Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Agronomic Information
2.2. Mycotoxin Analysis
2.3. Weather Data
2.4. Statistical Analyses
3. Results
3.1. Spatial Distribution of Contaminated Samples
3.2. Inter-Annual and Annual Variability of DON Contents
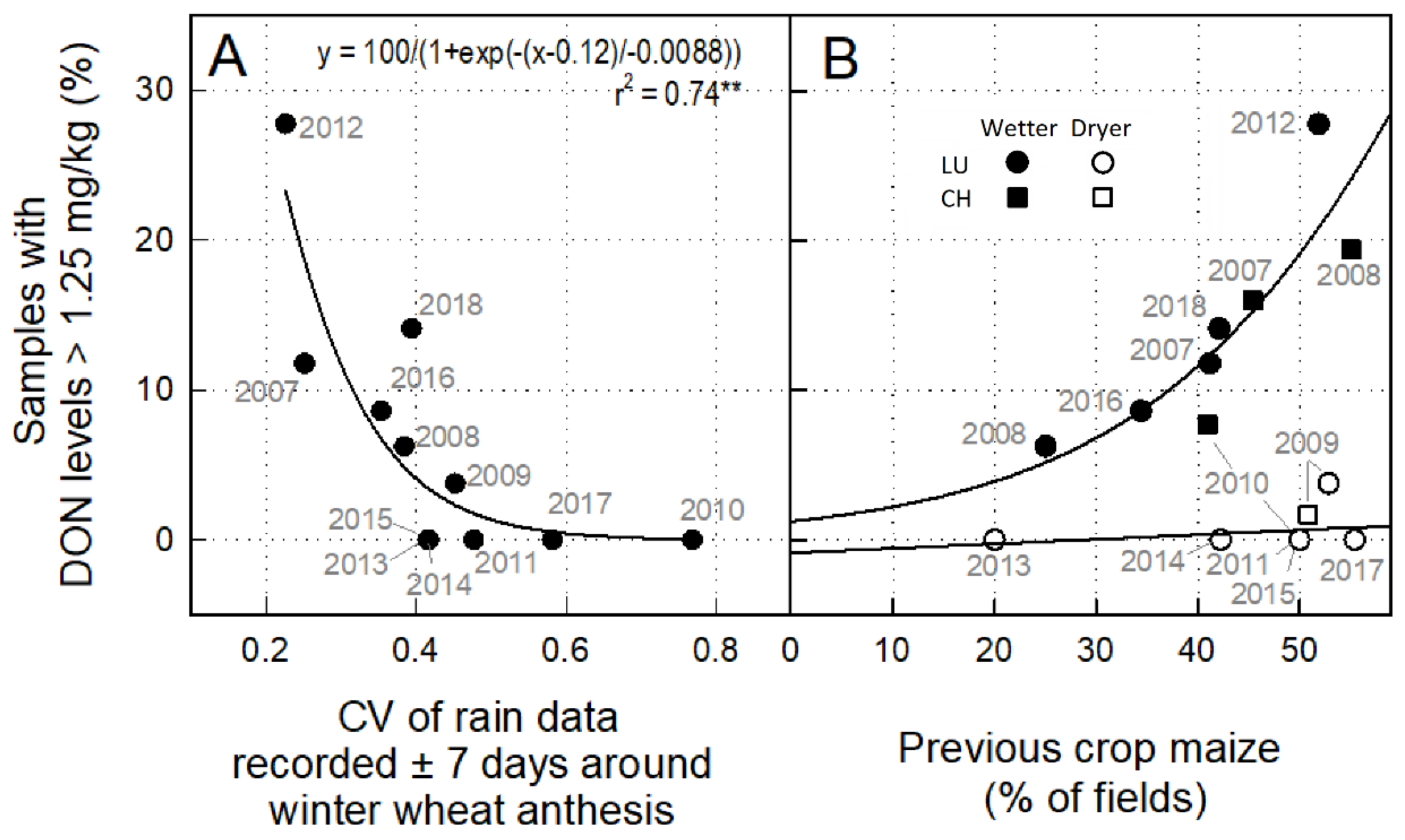
3.3. Effect of Weather and Previous Crop on the Percentage of Samples with DON > ML
4. Discussion
4.1. Considerations on Temporal Risk Dynamics
4.2. Novelty
4.3. Spatial Aspects
4.4. Considerations on the Role of Cultivars
4.5. Other Factors Affecting DON Production under Field Conditions
4.6. Comparison with Other European Surveys
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Ioos, R.; Belhadj, A.; Menez, M. Occurrence and Distribution of Microdochium nivale and Fusarium Species Isolated from Barley, Durum and Soft Wheat Grains in France from 2000 to 2002. Mycopathologia 2004, 158, 351. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vea, M.; Lizarraga, E.; González-Peñas, E.; López de Cerain, A. Co-Occurrence of Type-A and Type-B Trichothecenes in Barley from a Northern Region of Spain. Food Control 2012, 25, 81–88. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Foglia, P.; Gubbiotti, R.; Roccia, C.; Samperi, R.; Laganà, A. Development and Validation of a Liquid Chromatography/Atmospheric Pressure Photoionization-Tandem Mass Spectrometric Method for the Analysis of Mycotoxins Subjected to Commission Regulation (EC) No. 1881/2006 in Cereals. J. Chromatogr. A 2010, 1217, 6044–6051. [Google Scholar] [CrossRef]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium Mycotoxins’ Occurrence in Cereals Harvested from Croatian Fields. Food Control 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Siegel, D.; Babuscio, T. Mycotoxin Management in the European Cereal Trading Sector. Food Control 2011, 22, 1145–1153. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of Action, Human Exposure, and Toxicological Relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Krska, R.; Baumgartner, S.; Josephs, R. The State-of-the-Art in the Analysis of Type-A and -B Trichothecene Mycotoxins in Cereals. Fresenius J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Cui, L.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Zhou, L.; Liu, Y. A Minor Survey of Deoxynivalenol in Fusarium Infected Wheat from Yangtze–Huaihe River Basin Region in China. Food Control 2013, 30, 469–473. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of Deoxynivalenol and Its Acetylated and Modified Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified Mycotoxins: An Updated Review on Their Formation, Detection, Occurrence, and Toxic Effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- European Food Safety Authority. Deoxynivalenol in Food and Feed: Occurrence and Exposure. EFSA J. 2013, 11, 3379. [Google Scholar] [CrossRef]
- 2019 BIOMIN World Mycotoxin Survey Report. Available online: https://www.biomin.net/downloads/2019-biomin-world-mycotoxin-survey-report/ (accessed on 12 January 2021).
- Eskola, M.; Altieri, A.; Galobart, J. Overview of the Activities of the European Food Safety Authority on Mycotoxins in Food and Feed. World Mycotoxin J. 2018, 11, 277–289. [Google Scholar] [CrossRef]
- Ji, X.; Yang, H.; Wang, J.; Li, R.; Zhao, H.; Xu, J.; Xiao, Y.; Tang, B.; Qian, M. Occurrence of Deoxynivalenol (DON) in Cereal-Based Food Products Marketed through e-Commerce Stores and an Assessment of Dietary Exposure of Chinese Consumers to DON. Food Control 2018, 92, 391–398. [Google Scholar] [CrossRef]
- Jajić, I.; Jurić, V.; Abramović, B. First Survey of Deoxynivalenol Occurrence in Crops in Serbia. Food Control 2008, 19, 545–550. [Google Scholar] [CrossRef]
- Malachova, A.; Dzuman, Z.; Veprikova, Z.; Vaclavikova, M.; Zachariasova, M.; Hajslova, J. Deoxynivalenol, Deoxynivalenol-3-Glucoside, and Enniatins: The Major Mycotoxins Found in Cereal-Based Products on the Czech Market. J. Agric. Food Chem. 2011, 59, 12990–12997. [Google Scholar] [CrossRef] [PubMed]
- Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Deoxynivalenol and Nivalenol in Commercial Wheat Grain Related to Fusarium Head Blight Epidemics in Southern Brazil. Food Chem. 2012, 132, 1087–1091. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and Other Selected Fusarium Toxins in Swedish Oats—Occurrence and Correlation to Specific Fusarium Species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Determination of Aflatoxins, Deoxynivalenol, Ochratoxin A and Zearalenone in Wheat and Oat Based Bran Supplements Sold in the Spanish Market. Food Chem. Toxicol. 2013, 53, 133–138. [Google Scholar] [CrossRef]
- Běláková, S.; Benešová, K.; Čáslavský, J.; Svoboda, Z.; Mikulíková, R. The Occurrence of the Selected Fusarium Mycotoxins in Czech Malting Barley. Food Control 2014, 37, 93–98. [Google Scholar] [CrossRef]
- Kirinčič, S.; Škrjanc, B.; Kos, N.; Kozolc, B.; Pirnat, N.; Tavčar-Kalcher, G. Mycotoxins in Cereals and Cereal Products in Slovenia—Official Control of Foods in the Years 2008–2012. Food Control 2015, 50, 157–165. [Google Scholar] [CrossRef]
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and Other Selected Fusarium Toxins in Swedish Wheat—Occurrence and Correlation to Specific Fusarium Species. Int. J. Food Microbiol. 2013, 167, 284–291. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.W.; De Saeger, S.; Wu, A. Cumulative Health Risk Assessment of Co-Occurring Mycotoxins of Deoxynivalenol and Its Acetyl Derivatives in Wheat and Maize: Case Study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated Survey of Fusarium Species and Toxins in Finnish Cereal Grains. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food; FAO: Rome, Italy, 2001; ISBN 978-92-5-104664-7. [Google Scholar]
- Beyer, M.; Klix, M.B.; Klink, H.; Verreet, J.-A. Quantifying the Effects of Previous Crop, Tillage, Cultivar and Triazole Fungicides on the Deoxynivalenol Content of Wheat Grain—A Review. J. Plant Dis. Prot. 2006, 113, 241–246. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A. Effect of Agronomic Programmes with Different Susceptibility to Deoxynivalenol Risk on Emerging Contamination in Winter Wheat. Eur. J. Agron. 2017, 85, 12–24. [Google Scholar] [CrossRef]
- Bundessortenamt. Getreide, Mais, Öl-Und Faserpflanzen, Leguminosen, Rüben, Zwischenfrüchte. 2018. Available online: https://www.bundessortenamt.de/bsa/en/variety-testing/descriptive-variety-lists/downloading-descriptive-variety-lists (accessed on 12 January 2021).
- Prandini, A.; Sigolo, S.; Filippi, L.; Battilani, P.; Piva, G. Review of Predictive Models for Fusarium Head Blight and Related Mycotoxin Contamination in Wheat. Food Chem. Toxicol. 2009, 47, 927–931. [Google Scholar] [CrossRef]
- Birr, T.; Verreet, J.-A.; Klink, H. Prediction of Deoxynivalenol and Zearalenone in Winter Wheat Grain in a Maize-Free Crop Rotation Based on Cultivar Susceptibility and Meteorological Factors. J. Plant Dis. Prot. 2019, 126, 13–27. [Google Scholar] [CrossRef]
- Giroux, M.-E.; Bourgeois, G.; Dion, Y.; Rioux, S.; Pageau, D.; Zoghlami, S.; Parent, C.; Vachon, E.; Vanasse, A. Evaluation of Forecasting Models for Fusarium Head Blight of Wheat Under Growing Conditions of Quebec, Canada. Plant Dis. 2016, 100, 1192–1201. [Google Scholar] [CrossRef]
- Giraud, F.; Pasquali, M.; El Jarroudi, M.; Vrancken, C.; Brochot, C.; Cocco, E.; Hoffmann, L.; Delfosse, P.; Bohn, T. Fusarium Head Blight and Associated Mycotoxin Occurrence on Winter Wheat in Luxembourg in 2007/2008. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 825–835. [Google Scholar] [CrossRef]
- Aslanov, R.; El Jarroudi, M.; Gollier, M.; Pallez-Barthel, M.; Beyer, M. Yellow Rust Does Not like Cold Winters. But How to Find out Which Temperature and Time Frames Could Be Decisive in Vivo? J. Plant Pathol. 2019, 101, 539–546. [Google Scholar] [CrossRef]
- Beyer, M.; Junk, J.; Eickermann, M.; Clermont, A.; Kraus, F.; Georges, C.; Reichart, A.; Hoffmann, L. Winter Honey Bee Colony Losses, Varroa Destructor Control Strategies, and the Role of Weather Conditions: Results from a Survey among Beekeepers. Res. Vet. Sci. 2018, 118, 52–60. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.-R. An Eight-Year Survey of Wheat Shows Distinctive Effects of Cropping Factors on Different Fusarium Species and Associated Mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Xu, X. Effects of Environmental Conditions on the Development of Fusarium Ear Blight. Eur. J. Plant Pathol. 2003, 109, 683–689. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2007, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Pogoda, F.; Pallez, M.; Lazic, J.; Hoffmann, L.; Pasquali, M. Evidence for a Reversible Drought Induced Shift in the Species Composition of Mycotoxin Producing Fusarium Head Blight Pathogens Isolated from Symptomatic Wheat Heads. Int. J. Food Microbiol. 2014, 182–183, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.W.; Hooker, D.C. Climatic Models to Predict Occurrence of Fusarium Toxins in Wheat and Maize. Int. J. Food Microbiol. 2007, 119, 116–125. [Google Scholar] [CrossRef]
- Musa, T.; Hecker, A.; Vogelgsang, S.; Forrer, H.R. Forecasting of Fusarium Head Blight and Deoxynivalenol Content in Winter Wheat with FusaProg*. EPPO Bull. 2007, 37, 283–289. [Google Scholar] [CrossRef]
- Liu, C.; Manstretta, V.; Rossi, V.; Van der Fels-Klerx, H.J. Comparison of Three Modelling Approaches for Predicting Deoxynivalenol Contamination in Winter Wheat. Toxins 2018, 10, 267. [Google Scholar] [CrossRef]
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marín, S. Predicting Mycotoxins in Foods: A Review. Food Microbiol. 2009, 26, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Rämö, S.; Hietaniemi, V.; Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R. Molecular Quantification and Genetic Diversity of Toxigenic Fusarium Species in Northern Europe as Compared to Those in Southern Europe. Microorganisms 2013, 1, 162–174. [Google Scholar] [CrossRef]
- Logrieco, A.F.; Moretti, A. Between Emerging and Historical Problems: An Overview of the Main Toxigenic Fungi and Mycotoxin Concerns in Europe. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI: Wallingford, UK, 2008. [Google Scholar]
- Edwards, S.G. Fusarium Mycotoxin Content of UK Organic and Conventional Oats. Food Addit. Contam. Part A 2009, 26, 1063–1069. [Google Scholar] [CrossRef]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European Database of Fusarium Graminearum and F. Culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Beyer, M.; Klix, M.B.; Verreet, J.A. Estimating Mycotoxin Contents of Fusarium-Damaged Winter Wheat Kernels. Int. J. Food Microbiol. 2007, 119, 153–158. [Google Scholar] [CrossRef]
- Beyer, M.; Pogoda, F.; Ronellenfitsch, F.K.; Hoffmann, L.; Udelhoven, T. Estimating Deoxynivalenol Contents of Wheat Samples Containing Different Levels of Fusarium-Damaged Kernels by Diffuse Reflectance Spectrometry and Partial Least Square Regression. Int. J. Food Microbiol. 2010, 142, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Rod, K.S.; Bradley, C.A.; Van Sanford, D.A.; Knott, C.A. Integrating Management Practices to Decrease Deoxynivalenol Contamination in Soft Red Winter Wheat. Front. Plant Sci. 2020, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Škrbić, B.; Malachova, A.; Živančev, J.; Veprikova, Z.; Hajšlová, J. Fusarium Mycotoxins in Wheat Samples Harvested in Serbia: A Preliminary Survey. Food Control 2011, 22, 1261–1267. [Google Scholar] [CrossRef]
- Topi, D.; Babič, J.; Pavšič-Vrtač, K.; Tavčar-Kalcher, G.; Jakovac-Strajn, B. Incidence of Fusarium Mycotoxins in Wheat and Maize from Albania. Molecules 2021, 26, 172. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.J.; Klemsdal, S.; Hietaniemi, V.; Lindblad, M.; Ioannou-Kakouri, E.; Van Asselt, E.D. Mycotoxin Contamination of Cereal Grain Commodities in Relation to Climate in North West Europe. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2012, 29, 1581–1592. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Mañes, J.; Soler, C. Natural Co-Occurrence of Mycotoxins in Wheat Grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvähuoko, J.; Malachová, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sieviläinen, E.; Berthiller, F.; Peltonen, K. Simultaneous Determination of Major Type A and B Trichothecenes, Zearalenone and Certain Modified Metabolites in Finnish Cereal Grains with a Novel Liquid Chromatography-Tandem Mass Spectrometric Method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP Task 3.2.10 “Collection of Occurrence Data of Fusarium Toxins in Food and Assessment of Dietary Intake by the Population of EU Member States”: Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a Changing Climate: Semi-Quantitative Multi-Mycotoxin Analysis of Grain Grown in Exceptional Climatic Conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Musa, T.; Bänziger, I.; Kägi, A.; Bucheli, T.D.; Wettstein, F.E.; Pasquali, M.; Forrer, H.-R. Fusarium Mycotoxins in Swiss Wheat: A Survey of Growers’ Samples between 2007 and 2014 Shows Strong Year and Minor Geographic Effects. Toxins 2017, 9, 246. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium Species and Mycotoxins in Oats and Spring Wheat from Farmers’ Fields in Norway over a Six-Year Period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Chandelier, A.; Nimal, C.; André, F.; Planchon, V.; Oger, R. Fusarium Species and DON Contamination Associated with Head Blight in Winter Wheat over a 7-Year Period (2003–2009) in Belgium. Eur. J. Plant Pathol. 2011, 130, 403–414. [Google Scholar] [CrossRef]
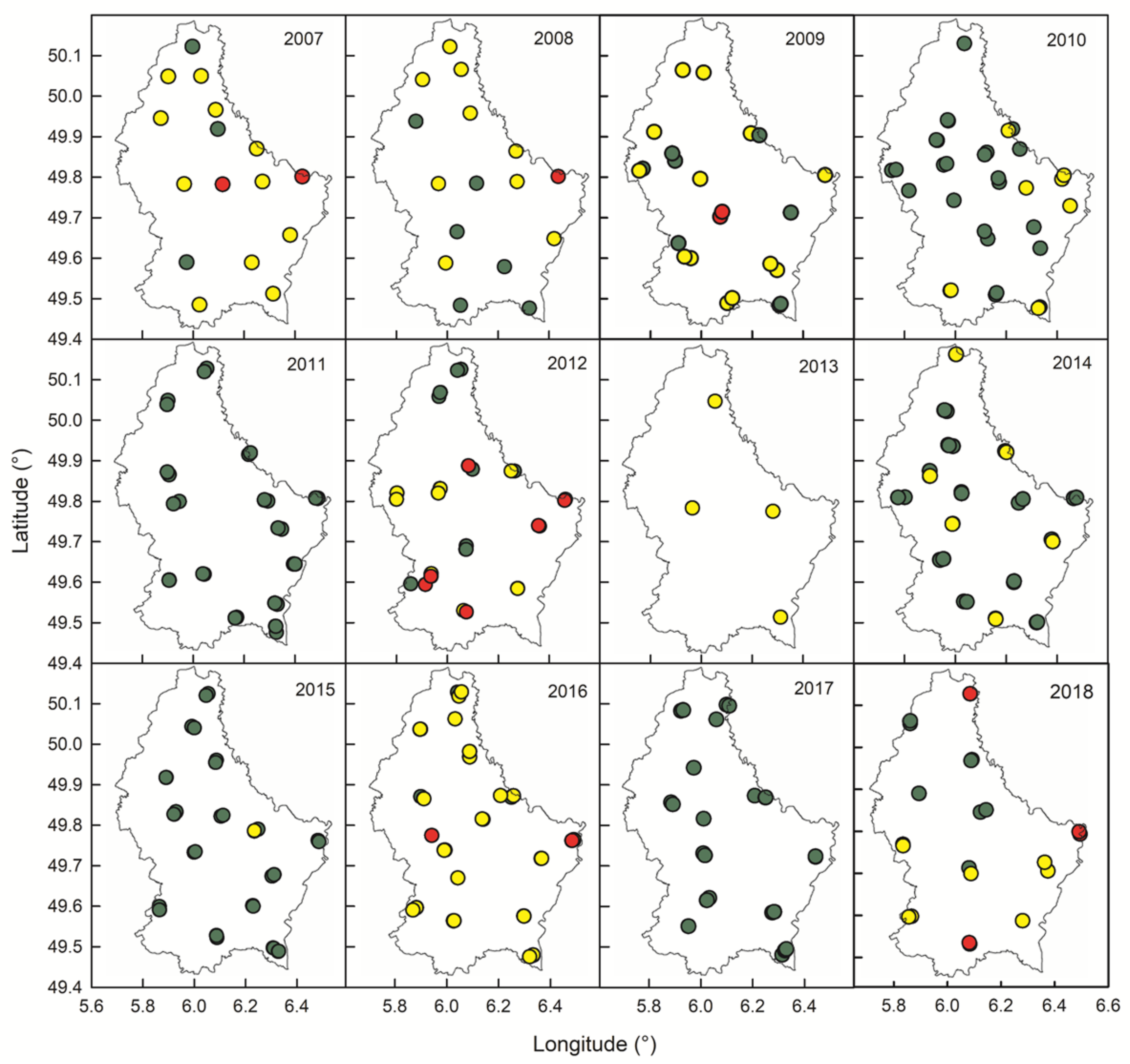

| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Numbers of locations sampled | 17 | 16 | 14 | 20 | 14 | 13 | 4 | 16 | 14 | 17 | 15 | 12 | 172 |
| Number of samples | 17 | 16 | 53 | 84 | 56 | 54 | 40 | 90 | 56 | 128 | 74 | 46 | 714 |
| Number of positive samples | 14 | 10 | 24 | 10 | 0 | 27 | 27 | 8 | 1 | 77 | 17 | 24 | 239 |
| % positive | 82 | 63 | 45 | 12 | 0 | 50 | 68 | 9 | 2 | 60 | 23 | 52 | 33 |
| Mean (µg/kg) | 704 | 813 | 230 | 109 | 76 | 1108 | 247 | 85 | 79 | 412 | 97 | 411 | |
| Median (µg/kg) | 278 | 248 | 76 | 76 | 76 | 93 | 181 | 76 | 76 | 120 | 76 | 91 | |
| Maximum (µg/kg) | 4506 | 8111 | 2092 | 845 | 76 | 9247 | 758 | 534 | 261 | 5145 | 349 | 2463 | |
| Number of samples above the MLs | 2 | 1 | 2 | 0 | 0 | 14 | 0 | 0 | 0 | 11 | 0 | 7 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallez-Barthel, M.; Cocco, E.; Vogelgsang, S.; Beyer, M. Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey. Agronomy 2021, 11, 960. https://doi.org/10.3390/agronomy11050960
Pallez-Barthel M, Cocco E, Vogelgsang S, Beyer M. Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey. Agronomy. 2021; 11(5):960. https://doi.org/10.3390/agronomy11050960
Chicago/Turabian StylePallez-Barthel, Marine, Emmanuelle Cocco, Susanne Vogelgsang, and Marco Beyer. 2021. "Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey" Agronomy 11, no. 5: 960. https://doi.org/10.3390/agronomy11050960
APA StylePallez-Barthel, M., Cocco, E., Vogelgsang, S., & Beyer, M. (2021). Frequency of Deoxynivalenol Concentrations above the Maximum Limit in Raw Winter Wheat Grain during a 12-Year Multi-Site Survey. Agronomy, 11(5), 960. https://doi.org/10.3390/agronomy11050960







