Relationship between Colonization by Onion Thrips (Thrips tabaci Lind.) and Leaf Colour Measures across Eight Onion Cultivars (Allium cepa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setup
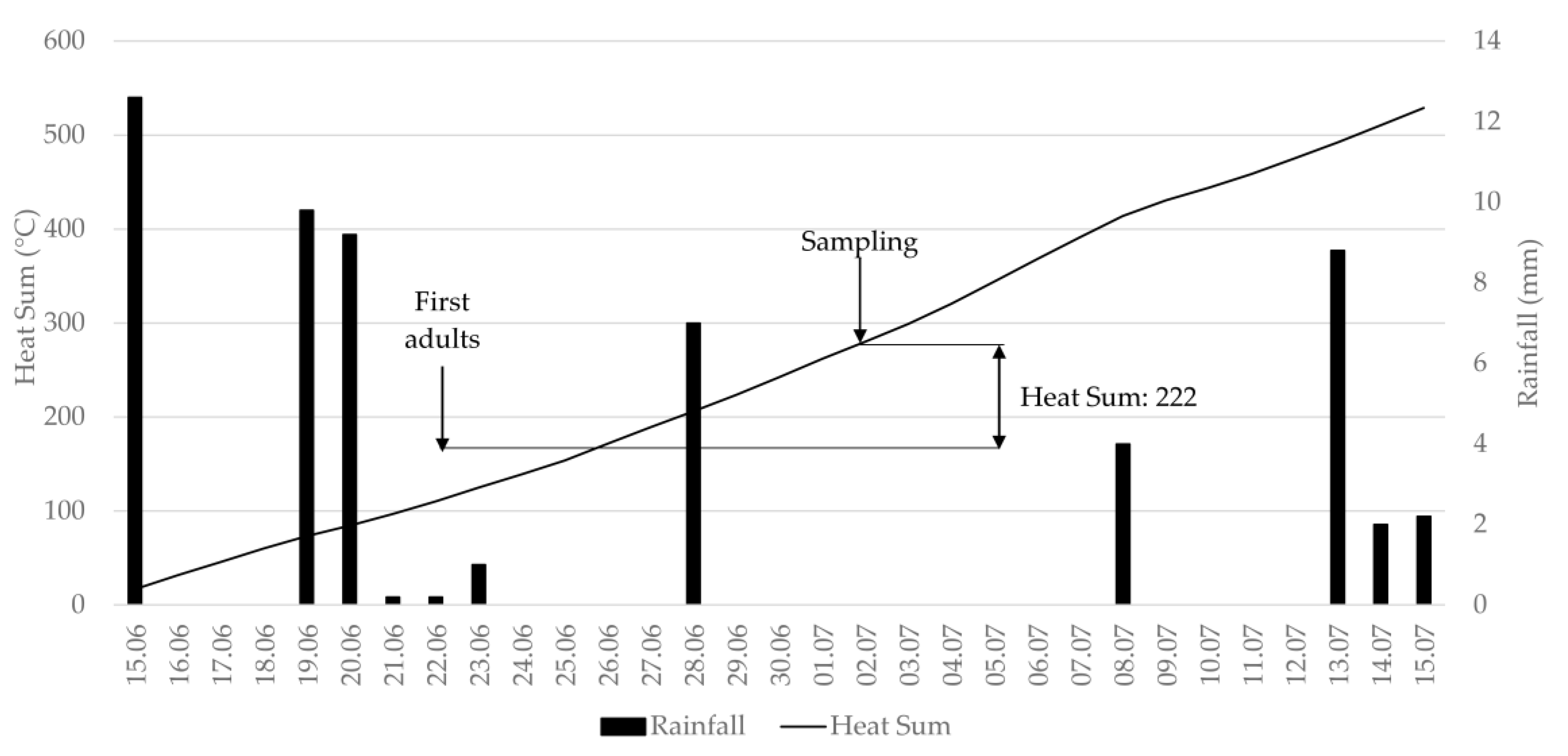
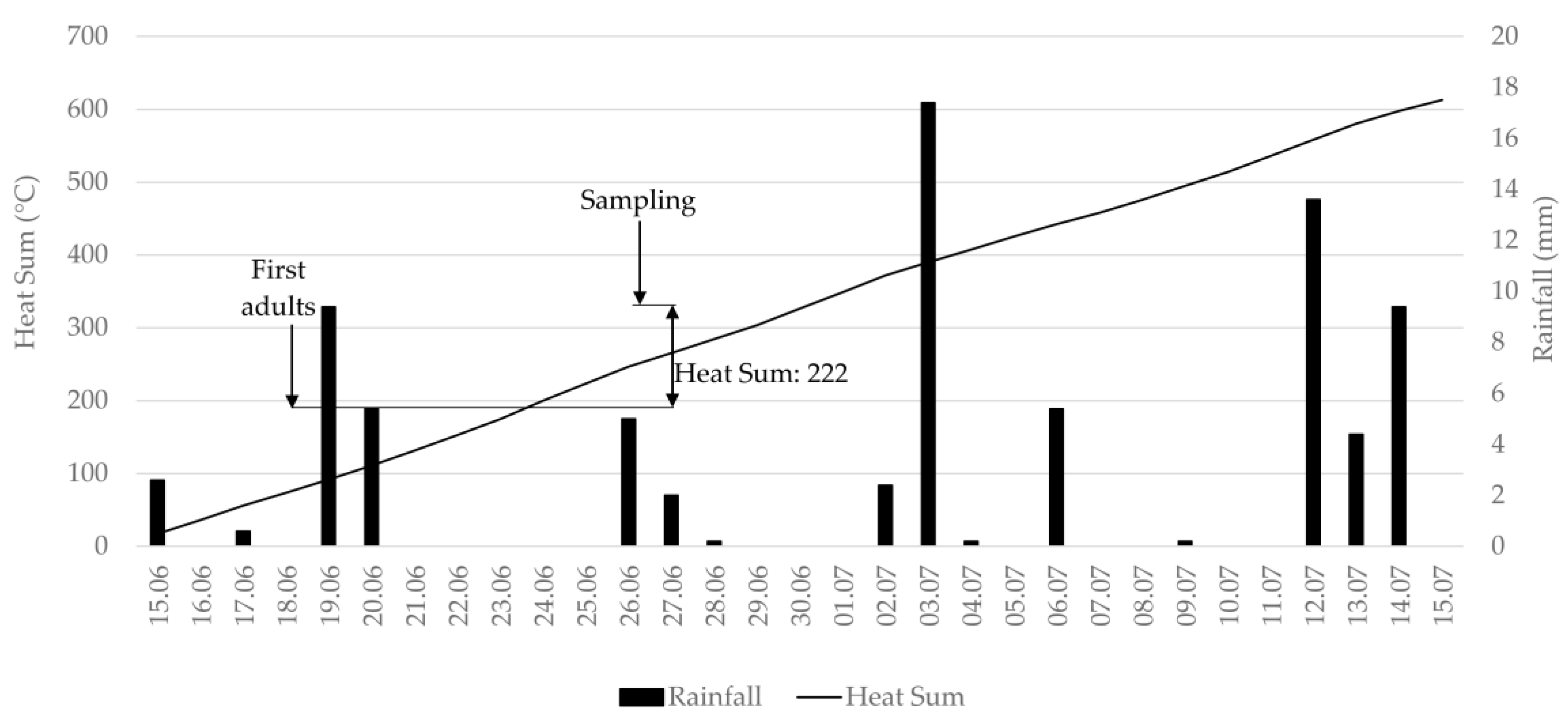
2.2. Evaluation of Thrips Abundance
2.3. Measurement of Leaf Colour
2.4. Statistical Analysis
3. Results
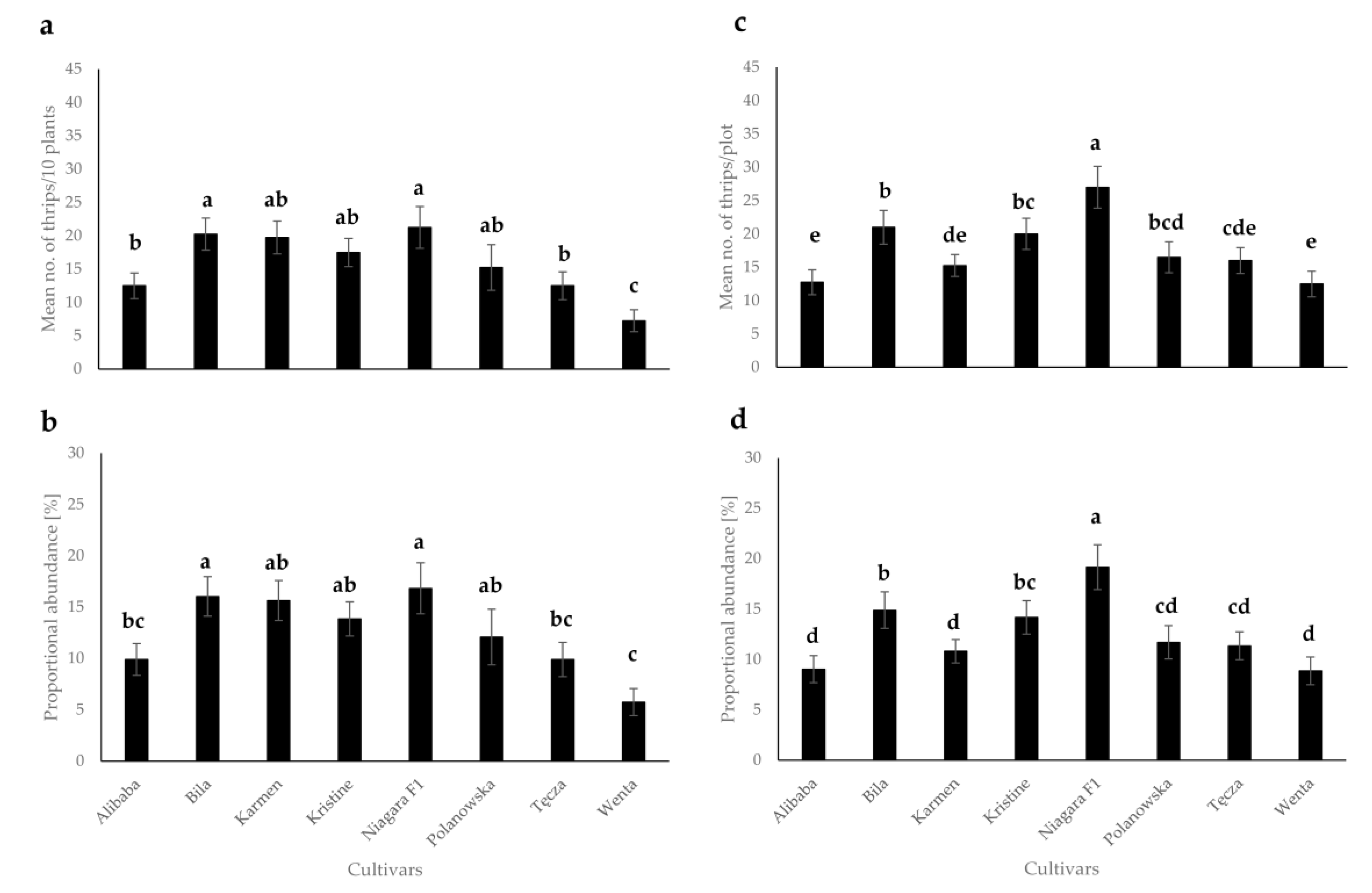
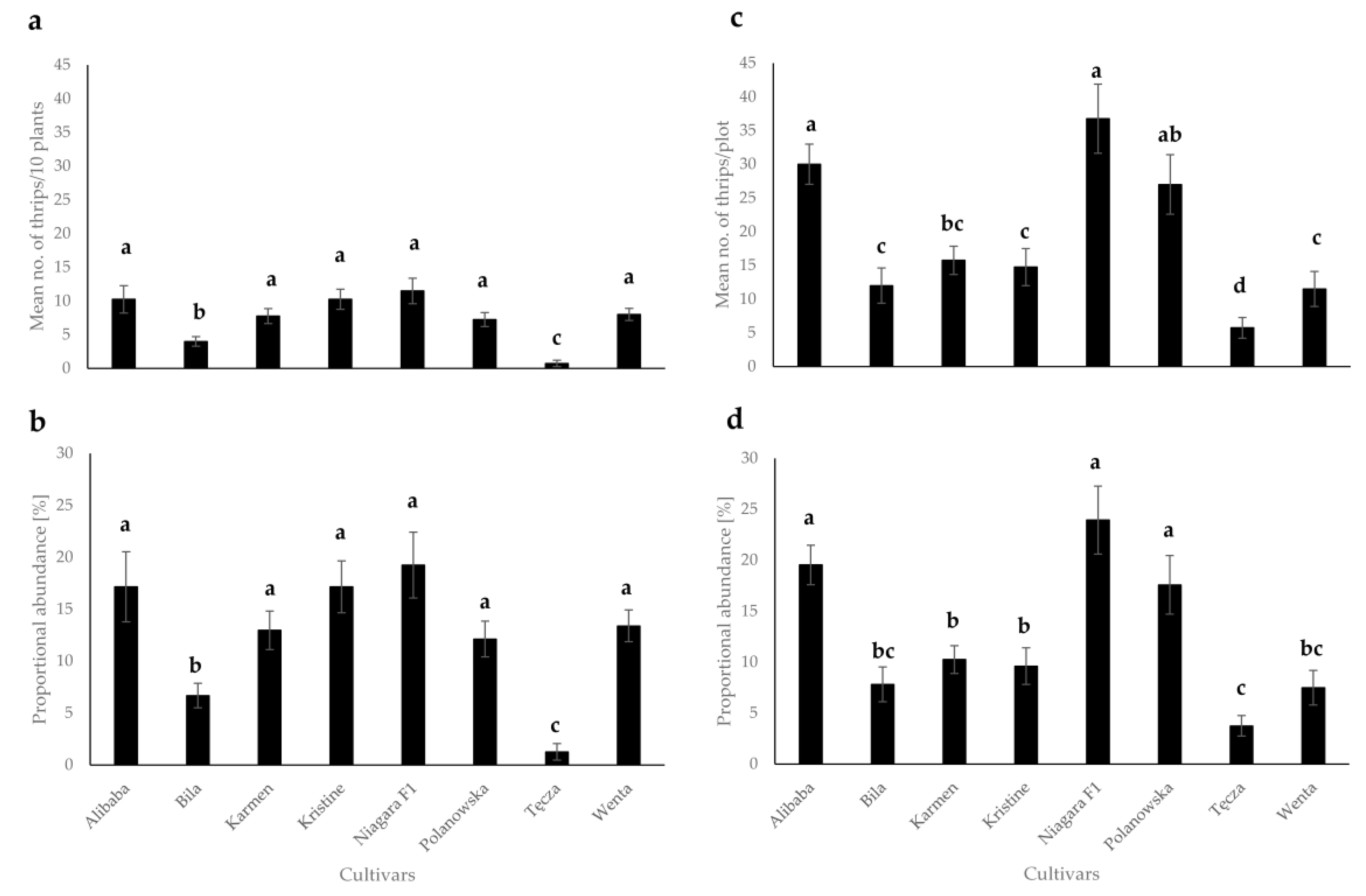
3.1. Evaluation of Thrips Abundance
3.2. Measurement of Onion Leaf Colour
3.3. Correlations between Thrips Occurrence and Colour Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat 2020. Available online: https://ec.europa.eu/info/departments/eurostat-european-statistics_pl (accessed on 26 February 2021).
- STATISTICS POLAND. Statistical Yearbook of Agriculture. Available online: https://stat.gov.pl/en/topics/statistical-yearbooks/ (accessed on 26 February 2021).
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Lewis, T. Pest thrips in perspective. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: New York, NY, USA, 1997; pp. 1–13. [Google Scholar]
- Szwejda, J.; Wrzodak, R. Phytophagous Entomofauna Occurring on Onion Plantations in Poland in Years 1919–2007. J. Fruit Ornam. Plant Res. 2009, 71, 5–14. [Google Scholar] [CrossRef]
- Boateng, C.O.; Schwartz, H.F.; Havey, M.J.; Otto, K. Evaluation of onion germplasm for resistance to Iris yellow spot (Iris yellow spot virus) and onion thrips Thrips tabaci. Southwest. Entomol. 2014, 39, 237–260. [Google Scholar] [CrossRef]
- Haider, K.; Ghulam, A.; Asifa, H.; Ghayour, A.; Amjad, A. Losses in onion (Allium cepa) due to onion thrips (Thrips tabaci) (Thysanoptera: Thripidae) and effect of weather factors on population dynamics of thrips. World Appl. Sci. J. 2014, 32, 2250–2258. [Google Scholar] [CrossRef]
- Fournier, F.; Boivin, G.; Stewart, R.K. Effect of Thrips tabaci (Thysanoptera: Thripidae) on yellow onion yields and economic thresholds for its management. J. Econ. Entomol. 1995, 88, 1401–1407. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H.; Gill, A.K.; Gillett-Kaufman, J.L.; Nault, B.A. Onion thrips (Thysanoptera: Thripidae) biology, ecology, and management in onion production systems. J. Integr. Pest Manag. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Evaluation of Onion Cultivars for Resistance to Onion Thrips (Thysanoptera: Thripidae) and Iris Yellow Spot Virus. J. Econ. Entomol. 2010, 103, 925–937. [Google Scholar] [CrossRef]
- Alston, D.G.; Drost, D. Onion Thrips (Thrips tabaci). ENT-117-08PR. Utah Pests Fact Sheet, Utah State University Extension. Utah State University Extension and Utah Plant Pest Diagnostic Laboratory, Logan, UT. Available online: http://extension.usu.edu/files/publications/factsheet/ent-117-08pr.pdf (accessed on 25 February 2021).
- MacIntyre Allen, J.K.; Scott-Dupree, C.D.; Tolman, J.H.; Ron Harris, C. Resistance of Thrips tabaci to pyrethroid and organophosphorus insecticides in Ontario, Canada. Pest Manag. Sci. 2005, 61, 809–815. [Google Scholar] [CrossRef]
- Shelton, A.M.; Zhao, J.Z.; Nault, B.A.; Plate, J.; Musser, F.R.; Larentzaki, E. Patterns of insecticide resistance in onion thrips (Thysanoptera: Thripidae) in onion fields in New York. J. Econ. Entomol. 2006, 99, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M. Plant resistance in pest management. In Introduction to Insect Pest Management, 3rd ed.; Metcalf, R.L., Luckmann, W.H., Eds.; Wiley: New York, NY, USA, 1994; pp. 73–118. [Google Scholar]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches, 1st ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Schoonhoven, L.M.; Van Loon, B.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Reeves, J.L. Vision should not be overlooked as an important sensory modality for finding host plants. Environ. Entomol. 2011, 40, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Introduction to Ecological Biochemistry, 1st ed.; Academic Press: Whiteknights, UK; University of Reading: Whiteknights, UK, 1994. [Google Scholar]
- Stenberg, J.A.; Ericson, L. Visual cues override olfactory cues in the host finding process of themonophagous leaf beetle Altica engstroemi. Entomol. Exp. Appl. 2007, 125, 81–88. [Google Scholar]
- Blake, A.J.; Go, M.C.; Hahn, G.S.; Grey, H.; Couture, S.; Gries, G. Polarization of foliar reflectance: Novel host plant cue for insect herbivores. Proc. R. Soc. B 2019, 286. [Google Scholar] [CrossRef]
- Terry, L.I. Host Selection, Communication and Reproductive Behaviour. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 65–118. [Google Scholar]
- Kirk, W.D. Ecologically selective coloured traps. Ecol. Entomol. 1984, 9, 35–41. [Google Scholar] [CrossRef]
- Lu, F.M. Color preference and using silver mulches to control the onion thrips, Thrips tabaci Lindeman. Chin. J. Entomol. 1990, 10, 337–342. [Google Scholar]
- Natwick, E.T.; Byers, J.A.; Chu, C.C.; Lopez, M.; Henneberry, T.J. Early Detection and Mass Trapping of Frankliniella occidentalis, and Thrips tabaci in Vegetable Crops. Southwest. Entomol. 2007, 32, 229–238. [Google Scholar] [CrossRef]
- Pobożniak, M.; Tokarz, K.; Musynov, K. Evaluation of sticky trap colour for thrips (Thysanoptera) monitoring in pea crops (Pisum sativum L.). J. Plant Dis. Prot. 2020, 127, 307–321. [Google Scholar] [CrossRef]
- Jenser, G.; Szénási, Á.; Zana, J. Investigation on the Colour Preference of Thrips tabaci Lindeman (Thysanoptera: Thripidae). Acta Phytopathol. Entomol. Hung. 2001, 36, 207–211. [Google Scholar] [CrossRef]
- Rőth, F.; Galli, Z.; Tóth, M.; Fail, J.; Jenser, G. The hypothesized visual system of Thrips tabaci (Lindeman) and Frankliniella occidentalis (Pergande) based on different coloured traps’ catches. North-West. J. Zool. 2016, 12, 40–49. [Google Scholar]
- Demirel, N.; Yildirim, A.E. Attraction of various sticky colour traps to Thrips tabaci Lindeman (Thysanoptera: Thripidae) and Empoasca decipiens Paoli (Homoptera: Cicadellidae) in cotton. J. Entomol. 2008, 5, 389–394. [Google Scholar]
- Westmore, G.W.; Allen, G.R.; Wilson, C.R. Colour preference and potato cultivar oviposition choice by onion thrips, Thrips tabaci (Thysanoptera: Thripidae). J. Asia-Pac. Entomol. 2019, 22, 25–32. [Google Scholar] [CrossRef]
- Menzel, R. Spectral Sensitivity and Color Vision in Invertebrates. In Comparative Physiology and Evolution of Vision in Invertebrates. Handbook of Sensory Physiology; Autrum, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1979; Volume 7/6/6 A, pp. 504–580. [Google Scholar] [CrossRef]
- Egri, Á.; Farkas, P.; Bernáth, B.; Guerin, M.P.; Fail, J. Spectral sensitivity of L2 biotype in the Thrips tabaci cryptic species complex. J. Insect Physiol. 2020, 121, 103999. [Google Scholar] [CrossRef]
- Makabe, T.; Futamura, T.; Noudomi, T.; Wakakuwa, M.; Arikawa, K. Phototaxis of Western Flower Thrips, Frankliniella occidentalis and Onion Thrips, Thrips tabaci and the Possibility of Controlling Thrips Using Ultraviolet-emitting Trap in the Greenhouse of Satsuma Mandarin (Citrus unshiu). Jpn. J. Appl. Entomol. Zool. 2014, 58, 187–195. [Google Scholar]
- Kogan, M.; Ortman, E.F. Antixenosis—A new term proposed to define Painter’s “nonpreference” modality of resistance. Bull. ESA 1978, 24, 175–176. [Google Scholar] [CrossRef]
- Jones, H.A.; Bailey, S.F.; Emsweller, S.L. Field studies of Thrips tabaci Lind. with special reference to resistance in onions. J. Econ. Entomol. 1935, 28, 678–680. [Google Scholar] [CrossRef]
- Coudriet, D.L.; Kishaba, A.N.; McCreight, J.D.; Bohn, G.W. Varietial Resistance in Onions to Thrips. J. Econ. Entomol. 1979, 72. [Google Scholar] [CrossRef]
- Patil, A.P.; Nawale, R.N.; Ajri, D.S.; Moholkar, P.R. Field screening of onion cultivars for their reaction to thrips. Ind. Cocoa Arec. Spices J. 1988, 12, 10–11. [Google Scholar]
- Hudák, K.; Pénzes, B. Factors influencing the population of the onion thrips on onion. Acta Phytopathol. Entomol. Hung. 2004, 39, 193–197. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fail, J.; Deutschlander, M.; Nault, B.A.; Shelton, A.M. Characterization of resistance, evaluation of the attractiveness of plant odors, and effect of leaf colour on different onion cultivars to onion thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2012, 105, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Fail, J.; Zana, J.; Pénzes, B. The role of plant characteristics in the resistance of white cabbage to onion thrips: Preliminary results. Acta Phytopathol. Entomol. Hung. 2008, 43, 267–275. [Google Scholar] [CrossRef]
- Bálint, J.; Nagy, B.V.; Fail, J. Correlations between Colonization of Onion Thrips and Leaf Reflectance Measures across Six Cabbage Varieties. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Pobożniak, M.; Leśniak, M.; Chuda, A.; Adamus, A. Field assessment of the susceptibility of onion cultivars to thrips attack–preliminary results. Pol. J. Entomol. 2016, 85, 121–133. [Google Scholar] [CrossRef][Green Version]
- Zur Strassen, R. Die terebranten thysanopteren Europas und des mittelmeer-gebietes. Tierwelt Dtschl. 2003, 74, 1–277. [Google Scholar]
- Zawirska, I. Thrips (Thysanoptera). In Diagnostics of Plant Pests and Their Natural Enemies; Kozłowski, M.W., Boczek, J., Eds.; SGGW: Warsaw, Poland, 1994; pp. 145–174. [Google Scholar]
- Edelson, J.V.; Magaro, J.J. Development of onion thrips, Thrips tabaci Lindeman, as a function of temperature. Southwest. Entomol. 1988, 13, 171–176. [Google Scholar]
- Murai, T. Effect of temperature on development and reproduction of the onion thrips, Thrips tabaci Lindeman (Thysanoptera: Thripidae), on pollen and honey solution. Appl. Entomol. Zool. 2000, 35, 499–504. [Google Scholar] [CrossRef]
- Spectrophotometer CM-2600d Simply Expands the Boundaries in Colour Control. Konica Minolta (2007). Konica Minolta Sensing, INC, Japan. Available online: https://www.konicaminolta.com/instruments/download/catalog/color/pdf/cm2600d_catalog_eng.pdf (accessed on 26 February 2021).
- Sanmartín, P.; Gambino, M.; Fuentes, E.; Serrano, M.A. Simple, Reliable, and Inexpensive Solution for Contact Color Measurement in Small Plant Samples. Sensors 2020, 20, 2348. [Google Scholar] [CrossRef]
- McLaren, K. The development of the CIE 1976 (L*a*b*) uniform colour-space and colour difference formula. J. Soc. Dyers Colour. 1976, 92, 338–341. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Smith, D.E. Colour Analysis. In Food Analysis; Nielson, S.S., Ed.; Springer Science + Business Media, LLC: New York, NY, USA, 2010; pp. 575–586. [Google Scholar]
- Fail, J.; Deutschlander, M.E.; Shelton, A.M. Antixenotic Resistance of Cabbage to Onion Thrips (Thysanoptera: Thripidae). I. Light Reflectance. J. Econ. Entomol. 2013, 106, 2602–2612. [Google Scholar] [CrossRef]
- Painter, R.H. Insect Resistance in Crop Plants; Macmillan: New York, NY, USA, 1951. [Google Scholar] [CrossRef]
- den Belder, E.; Elderson, J.; Agca, I. Is olfactory orientation of Thrips tabaci disrupted by a non-host plant. Proc. Exp. Appl. Entomol. NEV Amst. 2001, 12, 61–64. [Google Scholar]
- Lewis, T. Thrips, Their Biology, Ecology and Economic Importance; Academic Press: London, UK, 1973. [Google Scholar]
- Smith, E.A.; Fuchs, M.; Shields, E.J.; Nault, B.A. Long-Distance Dispersal Potential for Onion Thrips (Thysanoptera: Thripidae) and Iris yellow spot virus (Bunyaviridae: Tospovirus) in an Onion Ecosystem. Environ. Entomol. 2015, 44, 921–930. [Google Scholar] [CrossRef]
- Alimousavi, S.A.; Hassandokht, M.R.; Moharramipour, S.A.E.I.D. Evaluation of Iranian onion germplasms for resistance to thrips. Int. J. Agric. Biol. 2007, 9, 897–900. [Google Scholar]
- Birithia, R.K.; Subramanian, S.; Muthomi, J.W.; Narla, R.D. Resistance to Iris yellow spot virus and onion thrips among onion varieties grown in Kenya. Int. J. Trop. Insect Sci. 2014, 34, 73–79. [Google Scholar]
- Yousefi, M.; Abasifar, A.; Hafshejani, A.F.; Sendi, J.J. Resistance of eight Iranian onion cultivars to onion thrips (Thrips tabaci Lindeman) in the Markazi Province of Iran. Afr. J. Agric. Res. 2011, 6, 4925–4930. [Google Scholar]
- Pobożniak, M. The Species Composition, Harmfulness and Selected Aspects of the Occurrence and Feeding Preference of Thrips (Thysanoptera) on Pea (Pisum sativum L.) Cultivars; Wydawnictwo Uniwersytetu Rolniczego: Kraków, Poland, 2013; p. 391. [Google Scholar]
- Fairchild, M.D. Color Appearance Models, 3rd ed.; Wiley: Chichester, UK, 2013. [Google Scholar]
- Corney, D.; Haynes, J.-D.; Rees, G.; Lotto, R.B. The Brightness of Colour. PLoS ONE 2009, 4, e5091. [Google Scholar] [CrossRef]
- Ehleringer, J.; Björkman, O.; Mooney, H.A. Leaf pubescence: Effects on absorptance and photosynthesis in a desert shrub. Science 1976, 192, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Ustin, S.L.; Jacquemoud, S. How the Optical Properties of Leaves Modify the Absorption and Scattering of Energy and Enhance Leaf Functionality. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J.A., Townsend, P.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 349–384. [Google Scholar] [CrossRef]
- Woolley, J.T. Reflectance and transmittance of light by leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar]
- Ibrahim, N.D.; Adesiyun, A.A. Effects of age and height of onion (Allium cepa L.) plants on infestation thrips, Thrips tabaci Linderman (Thysanoptera: Thripidae) in Sokoto, Nigeria. Afr. J. Agric. Res. 2009, 4, 076–084. [Google Scholar] [CrossRef]
- Koschier, E.H.; Sedy, K.A.; Novak, J. Influence of plant volatiles on feeding damage caused by the onion thrips Thrips tabaci. Crop Prot. 2002, 21, 419–425. [Google Scholar] [CrossRef]
- Silva, V.C.P.; da Bettoni, M.M.; Bona, C.; Foerster, L.A. Morphological and chemical characteristics of onion plants (Allium cepa L.) associated with resistance to onion thrips. Acta Sci. Agron. 2014, 37, 85–92. [Google Scholar] [CrossRef]
- Njau, G.M.; Nyomora, A.M.S.; Dinssa, F.F.; Chang, J.-C.; Malini, P.; Subramanian, S.; Srinivasan, R. Evaluation of onion (Allium cepa) germplasm entries for resistance to onion thrips, Thrips tabaci (Lindeman) in Tanzania. Int. J. Trop. Insect Sci. 2017, 37, 98–113. [Google Scholar] [CrossRef]
| Cultivar | L*a*b* Colour Space | L*C*h Colour Space | |||
|---|---|---|---|---|---|
| L* 1 | a* | b* | C* | h* | |
| Mean [±SE] | |||||
| Alibaba | 45.98 ± 1.04 bc 2 | −6.89 ± 0.18 b | 9.88 ± 0.88 c | 12.07 ± 0.82 c | −54.75 ± 1.76 c |
| Bila | 48.64 ± 0.45 a | −7.44 ± 0.24 a | 15.08 ± 1.04 a | 16.82 ± 1.03 a | −63.58 ± 0.84 a |
| Karmen | 46.61 ± 0.39 bc | −6.41 ± 0.19 b | 10.16 ± 0.68 c | 12.02 ± 0.68 c | −57.56 ± 1.09 bc |
| Kristine | 44.89 ± 0.58 c | −6.88 ± 0.22 b | 10.64 ± 0.52 c | 12.68 ± 0.54 c | −57.03 ± 0.84 bc |
| Niagara F1 | 47.68 ± 0.73 ab | −6.57 ± 0.13 b | 10.54 ± 0.12 c | 12.42 ± 0.16 c | −58.07 ± 0.30 b |
| Polanowska | 45.29 ± 0.98 c | −6.39 ± 0.08 b | 9.20 ± 0.71 c | 11.22 ± 0.62 c | −54.89 ± 1.08 c |
| Tęcza | 45.80 ± 0.36 c | −6.83 ± 0.08 b | 13.36 ± 0.67 b | 15.01 ± 0.64 b | −62.78 ± 0.90 a |
| Wenta | 44.79 ± 0.36 c | −6.61 ± 0.16 b | 9.54 ± 0.56 c | 11.62 ± 0.55 c | −55.12 ± 2.14 bc |
| F cultivar | 5.740 | 4.520 | 13.384 | 12.183 | 13.170 |
| p cultivar | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 |
| F blocks | 4.010 | 2.380 | 5.344 | 4.961 | 5.230 |
| p blocks | 0.02 | 0.098 | 0.006 | 0.009 | 0.007 |
| Cultivar | L*a*b* Colour Space | L*C*h Colour Space | |||
|---|---|---|---|---|---|
| L* 1 | a* | b* | C* | h* | |
| Mean [±SE] | |||||
| Alibaba | 50.24 ± 0.72 a 2 | −9.87 ± 0.13 a | 22.27 ± 0.63 a | 24.36 ± 0.63 a | −66.05 ± 0.38 a |
| Bila | 46.05 ± 0.23 b | −8.98 ± 0.07 b | 17.75 ± 0.47 b | 19.92 ± 0.45 b | −63.14 ± 0.46 b |
| Karmen | 46.04 ± 0.41 b | −8.88 ± 0.22 b | 17.69 ± 0.76 b | 19.79 ± 0.77 b | −63.28 ± 0.55 b |
| Kristine | 49.10 ± 0.80 a | −9.78 ± 0.21 a | 20.51 ± 1.18 a | 22.73 ± 1.16 a | −64.37 ± 0.78 ab |
| Niagara F1 | 47.18 ± 0.66 b | −8.73 ± 0.25 b | 17.90 ± 1.22 b | 19.93 ± 0.55 b | −63.95 ± 1.02 ab |
| Polanowska | 46.11 ± 0.28 b | −9.05 ± 0.07 b | 17.45 ± 0.39 b | 19.66 ± 0.36 b | −62.56 ± 0.48 b |
| Tęcza | 46.15 ± 0.66 b | −8.76 ± 0.20 b | 16.83 ± 2.23 b | 18.98 ± 1.08 b | −62.34 ± 0.97 b |
| Wenta | 46.82 ± 0.35 | −8.92 ± 0.15 b | 17.90 ± 0.75 b | 20.01 ± 0.75 b | −63.44 ± 0.50 b |
| F cultivar | 8.040 | 6.270 | 4.961 | 5.230 | 2.660 |
| p cultivar | 0.000 | 0.000 | 0.001 | 0.001 | 0.039 |
| F blocks | 0.760 | 1.440 | 0.193 | 0.266 | 0.260 |
| p blocks | 0.526 | 0.259 | 0.900 | 0.849 | 0.851 |
| Cultivar | Alibaba | Bila | Karmen | Kristine | Niagara F1 | Polanowska | Tęcza | Wenta |
|---|---|---|---|---|---|---|---|---|
| Alibaba | x | 2.15 | 0.57 | 0.46 | 0.66 | −0.13 | 1.85 | 0.05 |
| Bila | −2.15 | x | −1.48 | −1.67 | −0.83 | −2.04 | −0.22 | −2.06 |
| Karmen | −0.57 | 1.48 | x | −0.13 | 0.09 | −0.53 | 1.21 | −0.51 |
| Kristine | −0.46 | 1.67 | 0.13 | x | 0.30 | −0.42 | 1.39 | −0.41 |
| Niagara F1 | −0.66 | 1.40 | −0.09 | 0.19 | x | −0.62 | 1.14 | −0.60 |
| Polanowska | −0.03 | 2.04 | 0.53 | 0.42 | 0.62 | x | 1.76 | 0.03 |
| Tęcza | −1.85 | 0.22 | −1.21 | −1.39 | −1.14 | −1.76 | x | −1.76 |
| Wenta | −0.05 | 2.06 | 0.51 | 0.41 | 0.60 | −0.03 | 1.76 | x |
| Cultivar | Alibaba | Bila | Karmen | Kristine | Niagara F1 | Polanowska | Tęcza | Wenta |
|---|---|---|---|---|---|---|---|---|
| Alibaba | x | −1.11 | −1.06 | −0.67 | −0.80 | −1.34 | −1.37 | −1.00 |
| Bila | 1.11 | x | 0.05 | 0.47 | 0.28 | −0.38 | −0.25 | 0.11 |
| Karmen | 1.06 | −0.05 | x | 0.42 | 0.22 | −0.25 | −0.30 | 0.06 |
| Kristine | 0.67 | −0.47 | −0.42 | x | −0.18 | −0.69 | −0.73 | −0.36 |
| Niagara F1 | 0.80 | −0.28 | −0.22 | 0.18 | x | −0.49 | −0.55 | −0.18 |
| Polanowska | 0.77 | 0.20 | 0.25 | 0.69 | 0.49 | x | −0.06 | 0.31 |
| Tęcza | 1.37 | 0.25 | 0.30 | 0.73 | 0.55 | 0.06 | x | 0.37 |
| Wenta | 1.00 | −0.11 | −0.06 | 0.36 | 0.18 | −0.31 | −0.37 | x |
| Colour Coordinates | Thrips Adults Collected Directly from Plants | Thrips Adults Collected with Sweeping Net | ||||||
|---|---|---|---|---|---|---|---|---|
| Actual Count | Proportional Abundance | Actual Count | Proportional Abundance | |||||
| r | p | r | p | r | p | r | p | |
| L* | 0.739 | 0.000 | 0.759 | 0.000 | 0.644 | 0.000 | 0.625 | 0.000 |
| a* | −0.441 | 0.011 | −0.454 | 0.009 | −0.365 | 0.040 | −0.343 ns | 0.055 |
| b* | 0.471 | 0.006 | 0.464 | 0.007 | 0.0466 | 0.007 | 0.428 | 0.014 |
| C* | 0.472 | 0.006 | 0.467 | 0.007 | 0.460 | 0.008 | 0.423 | 0.016 |
| h* | 0.448 | 0.010 | 0.448 | 0.010 | 0.441 | 0.011 | 0.441 | 0.011 |
| Colour Coordinates | Thrips Adults Collected Directly from Plants | Thrips Adults Collected with Sweeping Net | ||||||
|---|---|---|---|---|---|---|---|---|
| Actual Count | Proportional Abundance | Actual Count | Proportional Abundance | |||||
| r | p | r | p | r | p | r | p | |
| L* | 0.390 | 0.027 | 0.526 | 0.002 | 0.255 ns | 0.158 | 0.198 ns | 0.275 |
| a* | −0.333 ns | 0.062 | −0.417 | 0.017 | −0.174 ns | 0.341 | −0.732 ns | 0.691 |
| b* | 0.371 | 0.036 | 0.463 | 0.008 | 0.316 ns | 0.078 | 0.255 ns | 0.157 |
| C* | 0.372 | 0.036 | 0.464 | 0.007 | 0.305 ns | 0.089 | 0.239 ns | 0.186 |
| h* | 0.410 | 0.020 | 0.410 | 0.020 | 0.365 | 0.039 | 0.365 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pobożniak, M.; Olczyk, M.; Wójtowicz, T. Relationship between Colonization by Onion Thrips (Thrips tabaci Lind.) and Leaf Colour Measures across Eight Onion Cultivars (Allium cepa L.). Agronomy 2021, 11, 963. https://doi.org/10.3390/agronomy11050963
Pobożniak M, Olczyk M, Wójtowicz T. Relationship between Colonization by Onion Thrips (Thrips tabaci Lind.) and Leaf Colour Measures across Eight Onion Cultivars (Allium cepa L.). Agronomy. 2021; 11(5):963. https://doi.org/10.3390/agronomy11050963
Chicago/Turabian StylePobożniak, Maria, Marta Olczyk, and Tomasz Wójtowicz. 2021. "Relationship between Colonization by Onion Thrips (Thrips tabaci Lind.) and Leaf Colour Measures across Eight Onion Cultivars (Allium cepa L.)" Agronomy 11, no. 5: 963. https://doi.org/10.3390/agronomy11050963
APA StylePobożniak, M., Olczyk, M., & Wójtowicz, T. (2021). Relationship between Colonization by Onion Thrips (Thrips tabaci Lind.) and Leaf Colour Measures across Eight Onion Cultivars (Allium cepa L.). Agronomy, 11(5), 963. https://doi.org/10.3390/agronomy11050963







