of lipid content was observed in laser mutants of either FACHB 9 or FACHB 31 cultivated under autotrophic and heterotrophic conditions (Figure 5).
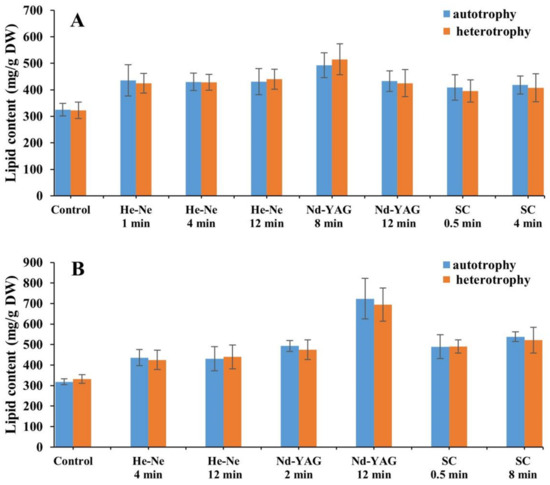
Figure 5.
Lipid contents of laser mutants of Chlorella vulgaris strain FACHB 9 (A) and C. pyrenoidesa strain FACHB 31 (B) cultivated under autotrophic and heterotrophic conditions.
Under heterotrophic cultivation for 21 days (three generations), the highest lipid content of 525.6 mg/g (DW) was obtained in the third generation of the FACHB 9 laser mutant treated with Nd:YAG for 8 min, which was a 0.67-fold increase compared with that of the control (Figure 6). The lipid contents were significantly increased by 0.35-fold and 0.35-fold in the first and third generations of laser mutants treated with He–Ne for 1 min, respectively; by 0.68-fold, 0.23-fold, and 0.36-fold in three generations of laser mutants of He–Ne for 4 min, respectively, and by 0.91-fold, 0.33-fold, and 0.37-fold for 12 min, respectively; by 1.22-fold, 0.48-fold, and 0.37-fold in three generations of laser mutants of Nd:YAG for 12 min, respectively; by 1.29-fold and 0.45-fold in the first and second generations of laser mutants of Nd:YAG for 8 min, respectively; by 0.76-fold and 0.33-fold in the first and second generations of laser mutants of SC for 0.5 min, respectively; and by 0.35-fold, 0.29-fold, and 0.30-fold in three generations of laser mutants of SC for 4 min, respectively.
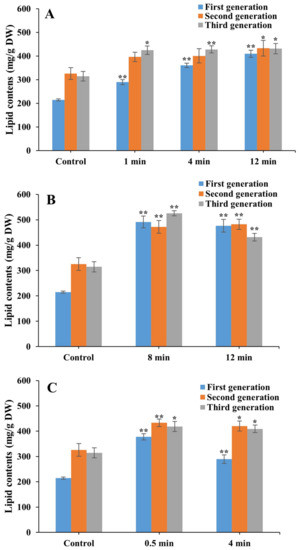
Figure 6.
Lipid contents of laser mutants of Chlorella vulgaris strain FACHB 9 treated with He–Ne (A), Nd:YAG (B), and SC (C) cultured under heterotrophic cultivation for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbols “*” and “**” indicate statistically significant differences compared to controls at p < 0.05 and p < 0.01, respectively.
Cultivated under heterotrophic conditions for 21 days (three generations), the highest lipid content of 780.0 mg/g DW was obtained in the first generation of the FACHB 31 laser mutant treated with He–Ne for 4 min, which was a 1.20-fold increase compared with that of the control (Figure 7). Significantly increased lipid contents were revealed in the second and third generations of the laser mutants treated with He–Ne for 4 min (increasing by 1.12-fold and 0.36-fold, respectively), in three generations of laser mutants treated with He–Ne for 12 min (increasing by 0.66-fold, 0.31-fold, and 0.29-fold, respectively), in the second and third generations of the laser mutants of Nd:YAG for 2 min (increasing by 0.77-fold and 0.51-fold, respectively), in the first and third generations of laser mutants of Nd:YAG for 12 min (increasing by 0.69-fold and 1.37-fold, respectively), and in three generations of laser mutants of SC for 0.5 min (increasing by 0.47-fold, 0.47-fold, and 0.36-fold, respectively) and 8 min (increasing by 1.27-fold, 0.01-fold, and 0.16-fold, respectively).
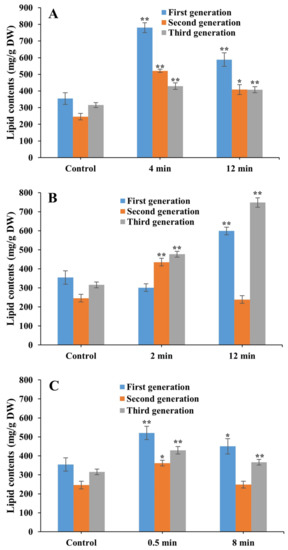
Figure 7.
Lipid contents of laser mutants of Chlorella pyrenoidesa strain FACHB 31 treated with He–Ne (A), Nd:YAG (B), and SC (C) cultured under heterotrophic cultivation for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbols “*” and “**” indicate statistically significant differences compared to controls at p < 0.05 and p < 0.01, respectively.
3.5. Determination of Lipid Productivity in Laser Mutants of FACHB 9 and FACHB 31
The maximum lipid productivities were increased in the third generation of the laser mutants of FACHB 9 treated with He–Ne for 1 min (increasing by 16.3-fold) and with SC for 0.5 and 4 min (increasing by 18.2-fold and 19.7-fold, respectively), compared with those of the controls (Figure 8). The lipid productivities were significantly increased in the second generation of the laser mutants treated with He–Ne for 1 min (increasing by 4.3-fold), in three generations of laser mutants of He–Ne for 4 min (increasing by 2.0-fold, 2.4-fold, and 11.6-fold, respectively) and 12 min (increasing by 2.0-fold, 1.6-fold, and 9.7-fold, respectively) and those of Nd:YAG for 12 min (increasing by 3.0-fold, 5.1-fold, and 3.2-fold, respectively), in the first and third generations of laser mutants treated with Nd:YAG for 8 min (increasing by 3.1-fold and 7.5-fold, respectively), and in the first and second generations of laser mutants of SC for 0.5 min (increasing by 1.5-fold and 8.5-fold, respectively) and 4 min (increasing by 1.4-fold and 1.0-fold, respectively).
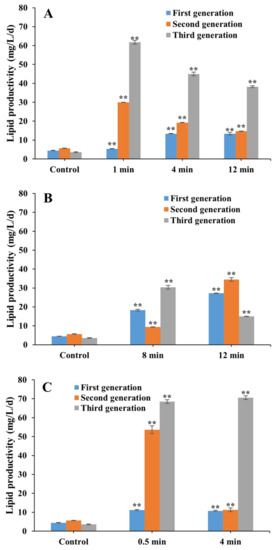
Figure 8.
Lipid productivity of laser mutants of Chlorella vulgaris strain FACHB 9 treated with He–Ne (A), Nd:YAG (B), and SC (C) cultured under heterotrophic cultivation for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbol “**” indicates statistically significant differences compared to controls at p < 0.01.
The maximum lipid productivity was increased by 3.1-fold in the first generation of the laser mutant of FACHB 31 treated with He–Ne for 12 min (Figure 9). Significantly increased lipid productivities were obtained in the second and third generations of the laser mutants treated with He–Ne for 4 min (increasing by 2.8-fold and 1.1-fold, respectively) and 12 min (increasing by 2.8-fold and 1.0-fold, respectively), in the second and third generations of laser mutants of Nd:YAG for 2 min (increasing by 2.9-fold and 1.3-fold, respectively), and in three generations of laser mutants of Nd:YAG for 12 min (increasing by 0.9-fold, 1.0-fold, and 0.8-fold, respectively) and SC for 0.5 min (increasing by 0.8-fold, 1.1-fold, and 1.1-fold, respectively) and 8 min (increasing by 1.1-fold, 1.1-fold, and 1.9-fold, respectively).
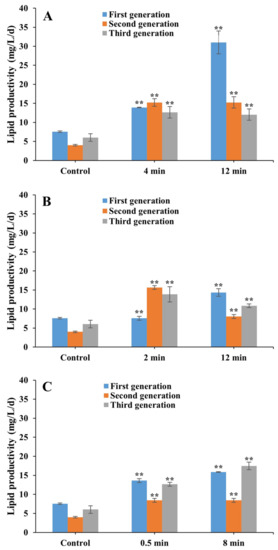
Figure 9.
Lipid productivity of laser mutants of Chlorella pyrenoidesa strain FACHB 31 treated with He–Ne (A), Nd:YAG (B), and SC (C) cultured under heterotrophic cultivation for 21 days (three generations). Data are presented as mean ± standard errors (n = 3). Symbol “**” indicates statistically significant differences compared to controls at p < 0.01.
3.6. Fatty Acids of FACHB 9 and FACHB 31 Mutants
Fatty acids are categorized into saturated fatty acids (SFAs) and unsaturated fatty acids, while the latter is further classified into monounsaturated fatty acids and polyunsaturated fatty acids (PUFAs) based on the number of double bonds, with PUFAs referring to straight chain fatty acids containing two or more double bonds and 18–22 carbon atoms [28]. Our results showed that two types of SFAs, i.e., butyric acid (C4:0) and caproic acid (C6:0), were detected in both the control and all seven laser mutants of strain FACHB 9, while one type of SFA, i.e., eicosanoic acid (C20:0), was detected only in the control and one type of laser mutant treated with Nd:YAG for 12 min (Table 2). Furthermore, a total of three types of SFAs, i.e., octanoic acid (C8:0), decanoic acid (C10:0), heptadecanoic acid (C17:0), and 4 types of PUFAs, i.e., linoleic acid (C18:2), eicosanoic acid (C20:2), conjugated linoleic acid (CLA), and γ-linolenic acid (γ-LNA), were not detected in the control, but in at least one laser mutant, and two types of fatty acids (i.e., C8:0 and C20:2) were revealed in all 7 types of laser mutants. Moreover, LNA was only detected in control but in none of the laser mutants (Table 2).

Table 2.
Effect of laser mutagenesis and heterotrophic cultivation on lipid components of Chlorella vulgaris strain FACHB 9. C4:0, butyric acid; C6:0, caproic acid; C8:0, octanoic acid; C10:0, undecanoic acid; C17:0, heptadecanoic acid; C18:2, linoleic acid; C20:0, eicosanoic acid; C20:2, eicosadienoic acid; LNA, linolenic acid; CLA, conjugated linoleic acid; γ-LNA, γ-linolenic acid.
Among the six types of fatty acids detected in strain FACHB 31, three types of SFAs, i.e., butyric acid (C4:0), caproic acid (C6:0), and heptadecanoic acid (C17:0), were detected in both the control and all seven types of laser mutants (Table 3). Furthermore, two types of fatty acids, i.e., linolenic acid (LNA) and octanoic acid (C8:0), were revealed in all seven types of the laser mutants but not in the control, while undecanoic acid (C11:0) was detected in only three types of laser mutants treated with Nd:YAG for 2 and 12 min and with SC for 0.5 min, respectively (Table 3).

Table 3.
Effect of laser mutagenesis and heterotrophic cultivation on lipid components Chlorella pyrenoidesa strain FACHB 31. C4:0, butyric acid; C6:0, caproic acid; C8:0, decanoic acid; C11:0, undecanoic acid; C17:0, heptadecanoic acid; LNA, linolenic acid.
4. Discussion
4.1. Effects of Laser Mutagenesis on Biomass and Lipid Contents in FACHB 9 and FACHB 31
Our results showed that the laser irradiation generated microalgae mutants with enhanced microalgae growth and accumulation of lipid contents in two Chlorella strains (i.e., FACHB 9 and FACHB 31). Specifically, the highest biomasses of 4.81 g/L (15.03-fold) and 4.66 g/L (7.32-fold) were obtained in the third generation of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and 12 min, respectively. The highest lipid contents of 525.6 mg/g DW (1.67-fold) and 780.0 mg/g DW (2.20-fold) were achieved in the third and the first generations of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and by He–Ne for 4 min, respectively.
As a novel breeding technique to screen beneficial phenotypes, laser mutagenesis shows promising prospects. The mechanisms of the biological effects of laser irradiation have been investigated with its wide applications reviewed, e.g., in accelerating seed germination, mutation breeding, and genetic engineering of forest trees and horticultural plants [12]. Studies have shown that the extended treatment of laser irradiation from 10 to 20 min causes not only the increased mortality from 60% to 78.7% in Chlorella, but also the increase of pathogenic single nucleotide polymorphisms (SNPs) [13]. Therefore, in our study, in order to explore the effect of laser irradiation on the growth and lipid accumulation in cells of Chlorella strains FACHB 9 and FACHB 31, the length of continuous irradiation treatment was set as 0.5, 1, 2, 4, 8, and 12 min, respectively.
Our results showed that several laser mutants were observed to accumulate significantly increased lipid contents compared with the control. For example, in FACHB 9, the highest lipid contents of 525.6 mg/g DW was obtained in the third generation of the laser mutants treated with Nd:YAG for 8 mins, while the lowest lipid contents of 290.0 mg/g DW was obtained in the first generation of the laser mutants treated with He–Ne for 1 min and SC for 4 min, respectively. In FACHB 31, the highest lipid contents of 780.0 mg/g DW was revealed in the first generation of laser mutants induced by laser irradiation of He–Ne for 4 min, while the lowest lipid contents of 247.3 mg/g DW was revealed in the second generation of laser mutants induced by laser irradiation of SC for 8 min. More importantly, although all the laser mutants were not stable in maintaining the derived phenotypes, the lipid contents in all the laser mutants screened in our study were significantly higher than those of the wild type (i.e., the controls). Furthermore, our results showed that laser mutagenesis significantly increased lipid accumulation under heterotrophic cultivation with much higher lipid contents (Figure 6 and Figure 7) than those previously reported in Chlorella vulgaris (e.g., 68.03 mg/g DW) [29], likely due to the laser mutations in our study.
It has been reported that some wild microalgae growing fast accumulated low lipid contents, while conversely, other microalgae accumulating high lipid contents showed a slowed growth rate [13], conflicting with the general expectation of having fast growing microalgae with high accumulation of lipid content. Notably, our results demonstrated that the third generation of the laser mutant of FACHB 9 treated with Nd:YAG for 8 min showed an increased growth rate with high lipid contents, likely due to the improvement of its growing mechanisms in the microalgae mutations caused by laser mutagenesis. Studies have shown that under certain energy levels, lasers can break the C–N bond to change the molecular structure, ultimately altering the metabolic process in vivo and resulting in the changes of several physiological parameters, including biomass, lipid contents, lipid productivity, and contents of PUFAs [30,31,32]. Therefore, it is speculated that the altered biomass, lipid contents, lipid productivity, and contents of PUFAs in the laser mutant strains of FACHB 9 and FACHB 31 treated with Nd:YAG, He–Ne, and SC irradiations might be due to the break of the C–N bond, accounting for a large proportion of the molecular structures in the DNA molecules [30,31,32].
Varied amounts of lipid contents have been reported in laser mutants of Chlorella sp., such as 680 mg/g DW [33], which is slightly lower than that of our study (780.0 mg/g DW). Similarly, an increased biomass of ~144% less than the increased biomass of 2.20-fold in our study was detected in the laser mutants of Chlorella sp. [34], while the laser mutant of Chlorella sp. achieved a higher biomass of 14.59 g/L than that of 4.81 g/L detected in our study [33], suggesting that laser mutagenesis randomly generates mutations with varied capabilities of accumulating lipids and biomass [13].
Our results have evidently shown that laser irradiation has the promising potential to generate microalgae mutants with enhanced biomass and accumulation of lipid contents. In particular, these technological innovations are expected to contribute significantly to the generation of microalgal biodiesel in the increasingly demanding market for renewable bioenergy worldwide [6]. Furthermore, with increased biomass and accumulation of lipid contents, the laser mutants of microalgae may develop into a type of promising feedstock for bioenergy production, ultimately replacing fossil energy [3].
4.2. Effects of Heterotrophic Cultivation on Biomass and Lipid Contents of FACHB 9 and FACHB 31
Our results showed that the heterotrophic cultivation (i.e., glucose) enhanced the microalgae growth and accumulation of lipid contents in two Chlorella strains (i.e., FACHB 9 and FACHB 31). Specifically, the highest biomass of 4.81 g/L (15.03-fold) and 4.66 g/L (7.32-fold) was obtained in the third generation of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and 12 min, respectively. The highest lipid contents of 525.6 mg/g DW (1.67-fold) and 780.0 mg/g DW (2.20-fold) were achieved in the third and the first generations of FACHB 9 and FACHB 31 induced by Nd:YAG for 8 min and by He–Ne for 4 min, respectively.
Heterotrophic cultivation has been considered as a potential method to reduce the production cost of economically important high-value products generated by microalgae [35]. Studies have shown that heterotrophic conditions play a critical role in enhancing biomass and lipid production [36], while glucose is the most common type of carbon and energy source for maintaining heterotrophic growth. It has been reported that Nannochloropsis sp. might utilize the carbon sources for their mixotrophic and heterotrophic growth, while glucose is the most commonly used carbon source in microalgal cultivation for lipid production [37]. In our study, we further investigated the biomass, lipid contents, lipid productivity, and the contents of PUFAs in the laser mutants of strains FACHB 9 and FACHB 31, cultured under heterotrophic conditions using glucose as the carbon source.
Our results showed that the cell growth in some laser mutants was significantly increased compared with that of the control under the heterotrophic condition (3 g/L glucose). In FACHB 9, the highest and lowest biomasses of 4.81 g/L (15.03-fold in comparison to that of the control) and 0.5 g/L, respectively were obtained in the third and the first generations of the laser mutants treated with Nd:YAG for 8 min and with He–Ne for 1 min, respectively. In FACHB 31, the maximum (4.66 g/L) and minimum (0.4 g/L) biomasses were detected in the third and the first generations of laser mutants treated with Nd:YAG for 12 min, respectively. Similarly, it was reported that the biomass was significantly increased, ranging from 4.9 to 31.2 g/L, in Chlorella protothecoides cultured with a much higher concentration of glucose (10–80 g/L) than that in our study, suggesting that these two strains of Chlorella may respond with similar mechanisms to the heterotrophic condition [38]. Furthermore, studies have shown that increased concentrations of glucose in the culture medium caused a reduction in the cell yield (0.39–0.49 g/g), indicating that a low concentration of glucose promoted cell growth, while a high concentration of glucose was not conducive to cell growth [39]. Similar results were revealed in our study, showing the same growth patterns in Chlorella heterotrophically cultured with a low concentration of glucose, with significantly increased cell growth compared with autotrophic cultivation (Figure 2). A much higher biomass of 15.5 g/L was obtained in Chlorella protothecoides heterotrophically cultured [40] than that of the highest biomass in our study (4.81 g/L), suggesting that Chlorella protothecoides may be a more suitable microalgal species for heterotrophic cultivation. These results provide additional experimental evidence to support the application of glucose with a low concentration in heterotrophic cultivation of microalgae.
Studies have shown that the microalgae Chlorella protothecoides and Crypthecodium cohnii are capable of directly uptaking and transforming carbohydrates (e.g., glucose) into lipids [41]. Furthermore, studies have shown that higher lipid productivity (37.1 ± 0.49 mg/L/d) was yielded in Nannochloropsis salina heterotrophically cultivated with various carbon and nitrogen sources than that of the photoautotrophic cultivation (22.16 ± 0.27 mg/L/d) [35]. Moreover, it has been reported that the lipid contents of Chlorella zofingiensis heterotrophically cultured with 30 g/L glucose was 900% higher than that of autotrophic cultivation, likely due to the participation of additional carbon sources in the biosynthetic pathway of lipids to generate lipids for storage, instead of converting carbon sources into the lipid membranes for organelle constructions [42]. These results are inconsistent with the results obtained in our study, showing no significant differences observed in lipid contents of laser mutants of FACHB 9 and FACHB 31 between autotrophic and heterotrophic cultivations (Figure 5), probably due to the laser mutations of related genes or regulatory elements in the selected laser mutants causing the alteration of gene expression involved in fatty acid synthesis or blocking the degradation pathway of fatty acids.
4.3. Effects of Laser Mutagenesis and Heterotrophic Cultivation on the Accumulation of Fatty Acids in FACHB 9 and FACHB 31
Our study showed that four types of PUFAs (i.e., linoleic acid, γ-linolenic acid, eicosadienoic acid, and conjugated linoleic acid) were accumulated in laser mutants of FACHB 9, while several types of SFAs (i.e., octanoic acid, decanoic acid, undecanoic acid, heptadecanoic acid) were accumulated in laser mutants of FACHB 9 or FACHB 31 with varied proportions in the total fatty acids. Studies have shown that a higher percentage of SFA content than PUFA content was observed in Nannochloropsis salina under the treatment of a mixture of various types of nitrogen sources, such as ammonium chloride, ammonium hydroxide, sodium nitrate, and urea [43]. In our study, results showed that laser mutagenesis altered the proportions of SFAs and PUFAs in laser mutants of FACHB 9 under heterotrophic conditions. The increased contents of linoleic acid (13.02–16.89%), eicosadienoic acid (3.11–6.78%), conjugated linoleic acid (4.16–8.82%), and γ-linolenic acid (6.23–11.78%) were obtained in laser mutants of FACHB 9 under heterotrophic cultivation, which were higher than those reported in N. salina cultivated under heterotrophic conditions [36]. Furthermore, six laser mutants of FACHB 31 (i.e., treated with He–Ne for 4 and 12 min, Nd:YAG for 2 and 12 min, and SC for 0.5 and 8 min) were revealed to accumulate linoleic acid with relatively low contents (1.45–2.89%), which was not detected in the wild type. These results indicate that different species and strains of microalgae may possess different potentials for high-value product accumulation under the treatment of laser irradiation.
Notably, in FACHB 9, four types of PUFAs, including linoleic acid (C18:2), γ-linolenic acid (γ-LNA), eicosadienoic acid (C20:2), and conjugated linoleic acid (CLA), and three types of SFAs, including octanoic acid (C8:0), decanoic acid (C10:0), and heptadecanoic acid (C17:0), were detected in laser mutants but not in the control. Similarly, two types of SFAs, i.e., octanoic acid (C8:0) and undecanoic acid (C11:0), and one type of PUFA, i.e., undecanoic acid (C11:0), were detected in laser mutants but not in the control of strain FACHB 31, probably due to the gene mutations caused by the laser irradiation, resulting in the alteration in the substrate preference of related enzymes in the biosynthesis of these fatty acids, ultimately catalyzing the substrate to produce these fatty acids that the wild types lack [44,45,46]. It has been commonly observed that fatty acids are detected in laser mutants but not in the wild types of microalgae. For example, studies have shown that the laser mutants of Chlorella vulgaris were revealed to accumulate high levels of linoleic acid (C18:2) [33], which is generally considered as an effective candidate for biodiesel [47]. These studies suggest that these microalgal laser strains contain promising potentials for the development of alternatives of biodiesel. Furthermore, it has been reported that the contents of eicosanoic acid (C20:0) was increased from 11.9% in the wild type of Chlorella to 19.7% under the treatment of UV mutagenesis [48]. Notably, our results showed higher contents of C20:0 (19.12%) in the wild type of Chlorella strain FACHB 9, which was slightly increased (19.45%) and detected in only one laser mutant (i.e., treated with Nd:YAG for 12 min), suggesting that Chlorella vulgaris in our study is an appropriate species for producing C20:0.
5. Conclusions
In this study, our results showed that laser mutagenesis was efficient to screen two strains of Chlorella to obtain mutants with enhanced accumulation of biomass and lipid contents. In C. vulgaris strain FACHB 9, the highest biomass of 4.81 g/L, the highest lipid contents of 525.6 mg/g DW, and the maximum lipid productivity of an increase by 20.7-fold were obtained in the third generation of the laser mutants treated with SC for 0.5 min, with Nd:YAG for 8 min, and with SC for 4 min, respectively. For C. pyrenoidesa strain FACHB 31, the highest biomass of 4.66 g/L was obtained in the third generation of the laser mutant derived by Nd:YAG for 12 min, while the highest lipid contents of 780.2 mg/g DW and the maximum lipid productivity of an increase by 4.1-fold were revealed in the first generation of the laser mutants treated with He–Ne for 4 min and 12 min, respectively. Furthermore, four types of PUFAs, including linoleic acid (C18:2), γ-linolenic acid (γ-LNA), eicosadienoic acid (C20:2), and conjugated linoleic acid (CLA), and three types of SFAs, including octanoic acid (C8:0), decanoic acid (C10:0), and heptadecanoic acid (C17:0), were detected in laser mutants but not in the control of strain FACHB 9, while two types of SFAs, i.e., octanoic acid (C8:0) and undecanoic acid (C11:0), and one type of PUFA, i.e., undecanoic acid (C11:0), were detected in laser mutants but not in the control of strain FACHB 31. Our study has strongly demonstrates that laser mutagenesis has shown a significant potential for economic microalgae breeding and screening for beneficial microalgal strains. In particular, our results have clearly shown that laser irradiation can generate microalgae mutants with enhanced biomass and accumulation of lipid contents. This study represents the technological innovations which are expected to make significant contributions to the generation of microalgal biodiesel in the increasingly demanding international market for renewable bioenergy. We foresee that the laser mutants of microalgae could be established as a type of renewable materials for bioenergy production, ultimately showing the promise to become an alternative to fossil energy.
Author Contributions
Conceptualization, C.M., Z.G.; methodology, W.X., R.Z., C.M., F.S., Z.G.; formal analysis, W.X., R.Z., Q.S., X.W., Z.W., F.S., C.W., K.C., B.Z.; resources, C.M., Z.G.; data curation, W.X., R.Z., C.M., F.S., Z.G.; writing—original draft preparation, W.X., C.M., Z.G.; writing—review and editing, W.X., R.Z., Q.S., C.M., X.W., Z.W., F.S., C.W., K.C., B.Z., Z.G.; project administration, C.M., F.S., Z.G.; funding acquisition, Z.G., C.M. All the authors commented on and approved the text. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31972815), the Major Basic Research Program of Shandong Province (ZR2019ZD17 and ZR2020ZD23), the Key Research and Development Program of Shandong Province (Food for Special Medical Purpose) (2018YYSP016), and the Open Fund of Shandong Provincial Key Laboratory of Plant Stress (SPKLPS202001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bajpai, P. Current Trends and the Future of the Algae-Based biofuels industry. In Third Generation Biofuels; SpringerBriefs in Energy; Springer: Singapore, 2019; pp. 67–70. [Google Scholar]
- Collotta, M.; Champagne, P.; Mabee, W.; Tomasoni, G.; Alberti, M. Life cycle analysis of the production of biodiesel from microalgae. In Life Cycle Assessment of Energy Systems and Sustainable Energy Technologies: Green Energy and Technology; Basosi, R., Cellura, M., Longo, S., Parisi, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 155–169. [Google Scholar]
- Rosenberg, J.N.; Oyler, G.A.; Wilkinson, L.; Betenbaugh, M.J. A green light for engineered algae: Redirecting metabolism to fuel a biotechnology revolution. Curr. Opin. Biotechnol. 2008, 19, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Ji, L.; Shi, Q.W.; Wu, H.Z.; Fan, J.H. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.X.; Liu, P.; Xia, J.L.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.Y.; Qiu, G.Z. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Queiroz Zepka, L.; Queiroz, M. Energy from Microalgae. In Green Energy and Technology; Biofuels from Microalgae: Biodiesel; Reijnders, L., Ed.; Springer: Cham, Switzerland, 2018; pp. 171–180. [Google Scholar]
- Hsieh, H.J.; Su, C.H.; Chien, L.J. Accumulation of lipid production in Chlorella minutissima by triacylglycerol biosynthesis-related genes cloned from Saccharomyces cerevisiae and Yarrowia lipolytica. J. Microbiol. 2012, 50, 526–534. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tohidfar, M.; Tabatabaei, M.; Bagheri, A.; Mohsenpor, M.; Mohtashami, S.K. Genetic manipulation, a feasible tool to enhance unique characteristic of Chlorella vulgaris as a feedstock for biodiesel production. Mol. Biol. Rep. 2013, 40, 4421–4428. [Google Scholar] [CrossRef]
- Du, H.M.; Ahmed, F.; Lin, B.; Li, Z.; Huang, Y.H.; Sun, G.; Ding, H.; Wang, C.; Meng, C.X.; Gao, Z.Q. The effects of plant growth regulators on cell growth, protein, carotenoid, PUFAs and lipid production of C. pyrenoidosa ZF strain. Energies 2017, 10, 1696. [Google Scholar] [CrossRef]
- Lin, B.; Ahmed, F.; Du, H.M.; Li, Z.M.; Yan, Y.C.; Huang, Y.H.; Cui, M.; Yin, Y.H.; Li, B.; Wang, M.M.; et al. Plant growth regulators promote lipid and carotenoid accumulation in Chlorella vulgaris. J. Appl. Phycol. 2018, 30, 1549–1561. [Google Scholar] [CrossRef]
- Ma, F.X.; Chen, X.Y. Laser applications in seed germination, mutation breeding and gene engineering for forestry and horticulture. Physics 2007, 36, 637–642. [Google Scholar]
- Liu, S.; Zhao, Y.; Liu, L.; Ao, X.; Ma, L.; Wu, M.; Ma, F. Improving Cell Growth and Lipid Accumulation in Green Microalgae Chlorella sp. via UV Irradiation. Appl. Biochem. Biotechnol. 2015, 175, 3507–3518. [Google Scholar] [CrossRef]
- Ouf, S.A.; Alsarrani, A.Q.; Al-Adly, A.A.; Ibrahim, M.K. Evaluation of low-intensity laser radiation on stimulating the cholesterol degrading activity: Part, I. Microorganisms isolated from cholesterol-rich materials. Saudi J. Biol. Sci. 2012, 19, 185–193. [Google Scholar] [CrossRef]
- Smith, V.H.; Sturm, B.S.M.; Denoyelles, F.G.; Billings, S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2010, 25, 301–309. [Google Scholar] [CrossRef]
- Zhang, H.N.; Gao, Z.Q.; Li, Z.; Du, H.M.; Lin, B.; Cui, M.; Yin, Y.H.; Lei, F.M.; Yu, C.Y.; Meng, C.X. Laser Radiation Induces Growth and Lipid Accumulation in the Seawater Microalga Chlorella pacifica. Energies 2017, 10, 1671. [Google Scholar] [CrossRef]
- Kishi, M.; Kawai, M.; Toda, T. Heterotrophic utilization of ethylene glycol and propylene glycol by Chlorella protothecoides. Algal Res. 2015, 11, 428–434. [Google Scholar] [CrossRef]
- Wu, G.X.; Gao, Z.Q.; Du, H.M.; Lin, B.; Yan, Y.C.; Li, G.Q.; Guo, Y.Y.; Fu, S.G.; Wei, G.X.; Wang, M.M.; et al. The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. J. Gen. Appl. Microb. 2018, 64, 42–49. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; De-Bashan, E.L.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Xie, T.H.; Xia, Y.; Zeng, Y.; Li, X.R.; Zhang, Y.K. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Sim, S.J.; Cheng, D.D. Recent advancements in mixotrophic bioprocessing for production of high value microalgal products. Bioresour. Technol. 2021, 320 Pt B, 124421. [Google Scholar] [CrossRef]
- Chi, Z.Y.; Zheng, Y.B.; Ma, J.W.; Chen, S.L. Oleaginous yeast Cryptococcus curvatus culture with dark fermentation hydrogen production effluent as feedstock for microbial lipid production. Int. J. Hydrogen Energy 2011, 36, 9542–9550. [Google Scholar] [CrossRef]
- Azma, M.; Mohamed, M.S.; Mohamad, R.; Rahim, R.A.; Arif, A.B. Improvement of medium composition for heterotrophic cultivation of green microalgae Tetraselmis suecica, using response surface methodology. Biochem. Eng. J. 2011, 53, 187–195. [Google Scholar] [CrossRef]
- Samek, O.; Zemanek, P.; Jona, A.; Telle, H.H. Characterization of oil-producing microalgae using Raman spectroscopy. Laser Phys. Lett. 2011, 8, 701–709. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canad. J. Physiol. Pharm. 1959, 37, 911–917. [Google Scholar]
- Cha, T.S.; Chen, J.W.; Goh, E.G.; Aziz, A.; Loh, S.H. Differential regulation of fatty acid biosynthesis in two Chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour. Technol. 2011, 102, 10633–10640. [Google Scholar] [CrossRef]
- White, B. Dietary fatty acids. Am. Fam. Phys. 2009, 80, 345–350. [Google Scholar]
- Kim, S.H.; Lim, S.R.; Hong, S.J.; Cho, B.K.; Lee, H.; Lee, C.G.; Choi, H.K. Effect of ethephon as an ethylene-releasing compound on the metabolic profile of Chlorella vulgaris. J. Agric. Food Chem. 2016, 64, 4807–4816. [Google Scholar] [CrossRef]
- Bilian, C.; Huiru, Z.; Mingzi, W.; Rong, C. Mutagenesis of Spirulina platensis by Nd:YAG laser. Acta Laser Biol. Sin. 2000, 9, 125–128. [Google Scholar]
- Zhao, M.M.; Wang, W.W. Studies on the mutagenesis of Spirulina platensis using He-Ne laser. Acta Photonica Sin. 2005, 34, 400. [Google Scholar]
- Bilian, C.; Mingzi, W.; Huiru, Z.; Rong, C. Effects of LD laser on the growth and morphology of Spirulina platensis. Acta Photonica Sin. 2000, 29, 411. [Google Scholar]
- Muthuraj, M.; Selvaraj, B.; Palabhanvi, B.; Kumar, V.; Das, D. Enhanced lipid content in chlorella sp. FC2 IITG via high energy irradiation mutagenesis. Korean J. Chem. Eng. 2018, 36, 63–70. [Google Scholar] [CrossRef]
- Rahman, D.Y.; Rachmayati, R.; Widyaningrum, D.N.; Susilaningsih, D. Enhancement of lipid production of chlorella sp. 042 by mutagenesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 439, 012021. [Google Scholar] [CrossRef]
- Eriksen, N.T. The technology of microalgal culturing. Biotechnol. Lett. 2008, 30, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandi, T. Heterotrophic cultivation of Nannochloropsis salina for enhancing biomass and lipid production. Biotechnol. Rep. 2016, 10, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wei, C.; Zhao, L.C.; Fan, O.Y. Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 2004, 16, 499–503. [Google Scholar] [CrossRef]
- Shi, X.M.; Liu, H.J.; Zhang, X.W.; Chen, F. Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Proc. Biochem. 1999, 34, 341–347. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.F.; Xiang, J.Y.; Wu, Q.Y. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Swaaf, M.E.D.; Pronk, J.T.; Sijtsma, L. Fed-batch cultivation of the docosahexaenoic- acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl. Microbiol. Biotechnol. 2003, 61, 40–43. [Google Scholar] [CrossRef]
- Jin, L.; Huang, J.; Zheng, S.; Zhong, Y.; Yue, J.; Feng, C. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar]
- Campos, H.; Boeing, W.J.; Dungan, B.N.; Schaup, T. Cultivating the marine microalga Nannochloropsis salina under various nitrogen sources: Effect on biovolume yields, lipid content and composition, and invasive organism. Biomass Bioenergy 2014, 66, 301–307. [Google Scholar] [CrossRef]
- Takatsuka, Y.; Yamaguchi, Y.; Ono, M.; Kamio, Y. Gene cloning and molecular characterization of lysine decarboxylase from selenomonas ruminantium delineate its evolutionary relationship to ornithine decarboxylases from eukaryotes. J. Bacteriol. 2000, 182, 6732–6741. [Google Scholar] [CrossRef]
- Mahan, S.D.; Ireton, G.C.; Stoddard, B.L.; Black, M.E. Alanine-scanning mutagenesis reveals a cytosine deaminase mutant with altered substrate preference. Biochemistry 2004, 43, 8957–8964. [Google Scholar] [CrossRef]
- Huang, R.; Hippauf, F.; Rohrbeck, D.; Haustein, M.; Wenke, K.; Feike, J.; Barkman, T.J. Enzyme functional evolution through improved catalysis of ancestrally nonpreferred substrates. Proc. Natl. Acad. Sci. USA 2012, 109, 2966–2971. [Google Scholar] [CrossRef]
- Muthuraj, M.; Kumar, V.; Palabhanvi, B.; Das, D. Evaluation of indigenous microalgal isolate Chlorella sp. FC2 IITG as a cell factory for biodiesel production and scale up in outdoor conditions. J. Ind. Microbiol. Biotechnol. 2014, 41, 499–511. [Google Scholar] [CrossRef]
- Anthony, J.; Rangamaran, V.R.; Gopal, D.; Shivasankarasubbiah, K.T.; Thilagam, M.; Dhassiah, M.P. Ultraviolet and 5′ fluorodeoxyuridine induced random mutagenesis in chlorella vulgaris and its impact on fatty acid profile: A new insight on lipid-metabolizing genes and structural characterization of related proteins. Mar. Biotechnol. 2015, 17, 66–80. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).