Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Ethylene Production, Respiration Rate, and Quality Parameters
2.3. Total and Individual Phenolic Quantification
2.4. Statistical Analysis
3. Results
3.1. Fruit Weight and Crop Yield
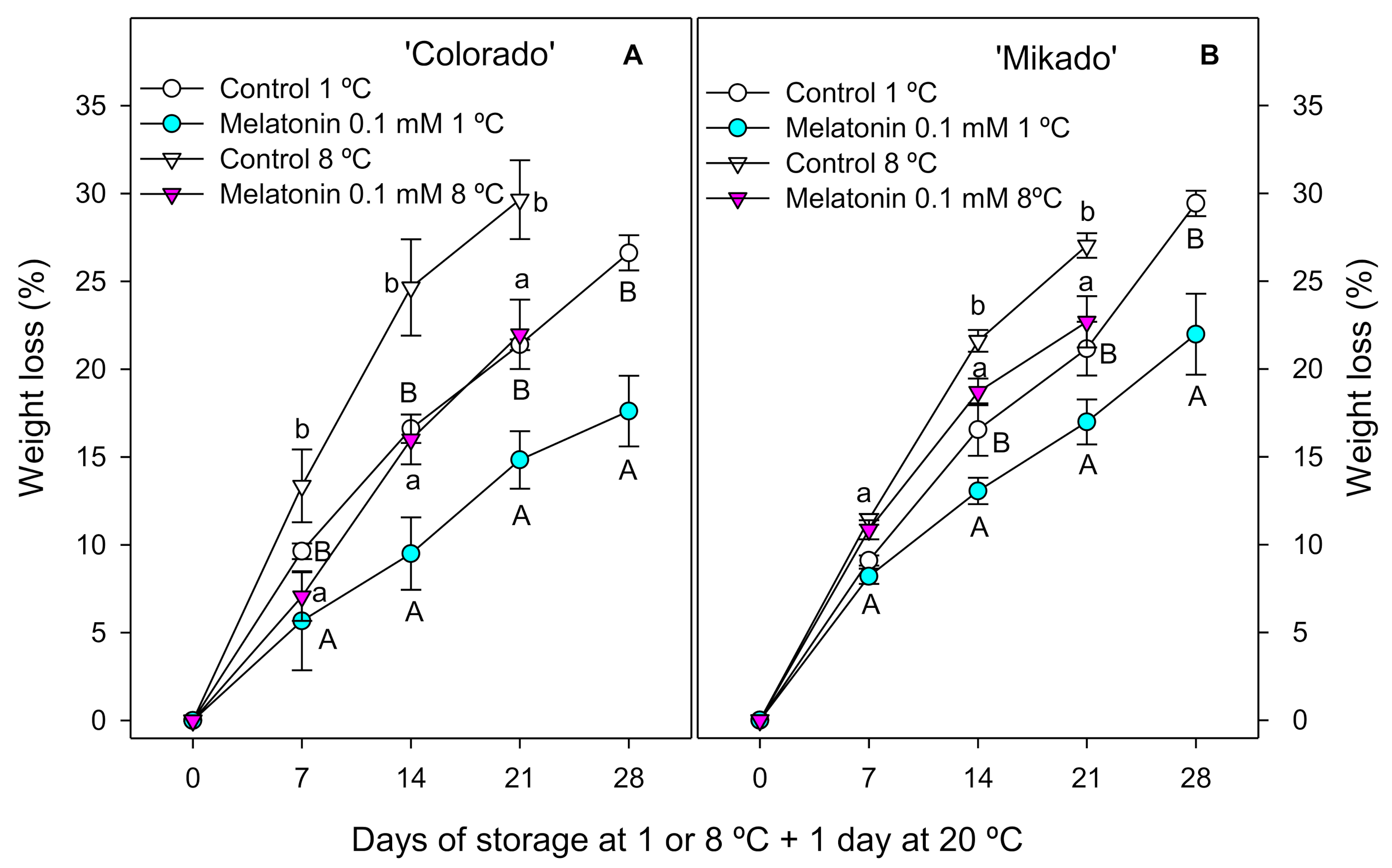
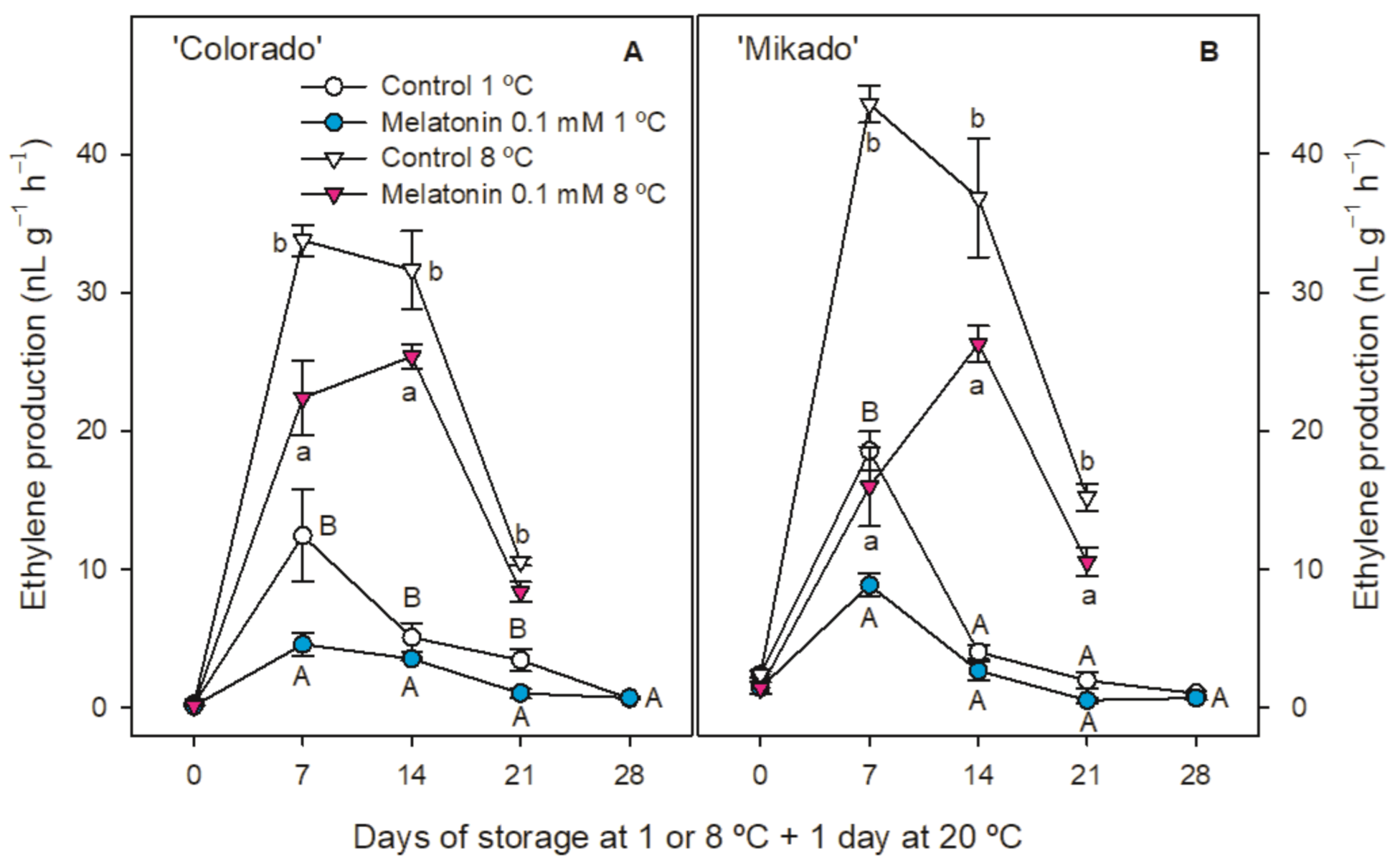
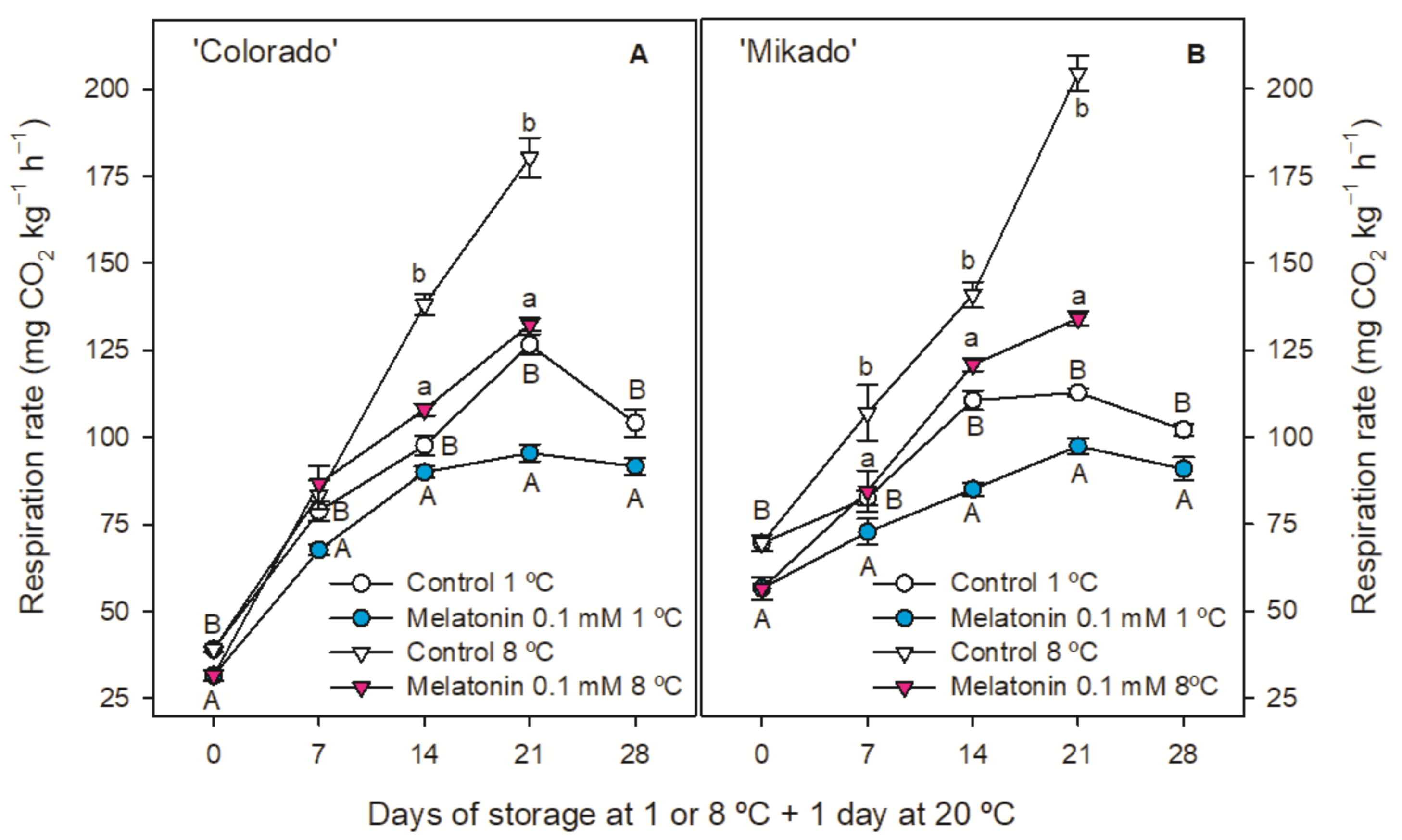
3.2. Weight Loss, Ethylene Production and Respiration Rate
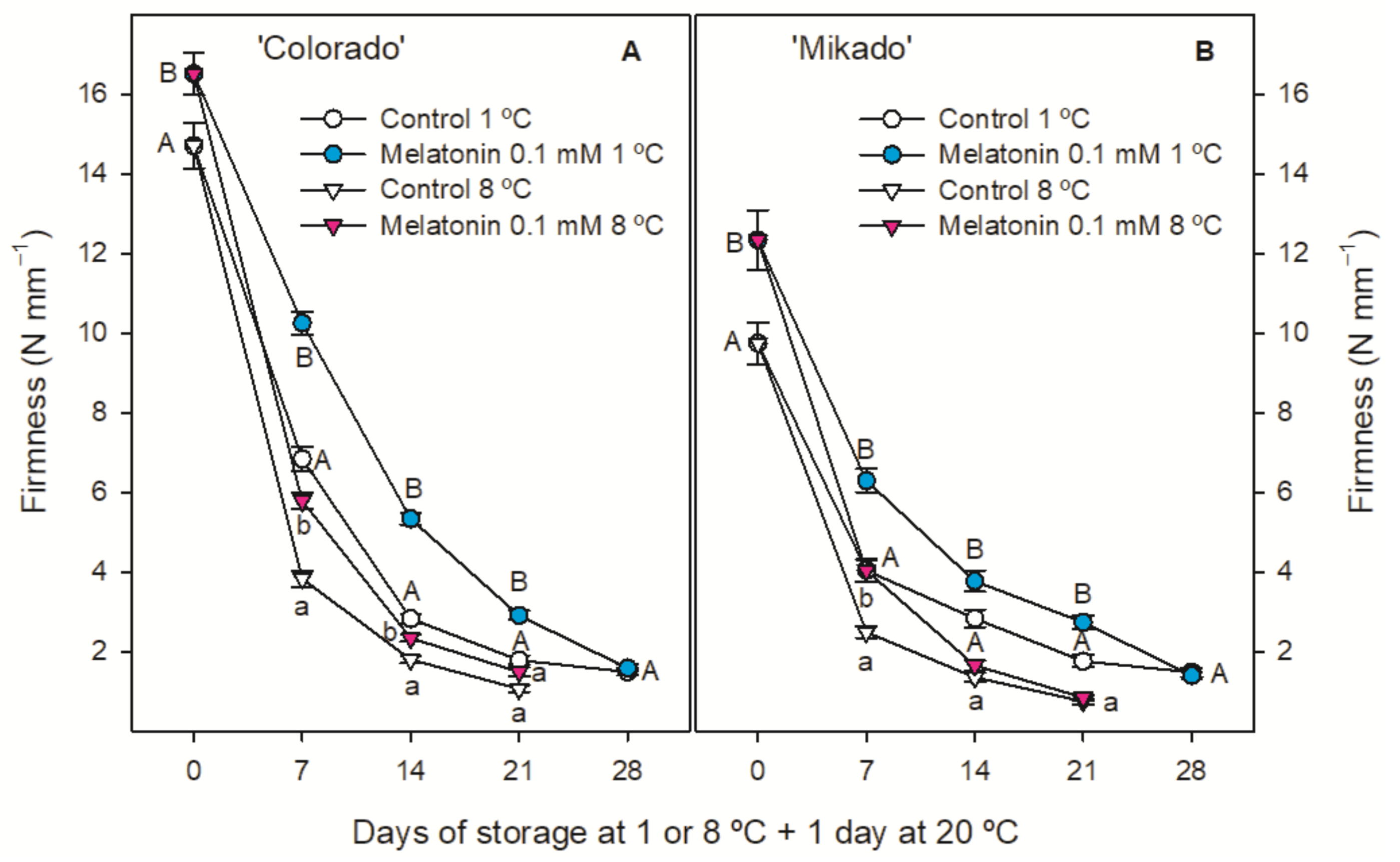
3.3. Quality Parameters
3.4. Individual and Total Phenolic Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Fan, X.; Xi, Y.; Zhao, H.; Liu, B.; Cao, J.; Jiang, W. Improving fresh apricot (Prunus armeniaca L.) quality and antioxidant capacity by storage at near freezing temperature. Sci. Hortic. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Stanley, J.; Prakash, R.; Marshall, R.; Schröder, R. Effect of harvest maturity and cold storage on correlations between fruit properties during ripening of apricot (Prunus armeniaca). Postharvest Biol. Technol. 2013, 82, 39–50. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Pretel, M.T.; Serrano, M.; Amorós, A.; Romojaro, F. Ripening and ethylene biosynthesis in controlled atmosphere stored apricots. Eur. Food Res. Technol. 1999, 209, 130–134. [Google Scholar] [CrossRef]
- Egea, I.; Flores, F.B.; Martínez-Madrid, M.C.; Romojaro, F.; Sánchez-Bel, P. 1-Methylcyclopropene affects the antioxidant system of apricots (Prunus armeniaca L. cv. Búlida) during storage at low temperature. J. Sci. Food Agric. 2010, 90, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehleers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef]
- Shi, H.; Chen, K.; Wei, Y.; He, C. Fundamental issues of melatonin-mediated stress signaling in plants. Front. Plant Sci. 2016, 7, 1124. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Is Phytomelatonin a New Plant Hormone? Agronomy 2020, 10, 95. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin is a potential target for improving post-harvest preservation of fruits and vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.X.; et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bal, E. Effect of melatonin treatments on biochemical quality and postharvest life of nectarines. J. Food Meas. Charact. 2021, 15, 288–295. [Google Scholar] [CrossRef]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Abd El-Naby, S.K.M.A.; Mohamed, A.A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci. Pol. Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Giménez, M.J.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest application of oxalic acid improved pomegranate fruit yield, quality, and bioactive compounds at harvest in a concentration-dependent manner. Agronomy 2020, 10, 1522. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Li, M.; Lu, X.; Sun, Y.; Qiu, D. Exogenous melatonin improves fruit quality features, health promoting antioxidant compounds and yield traits in tomato fruits under acid rain stress. Molecules 2018, 23, 1868. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; Abd Elbar, O.H.; Farag, R.; Hikal, M.; El-Kelish, A.; El-Yazied, A.A.; Alkahtani, J.; Abd El-Gawad, H.G. Melatonin counteracts drought induced oxidative damage and stimulates growth, productivity and fruit quality properties of tomato plants. Plants 2020, 9, 1276. [Google Scholar] [CrossRef]
- Egea, M.I.; Sánchez-Bel, P.; Martínez-Madrid, M.C.; Flores, F.B.; Romojaro, F. The effect of beta ionization on the antioxidant potential of “Búlida” apricot and its relationship with quality. Postharvest Biol. Technol. 2007, 46, 63–70. [Google Scholar] [CrossRef]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, W.; Gong, H.; Yang, Y.; Zhang, A.; Liu, H.; Guo, F.; Cui, K. Cell wall polysaccharides degradation and ultrastructure modification of apricot during storage at a near freezing temperature. Food Chem. 2019, 300, 125194. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean diet and inflammatory markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Tarangon, E.; Moreno, J.J. Polyphenols and taste 2 receptors. Physiological, pathophysiological and pharmacological implications. Biochem. Pharmacol. 2020, 78, 114086. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Gil, M.; Tomas-Barberan, F.A. Characterization and quantification of phenolic compounds in new apricot. (Prunus armeniaca L.) varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical characters and antioxidant, sugar and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the effect of maturity at harvest on the phenolic and carotenoid content of northeast USA apricot (Prunus armeniaca) varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef]
- Kan, T.; Gundogdu, M.; Ercisli, S.; Muradoglu, F.; Celik, F.; Gecer, M.K.; Kodad, O.; Zia-Ul-Haq, M. Phenolic compounds and vitamins in wild and cultivated apricot (Prunus armeniaca L.) fruits grown in irrigated and dry farming conditions. Biol. Res. 2014, 47, 46. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Arzani, K.; Barzegar, M. Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef] [PubMed]
- Jannatizadeh, A. Exogenous melatonin applying confers chilling tolerance in pomegranate fruit during cold storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Jiao, J.; Guo, L.; Liu, H.; Wang, Y.; He, Y.; Jin, M.; Rao, J. Effect of different packaging film thicknesses on chilling injury in postharvest ‘Cuixiang’ Kiwifruit. N. Z. J. Crop Hortic. Sci. 2020, in press. [Google Scholar] [CrossRef]
- Yu, W.; Sheng, J.; Zhao, R.; Wang, Q.; Ma, P.; Shen, L. Ethylene biosynthesis is involved in regulating chilling tolerance and SlCBF1 gene expression in tomato fruit. Postharvest Biol. Technol. 2019, 149, 139–147. [Google Scholar] [CrossRef]
- Kashash, Y.; Holland, D.; Porat, R. Molecular mechanisms involved in postharvest chilling tolerance of pomegranate fruit. J. Sci. Food Agric. 2019, 99, 5617–5623. [Google Scholar] [CrossRef]
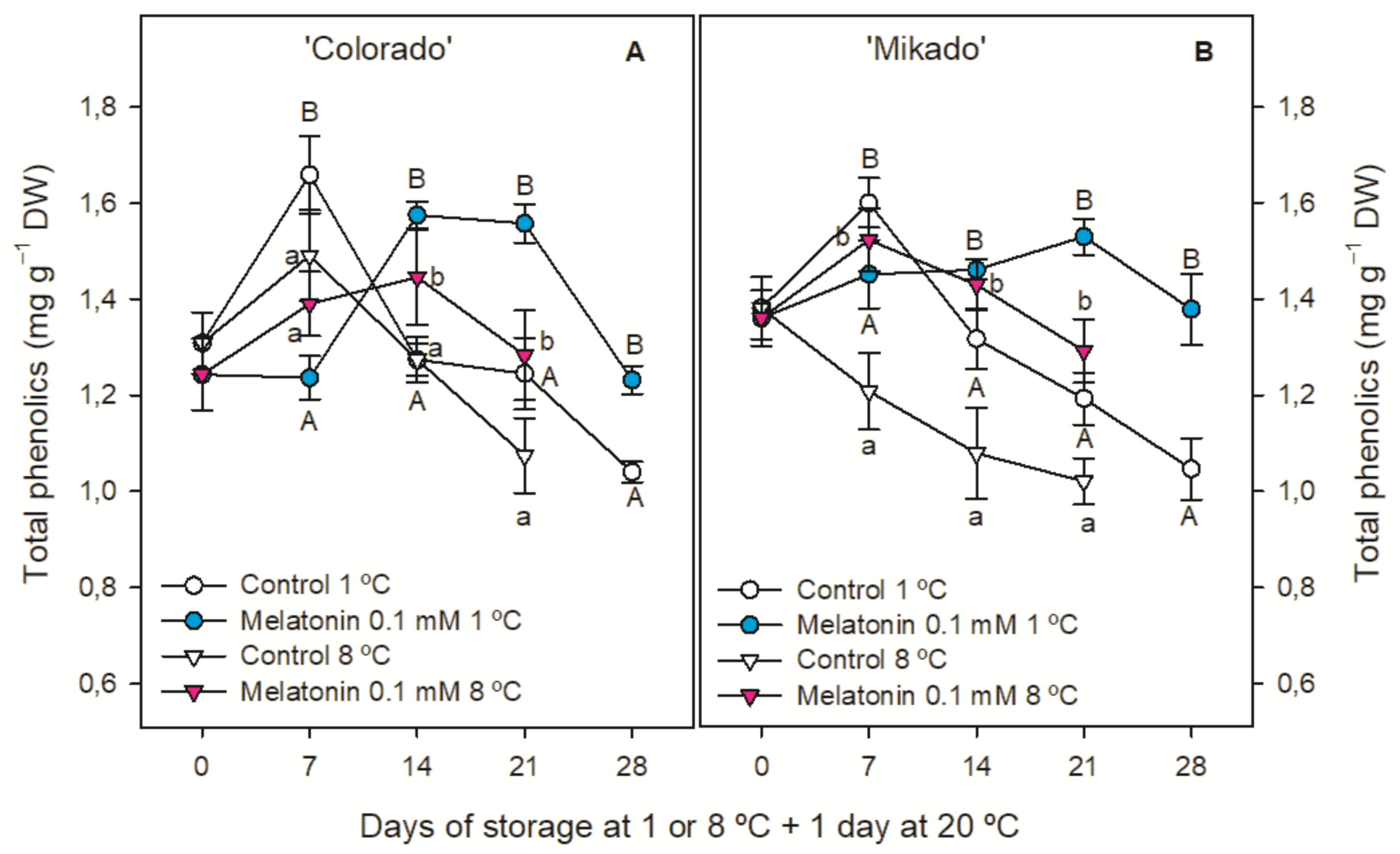
| ‘Colorado’ | ‘Mikado’ | ||||
|---|---|---|---|---|---|
| Days | Control | Melatonin | Control | Melatonin | |
| Yield | 26.25 ± 0.71 a | 28.35 ± 0.75 b | 19.65 ± 0.85 a | 21.42 ± 0.83 b | |
| FW | 0 | 54.77 ± 1.75 a | 59.46 ± 1.08 b | 59.84 ± 1.11 a | 67.38 ± 1.54 b |
| Colour a* | 0 | 36.35 ± 0.99 aA | 37.22 ± 0.48 aA | 27.53 ± 1.02 aA | 26.24 ± 1.08 aA |
| 21 at 1 °C | 40.73 ± 1.34 aB | 42.81 ± 0.60 aB | 34.74 ± 1.06 aB | 34.62 ± 0.59 aB | |
| 21 at 8 °C | 44.84 ± 0.49 aC | 45.35 ± 0.41 aC | 36.4 ± 1.12 aB | 35.68 ± 0.93 aB | |
| Colour b* | 0 | 61.01 ± 1.10 aB | 60.72 ± 0.56 aA | 61.91 ± 1.27 aA | 64.32 ± 1.38 aB |
| 21 at 1 °C | 62.8 ± 0.72 aB | 62.37 ± 0.40 aA | 62.97 ± 1.22 aA | 59.01 ± 1.02 aA | |
| 21 at 8 °C | 58.95 ± 0.60 aA | 60.06 ± 1.03 aA | 59.52 ± 1.79 aA | 61.68 ± 1.45 aA | |
| Colour L* | 0 | 61.02 ± 1.01 aC | 60.46 ± 0.63 aC | 61.71 ± 0.98 aC | 63.61 ± 1.14 aB |
| 21 at 1 °C | 58.84 ± 0.58 aB | 58.18 ± 0.49 aB | 58.69 ± 1.09 aB | 54.77 ± 1.06 aA | |
| 21 at 8 °C | 52.71 ± 0.55 aA | 53.88 ± 0.97 aA | 53.95 ± 1.56 aA | 56.09 ± 1.35 aA | |
| ‘Colorado’ | ‘Mikado’ | ||||
|---|---|---|---|---|---|
| Control | Melatonin | Control | Melatonin | ||
| TSS | Day 0 | 10.88 ± 0.21 aA | 10.25 ± 0.31 aA | 9.02 ± 0.25 aA | 9.23 ± 0.08 aA |
| 21 d 1 °C + 1 d 20 °C | 13.88 ± 0.26 aB | 12.60 ± 0.15 bB | 10.15 ± 0.14 aB | 9.92 ± 0.12 bB | |
| 21 d 8 °C + 1 d 20 °C | 14.53 ± 0.19 aC | 13.30 ± 0.26 bC | 11.47 ± 0.18 aC | 10.55 ± 0.04 bC | |
| TA | Day 0 | 2.55 ± 0.03 aC | 2.66 ± 0.04 aB | 1.83 ± 0.05 aC | 1.78 ± 0.07 aB |
| 21 d 1 °C + 1 d 20 °C | 2.32 ± 0.04 aB | 2.69 ± 0.06 bB | 1.65 ± 0.04 aB | 1.82 ± 0.06 bB | |
| 21 d 8 °C + 1 d 20 °C | 1.70 ± 0.06 aA | 2.13 ± 0.02 bA | 1.32 ± 0.11 aA | 1.68 ± 0.05 bA | |
| Control | Melatonin | |
|---|---|---|
| Colorado | 2.42 ± 0.09 b | 1.88 ± 0.08 a |
| Mikado | 2.83 ± 0.11 b | 1.63 ± 0.08 a |
| Scale for chilling injury damage | ||
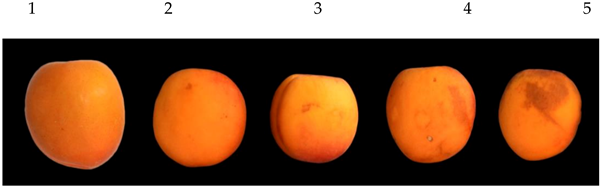 | ||
| ‘Colorado’ | ‘Mikado’ | |||
|---|---|---|---|---|
| Control | Melatonin | Control | Melatonin | |
| Neochlorogenic acid | 3.60 ± 0.28 a | 2.52 ± 0.16 a | 2.10 ± 0.06 a | 2.16 ± 0.18 a |
| Chlorogenic acid | 15.78 ± 1.08 a | 14.73 ± 0.46 a | 12.25 ± 0.21 a | 12.61 ± 0.60 a |
| Rutin | 0.22 ± 0.01 a | 0.21 ± 0.03 a | 0.08 ± 0.01 a | 0.12 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures. Agronomy 2021, 11, 917. https://doi.org/10.3390/agronomy11050917
Medina-Santamarina J, Zapata PJ, Valverde JM, Valero D, Serrano M, Guillén F. Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures. Agronomy. 2021; 11(5):917. https://doi.org/10.3390/agronomy11050917
Chicago/Turabian StyleMedina-Santamarina, Jorge, Pedro Javier Zapata, Juan Miguel Valverde, Daniel Valero, María Serrano, and Fabián Guillén. 2021. "Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures" Agronomy 11, no. 5: 917. https://doi.org/10.3390/agronomy11050917
APA StyleMedina-Santamarina, J., Zapata, P. J., Valverde, J. M., Valero, D., Serrano, M., & Guillén, F. (2021). Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures. Agronomy, 11(5), 917. https://doi.org/10.3390/agronomy11050917











