Dispensing a Synthetic Green Leaf Volatile to Two Plant Species in a Common Garden Differentially Alters Physiological Responses and Herbivory
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plants
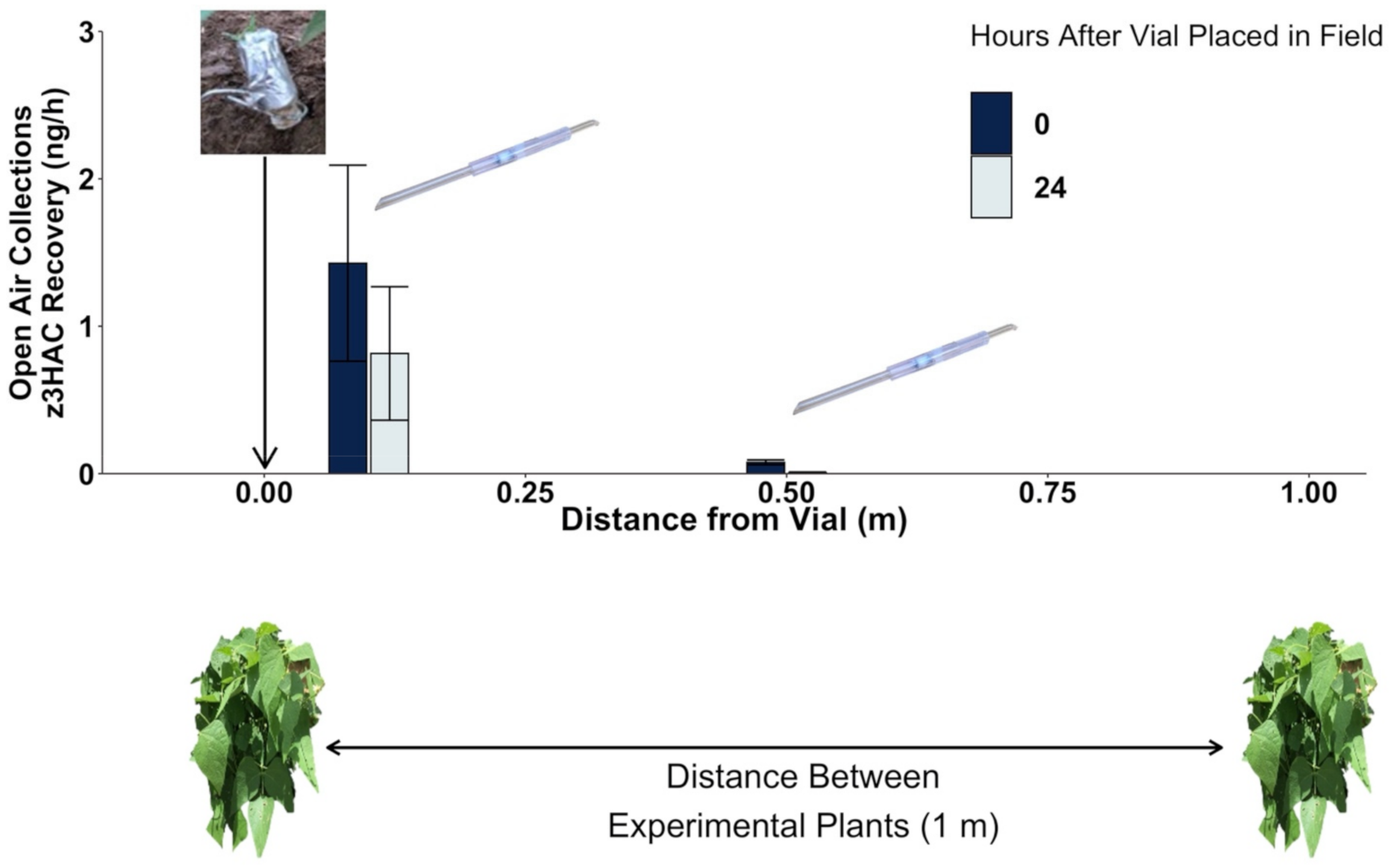
2.2. Volatile Exposure Manipulations
2.3. Growth, Biomass, and Reproduction Measurements
2.4. Herbivory
2.5. Leaf Collections and Cyanide Measurements
2.6. Statistical Analyses
3. Results
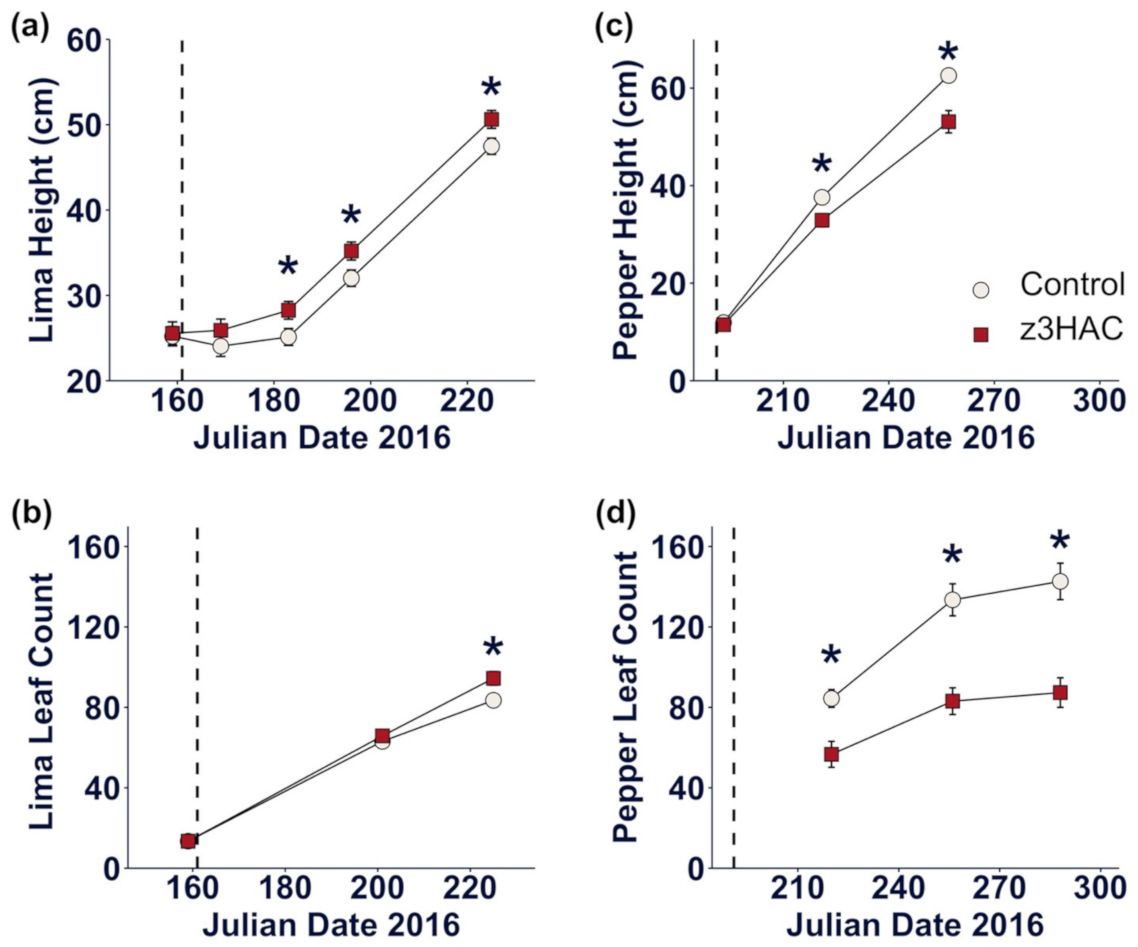
3.1. z3HAC Differentially Affects Growth of Lima Bean and Pepper Plants
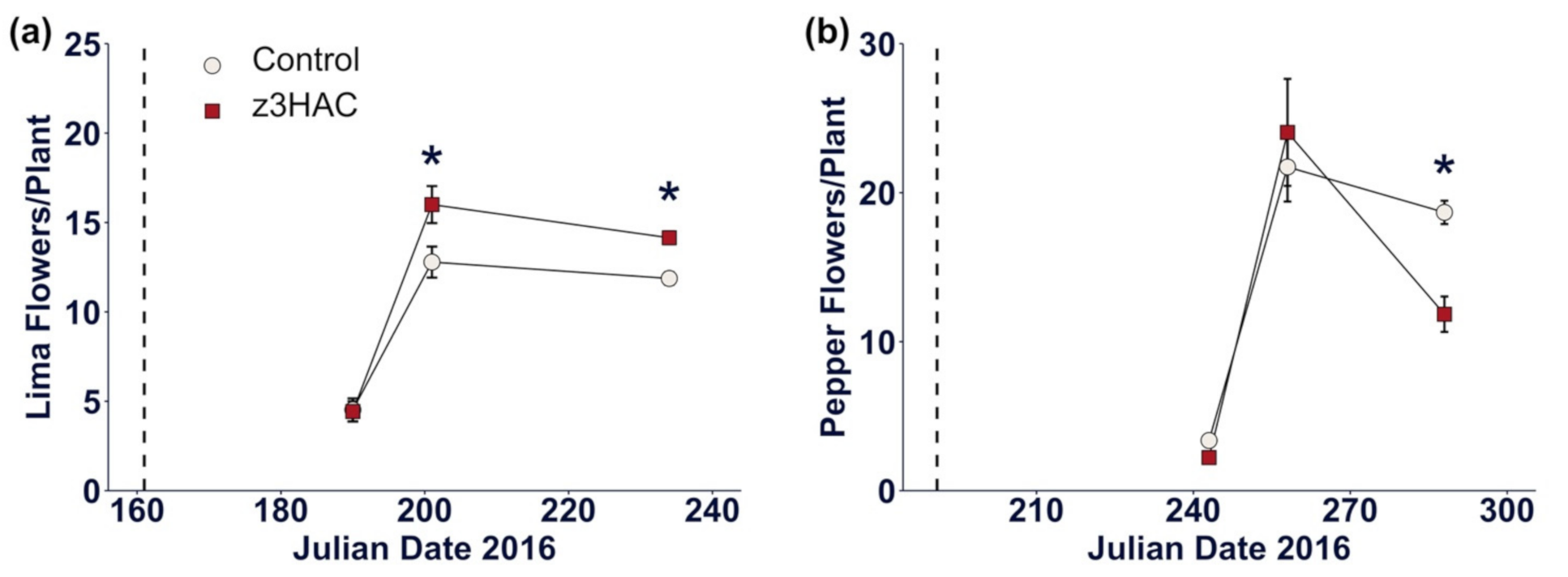
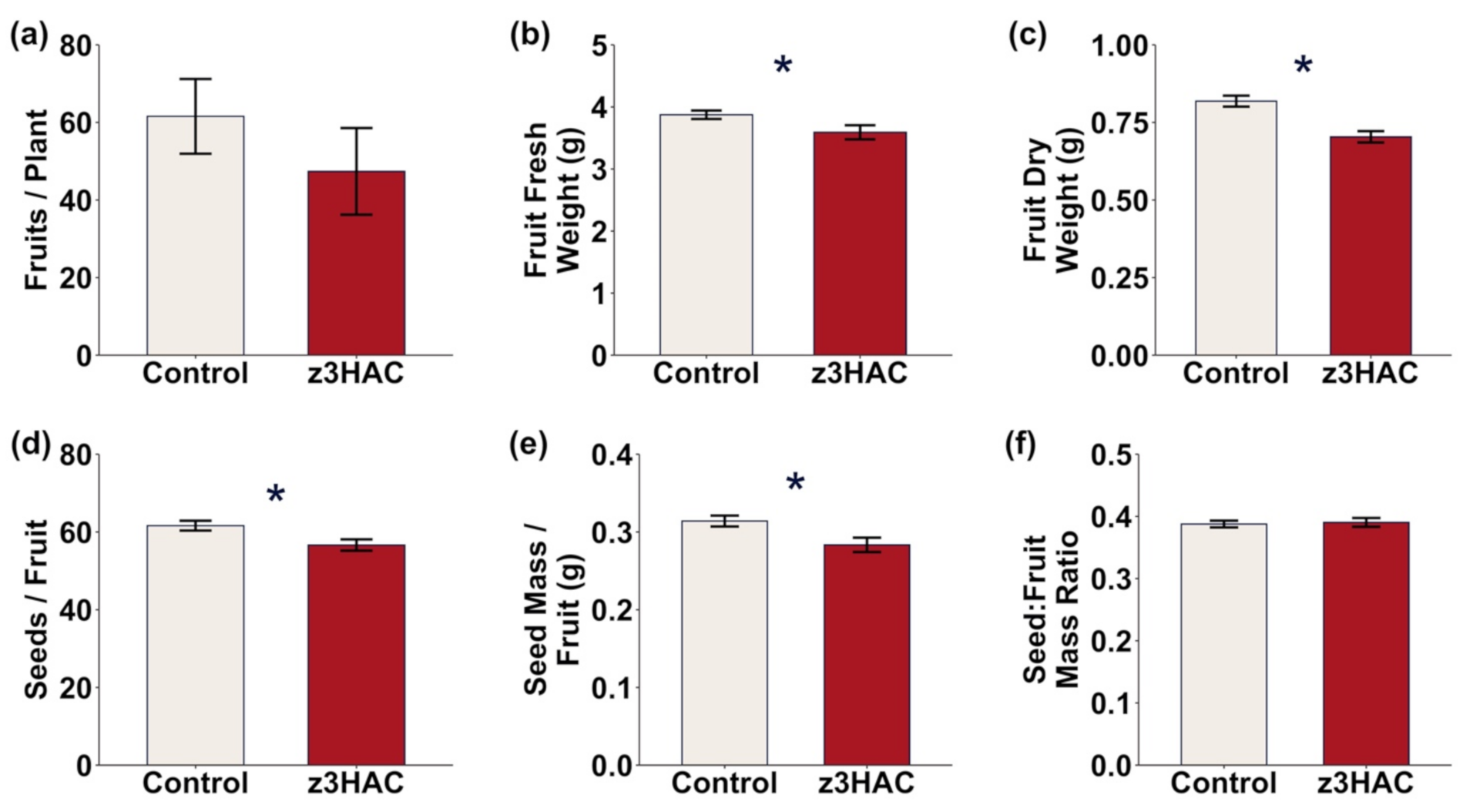
3.2. z3HAC Reduces Reproductive Output in Pepper Plants
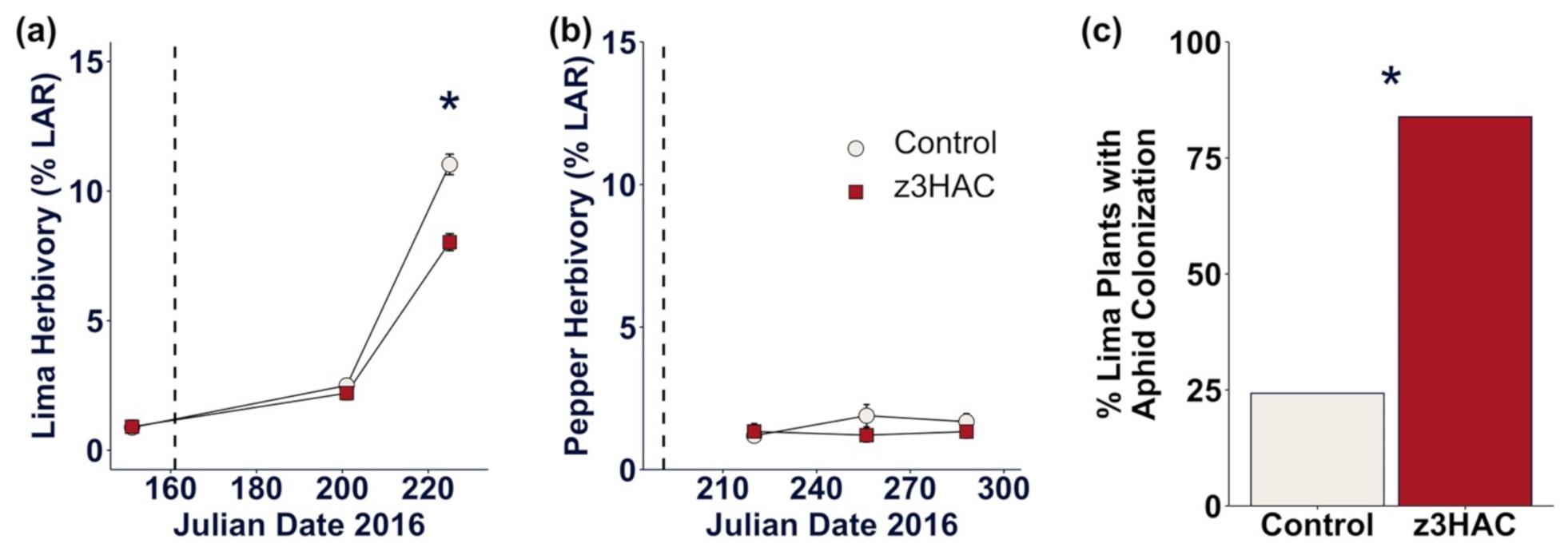
3.3. z3HAC Exposure Reduces Herbivory on Lima Bean
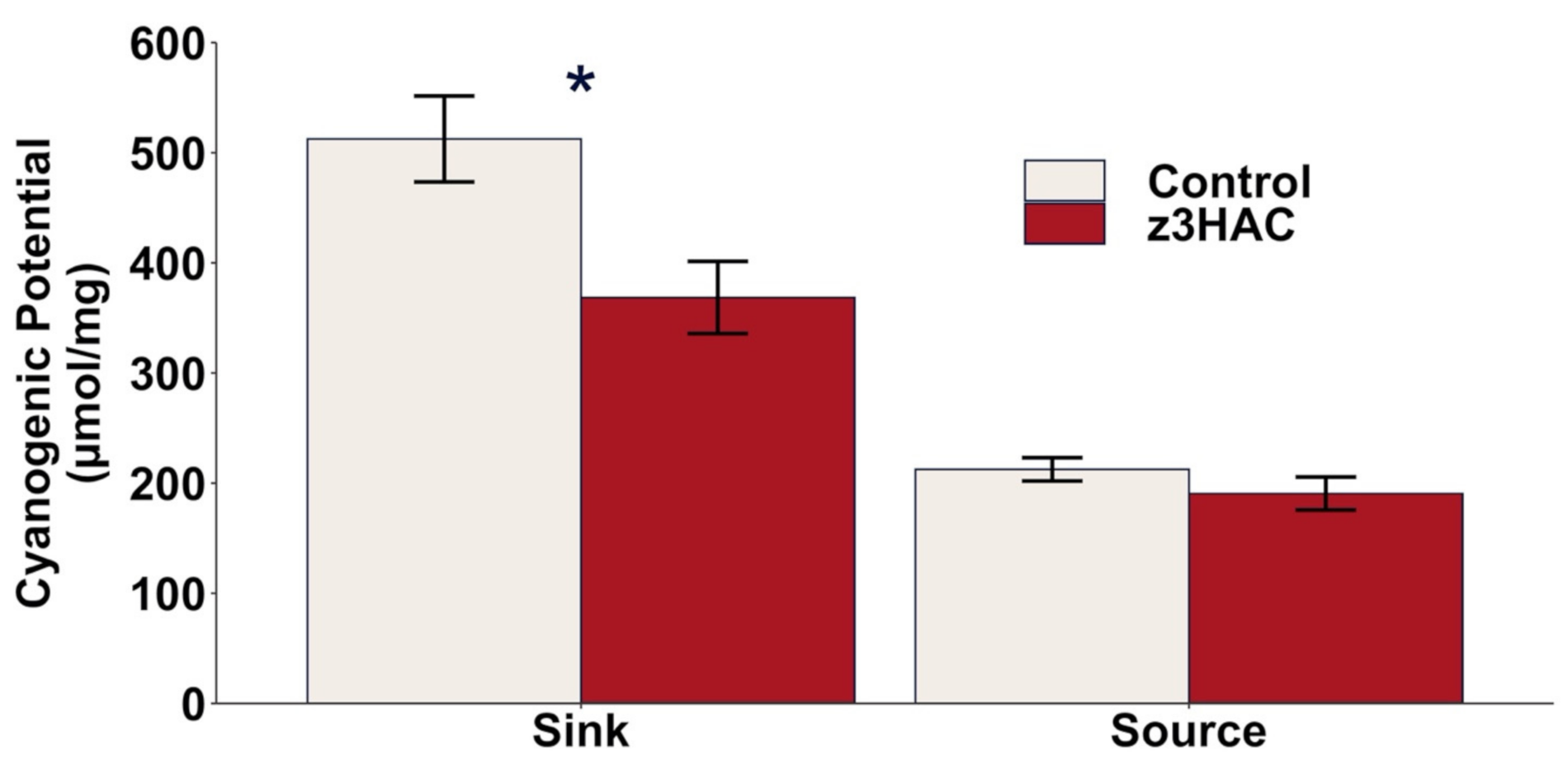
3.4. Cyanogenic Potential in Lima Bean Is Decreased by z3HAC Exposure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense: The present review is one in the special series of reviews on animal–plant. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhang, H.; Ryu, C.-M. Dynamic Chemical Communication between Plants and Bacteria through Airborne Signals: Induced Resistance by Bacterial Volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; de Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis -3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef]
- Turlings, T.C.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Frost, C.J. New evidence for a multi-functional role of herbivore-induced plant volatiles in defense against herbivores. Plant Signal. Behav. 2010, 5, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; de Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Bueno, J.C.S. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef]
- Pearse, I.S.; Hughes, K.; Shiojiri, K.; Ishizaki, S.; Karban, R. Interplant volatile signaling in willows: Revisiting the original talking trees. Oecologia 2013, 172, 869–875. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Ozawa, R.; Nishioka, T.; Boland, W.; Koch, T.; Kühnemann, F.; Takabayashi, J. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J. 2002, 29, 87–98. [Google Scholar] [CrossRef]
- Farag, M.A.; Fokar, M.; Abd, H.; Zhang, H.; Allen, R.D. (Z)-3-Hexenol induces defense genes and downstream metabolites in maize. Planta 2004, 220, 900–909. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Farag, M.A.; Paré, P.W. C6-Green leaf volatiles trigger local and systemic VOC emissions in tomato. Phytochemistry 2002, 61, 545–554. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Rodriguez-Saona, L.E.; Frost, C.J. Herbivore-Induced Volatiles in the Perennial Shrub, Vaccinium corymbosum, and Their Role in Inter-branch Signaling. J. Chem. Ecol. 2009, 35, 163–175. [Google Scholar] [CrossRef]
- Girón-Calva, P.S.; Molina-Torres, J.; Heil, M. Volatile Dose and Exposure Time Impact Perception in Neighboring Plants. J. Chem. Ecol. 2012, 38, 226–228. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Köpke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of Feeding Spodoptera littoralis on Lima Bean Leaves: IV. Diurnal and Nocturnal Damage Differentially Initiate Plant Volatile Emission. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Matsui, K.; Takabayashi, J. Chemical and Molecular Ecology of Herbivore-Induced Plant Volatiles: Proximate Factors and Their Ultimate Functions. Plant Cell Physiol. 2009, 50, 911–923. [Google Scholar] [CrossRef]
- Copolovici, L.; Kännaste, A.; Remmel, T.; Vislap, V.; Niinemets, Ü. Volatile Emissions from Alnus glutionosa Induced by Herbivory are Quantitatively Related to the Extent of Damage. J. Chem. Ecol. 2011, 37, 18–28. [Google Scholar] [CrossRef]
- Boggia, L.; Sgorbini, B.; Bertea, C.M.; Cagliero, C.; Bicchi, C.; Maffei, M.E.; Rubiolo, P. Direct Contact—Sorptive Tape Extraction coupled with Gas Chromatography—Mass Spectrometry to reveal volatile topographical dynamics of lima bean (Phaseolus lunatus L.) upon herbivory by Spodoptera littoralis Boisd. BMC Plant Biol. 2015, 15, 102. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Paschold, A.; Halitschke, R.; Baldwin, I.T. Using ‘mute’ plants to translate volatile signals. Plant J. 2005, 45, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential Metabolisms of Green Leaf Volatiles in Injured and Intact Parts of a Wounded Leaf Meet Distinct Ecophysiological Requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Balmer, A.; Gamir, J.; Flors, V.; Mauch-Mani, B. Preparing to fight back: Generation and storage of priming compounds. Front. Plant Sci. 2014, 5, 295. [Google Scholar] [CrossRef]
- D’Auria, J.C.; Pichersky, E.; Schaub, A.; Hansel, A.; Gershenzon, J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant J. 2006, 49, 194–207. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Alborn, H.T. Attraction of pepper weevil to volatiles from damaged pepper plants. Èntomol. Exp. Appl. 2010, 138, 1–11. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Tumlinson, J.H. Compatible and Incompatible Xanthomonas Infections Differentially Affect Herbivore-Induced Volatile Emission by Pepper Plants. J. Chem. Ecol. 2006, 32, 1755–1768. [Google Scholar] [CrossRef]
- McCormick, A.C.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef]
- Accamando, A.K.; Cronin, J.T. Costs and Benefits of Jasmonic Acid Induced Responses in Soybean. Environ. Èntomol. 2012, 41, 551–561. [Google Scholar] [CrossRef]
- Cipollini, D.; Purrington, C.B.; Bergelson, J. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–89. [Google Scholar] [CrossRef]
- Cipollini, D.; Heil, A.M. Costs and benefits of induced resistance to herbivores and pathogens in plants. CAB Int. 2010, 5, 1–25. [Google Scholar] [CrossRef]
- Douma, J.C.; Vermeulen, P.J.; Poelman, E.H.; Dicke, M.; Anten, N.P.R. When does it pay off to prime for defense? A modeling analysis. New Phytol. 2017, 216, 782–797. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; de Moraes, C.M. Plant Defense Priming against Herbivores: Getting Ready for a Different Battle: Figure 1. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef]
- Van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef]
- Orrock, J.L.; Connolly, B.M.; Choi, W.-G.; Guiden, P.W.; Swanson, S.J.; Gilroy, S. Plants eavesdrop on cues produced by snails and induce costly defenses that affect insect herbivores. Oecologia 2018, 186, 703–710. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.-M. Two Volatile Organic Compounds Trigger Plant Self-Defense against a Bacterial Pathogen and a Sucking Insect in Cucumber under Open Field Conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef]
- Karban, R.; Maron, J. The fitness consequences of interspecific eavesdropping between plants. Ecology 2002, 83, 1209–1213. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Schiwy, S.; Jensen, M.; Heil, M. Quantitative Variability of Direct Chemical Defense in Primary and Secondary Leaves of Lima Bean (Phaseolus lunatus) and Consequences for a Natural Herbivore. J. Chem. Ecol. 2008, 34, 1298–1301. [Google Scholar] [CrossRef]
- Zachariah, T.J.; Safeer, A.; Jayarajan, K.; Leela, N.; Vipin, T.; Saji, K.; Shiva, K.; Parthasarathy, V.; Mammootty, K. Correlation of metabolites in the leaf and berries of selected black pepper varieties. Sci. Hortic. 2010, 123, 418–422. [Google Scholar] [CrossRef]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; de Moraes, C.M. Why do distance limitations exist on plant-plant signaling via airborne volatiles? Plant Signal. Behav. 2008, 3, 466–468. [Google Scholar] [CrossRef]
- Dolch, R.; Tscharntke, T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours. Oecologia 2000, 125, 504–511. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Huntzinger, M.; McCall, A.C. Damage-induced resistance in sagebrush: Volatiles are key to intra- and interplant communication. Ecology 2006, 87, 922–930. [Google Scholar] [CrossRef]
- Heil, M.; Adame-Álvarez, R.M. Short signalling distances make plant communication a soliloquy. Biol. Lett. 2010, 6, 843–845. [Google Scholar] [CrossRef]
- Engelberth, J.; Seidl-Adams, I.; Schultz, J.C.; Tumlinson, J.H. Insect Elicitors and Exposure to Green Leafy Volatiles Differentially Upregulate Major Octadecanoids and Transcripts of 12-Oxo Phytodienoic Acid Reductases in Zea mays. Mol. Plant Microbe Interact. 2007, 20, 707–716. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Matsui, K.; Sabelis, M.W.; Takabayashi, J. Intermittent exposure to traces of green leaf volatiles triggers a plant response. Sci. Rep. 2012, 2, 378. [Google Scholar] [CrossRef]
- Von Mérey, G.; Veyrat, N.; Mahuku, G.; Valdez, R.L.; Turlings, T.C.; D’Alessandro, M. Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects. Phytochemistry 2011, 72, 1838–1847. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect Canopy Herbivory and Frass Deposition Affect Soil Nutrient Dynamics and Export in Oak Mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol. 2008, 178, 835–845. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic Glycosides: Synthesis, Physiology, and Phenotypic Plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Lieberei, R.; Ganzhorn, J.U. Plant Cyanogenesis of Phaseolus lunatus and its Relevance for Herbivore–Plant Interaction: The Importance of Quantitative Data. J. Chem. Ecol. 2005, 31, 1445–1473. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-López, S.; Villamil, N.; Zedillo-Avelleyra, P.; Boege, K. Plant defence as a complex and changing phenotype throughout ontogeny. Ann. Bot. 2015, 116, 797–806. [Google Scholar] [CrossRef]
- Schultz, J.C.; Appel, H.M.; Ferrieri, A.P.; Arnold, T.M. Flexible resource allocation during plant defense responses. Front. Plant Sci. 2013, 4, 324. [Google Scholar] [CrossRef] [PubMed]
- Brinker, A.M.; Seigler, D. Methods for the detection and quantitative determination of cyanide in plant materials. Phyto Chem. Bull. 1989, 21, 24–31. [Google Scholar]
- Gleadow, R.; Bjarnholt, N.; Jørgensen, K.; Fox, J. Cyanogenic Glycosides. In Soil Allelochemicals; Narwal, S.S., Ed.; Studium Press LLC: New Delhi, India, 2011; Volume 1, pp. 283–310. [Google Scholar]
- Agrawal, A.A. Induced Responses to Herbivory in Wild Radish: Effects on Several Herbivores and Plant Fitness. Ecology 1999, 80, 1713. [Google Scholar] [CrossRef]
- Baldwin, I.T. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proc. Natl. Acad. Sci. USA 1998, 95, 8113–8118. [Google Scholar] [CrossRef] [PubMed]
- Didiano, T.J.; Turley, N.E.; Everwand, G.; Schaefer, H.; Crawley, M.J.; Johnson, M.T.J. Experimental test of plant defence evolution in four species using long-term rabbit exclosures. J. Ecol. 2014, 102, 584–594. [Google Scholar] [CrossRef]
- Koricheva, J. Meta-Analysis of Sources of Variation in Fitness Costs of Plant Antiherbivore Defenses. Ecology 2002, 83, 176–190. [Google Scholar] [CrossRef]
- Mauricio, R. Costs of Resistance to Natural Enemies in Field Populations of the Annual Plant Arabidopsis thaliana. Am. Nat. 1998, 151, 20–28. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2015, 91, 1118–1133. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. The costs of green leaf volatile-induced defense priming: Temporal diversity in growth re-sponses to mechanical wounding and insect herbivory. Plants 2019, 8, 23. [Google Scholar] [CrossRef]
- Yip, E.C.; de Moraes, C.M.; Mescher, M.C.; Tooker, J.F. The volatile emission of a specialist herbivore alters patterns of plant defence, growth and flower production in a field population of goldenrod. Funct. Ecol. 2017, 31, 1062–1070. [Google Scholar] [CrossRef]
- Ameye, M.; Audenaert, K.; de Zutter, N.; Steppe, K.; van Meulebroek, L.; Vanhaecke, L.; de Vleesschauwer, D.; Haesaert, G.; Smagghe, G. Priming of Wheat with the Green Leaf Volatile Z-3-Hexenyl Acetate Enhances Defense against Fusarium graminearum But Boosts Deoxynivalenol Production. Plant Physiol. 2015, 167, 1671–1684. [Google Scholar] [CrossRef]
- Walters, D.R.; Paterson, L.; Walsh, D.J.; Havis, N.D. Priming for plant defense in barley provides benefits only under high disease pressure. Physiol. Mol. Plant Pathol. 2008, 73, 95–100. [Google Scholar] [CrossRef]
- Choh, Y.; Takabayashi, J. Herbivore-induced extrafloral nectar production in lima bean plants enhanced by previous exposure to volatiles from infested conspecifics. J. Chem. Ecol. 2006, 32, 2073–2077. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Peiffer, M.; Tan, C.-W.; Stanley, B.A.; Stanley, A.; Wang, J.; Jones, A.G.; Hoover, K.; Rosa, C.; Luthe, D.; et al. Fall Armyworm-Associated Gut Bacteria Modulate Plant Defense Responses. Mol. Plant Microbe Interact. 2017, 30, 127–137. [Google Scholar] [CrossRef]
- Bissmeyer, S.; Freundlich, G.; Frost, C. The influence of dose of a plant-derived volatile cue on Arabidopsis thaliana resistance against insect herbivores. Ky. J. Undergrad. Res. 2018, 2, 84–95. [Google Scholar]
- Lucas-Barbosa, D. Integrating Studies on Plant–Pollinator and Plant–Herbivore Interactions. Trends Plant Sci. 2016, 21, 125–133. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Strauss, S.Y.; Stout, M.J. Costs of Induced Responses and Tolerance to Herbivory in Male and Female Fitness Components of Wild Radish. Evolution 1999, 53, 1093–1104. [Google Scholar] [CrossRef]
- Pashalidou, F.G.; Lucas-Barbosa, D.; van Loon, J.J.A.; Dicke, M.; Fatouros, N.E. Phenotypic plasticity of plant response to herbivore eggs: Effects on resistance to caterpillars and plant development. Ecology 2013, 94, 702–713. [Google Scholar] [CrossRef]
- Agrawal, A.A. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends Plant Sci. 2000, 5, 309–313. [Google Scholar] [CrossRef]
- Heath, J.J.; Kessler, A.; Woebbe, E.; Cipollini, D.; Stireman, J.O. Exploring plant defense theory in tall goldenrod, Solidago altissima. New Phytol. 2014, 202, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Cuny, M.A.C.; Gendry, J.; Hernández-Cumplido, J.; Benrey, B. Changes in plant growth and seed production in wild lima bean in response to herbivory are attenuated by parasitoids. Oecologia 2018, 187, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Godschalx, A.L.; Stady, L.; Watzig, B.; Ballhorn, D.J. Is protection against florivory consistent with the optimal defense hypothesis? BMC Plant Biol. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Ferrieri, A.P.; Robert, C.A.M.; Glauser, G.; Kallenbach, M.; Baldwin, I.T.; Erb, M. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol. 2013, 200, 1234–1246. [Google Scholar] [CrossRef]
- Eichenberg, D.; Purschke, O.; Ristok, C.; Wessjohann, L.A.; Bruelheide, H. Trade-offs between physical and chemical carbon-based leaf defence: Of intraspecific variation and trait evolution. J. Ecol. 2015, 103, 1667–1679. [Google Scholar] [CrossRef]
- Gómez, S.; Ferrieri, R.A.; Schueller, M.; Orians, C.M. Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol. 2010, 188, 835–844. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.-M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003, 54, 1931–1939. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S.; Zhang, W.; Xu, C.; Li, B.; Zhang, D.; Mu, W.; Liu, F. Nematicidal Activity of trans-2-Hexenal against Southern Root-Knot Nematode (Meloidogyne incognita) on Tomato Plants. J. Agric. Food Chem. 2017, 65, 544–550. [Google Scholar] [CrossRef]
- Cipollini, N. Constitutive expression of methyl jasmonate-inducible responses delays reproduction and constrains fitness responses to nutrients in Arabidopsis thaliana. Evol. Ecol. 2008, 24, 59–68. [Google Scholar] [CrossRef]
- Karban, R. Tradeoff between resistance induced by volatile communication and over-topping vertical growth. Plant Signal. Behav. 2017, 12, e1309491. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Heil, M.; Lieberei, R. Phenotypic Plasticity of Cyanogenesis in Lima Bean Phaseolus lunatus—Activity and Activation of β-Glucosidase. J. Chem. Ecol. 2006, 32, 261–275. [Google Scholar] [CrossRef][Green Version]
- Kessler, A. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.; Gonçalves, F.G.; Colepicolo, C.; Sanches, P.A.; Bento, J.M.S. Effects of single and multiple herbivory by host and non-host caterpillars on the attractiveness of herbivore-induced volatiles of sugarcane to the generalist parasitoid Cotesia flavipes. Èntomol. Exp. Appl. 2017, 165, 83–93. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Heil, M.; Åhman, I.; Björkman, C. Optimizing Crops for Biocontrol of Pests and Disease. Trends Plant Sci. 2015, 20, 698–712. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.V.; Bento, J.M.S. Herbivore-Induced Plant Volatiles to Enhance Biological Control in Agriculture. Neotrop. Èntomol. 2013, 42, 331–343. [Google Scholar] [CrossRef]
- Lucchi, A.; Loni, A.; Gandini, L.M.; Scaramozzino, P.; Ioriatti, C.; Ricciardi, R.; Shearer, P.W. Using herbivore-induced plant volatiles to attract lacewings, hoverflies and parasitoid wasps in vineyards: Achievements and constraints. Bull. Insectol. 2017, 70, 273–282. [Google Scholar]
- Webster, B.; Bruce, T.; Dufour, S.; Birkemeyer, C.; Birkett, M.; Hardie, J.; Pickett, J. Identification of Volatile Compounds Used in Host Location by the Black Bean Aphid, Aphis fabae. J. Chem. Ecol. 2008, 34, 1153–1161. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Wei, J.; van Loon, J.J.A.; Gols, R.; Menzel, T.R.; Li, N.; Kang, L.; Dicke, M. Reciprocal crosstalk between jasmonate and salicylate defence-signalling pathways modulates plant volatile emission and herbivore host-selection behaviour. J. Exp. Bot. 2014, 65, 3289–3298. [Google Scholar] [CrossRef]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 220, 666–683. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Caparrotta, S.; Boni, S.; Taiti, C.; Palm, E.; Mancuso, S.; Pandolfi, C. Induction of priming by salt stress in neighboring plants. Environ. Exp. Bot. 2018, 147, 261–270. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Muroi, A.; Nishihara, M. Plant–plant–plant communications, mediated by (E)-β-ocimene emitted from transgenic tobacco plants, prime indirect defense responses of lima beans. J. Plant Interact. 2012, 7, 193–196. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freundlich, G.E.; Shields, M.; Frost, C.J. Dispensing a Synthetic Green Leaf Volatile to Two Plant Species in a Common Garden Differentially Alters Physiological Responses and Herbivory. Agronomy 2021, 11, 958. https://doi.org/10.3390/agronomy11050958
Freundlich GE, Shields M, Frost CJ. Dispensing a Synthetic Green Leaf Volatile to Two Plant Species in a Common Garden Differentially Alters Physiological Responses and Herbivory. Agronomy. 2021; 11(5):958. https://doi.org/10.3390/agronomy11050958
Chicago/Turabian StyleFreundlich, Grace E., Maria Shields, and Christopher J. Frost. 2021. "Dispensing a Synthetic Green Leaf Volatile to Two Plant Species in a Common Garden Differentially Alters Physiological Responses and Herbivory" Agronomy 11, no. 5: 958. https://doi.org/10.3390/agronomy11050958
APA StyleFreundlich, G. E., Shields, M., & Frost, C. J. (2021). Dispensing a Synthetic Green Leaf Volatile to Two Plant Species in a Common Garden Differentially Alters Physiological Responses and Herbivory. Agronomy, 11(5), 958. https://doi.org/10.3390/agronomy11050958




