Herbicides Efficacy against Volunteer Oilseed Rape as Influenced by Spray Solution pH
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Efficacy of Herbicides Based on Visual Assessment and Fresh Weight Reduction
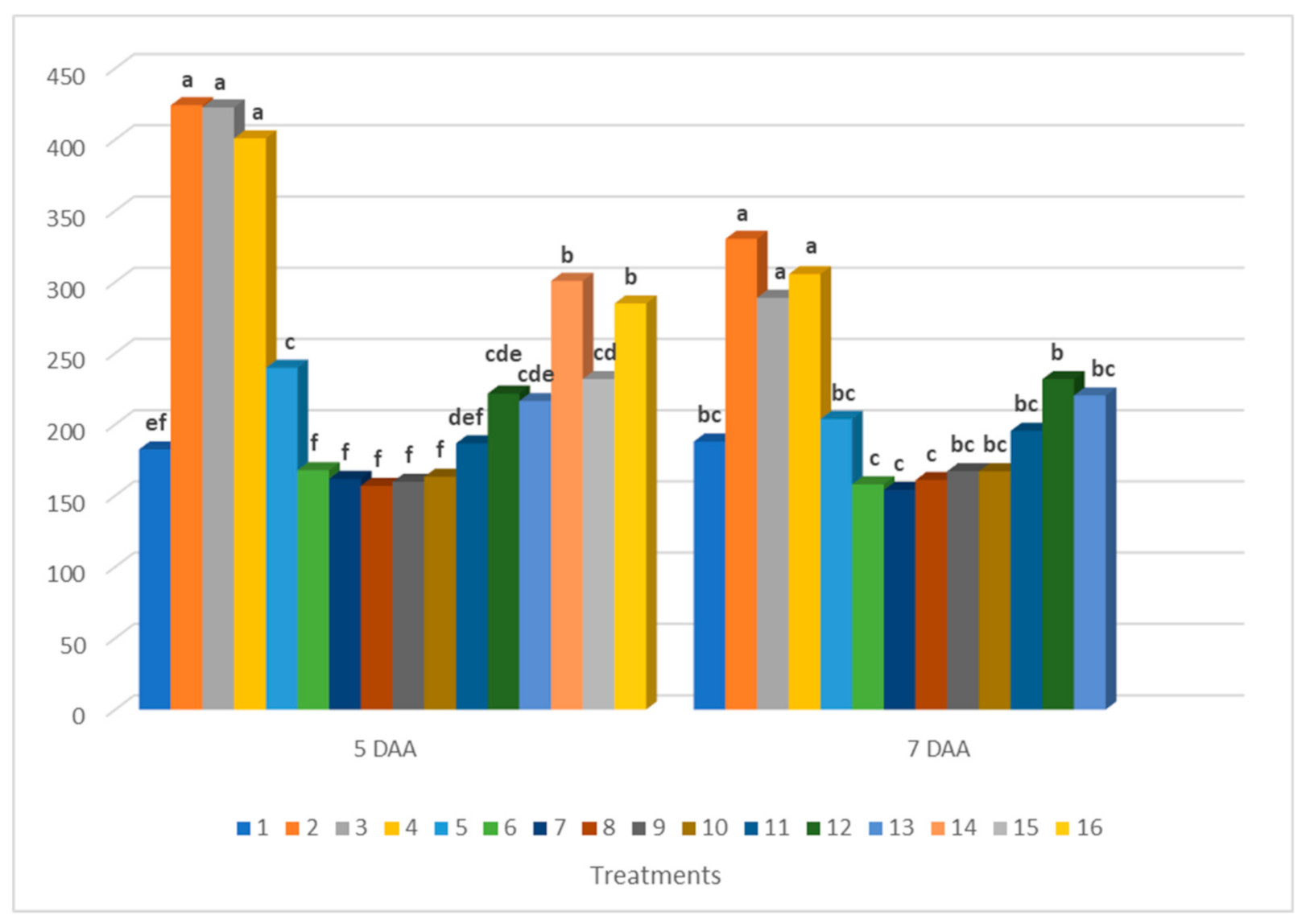
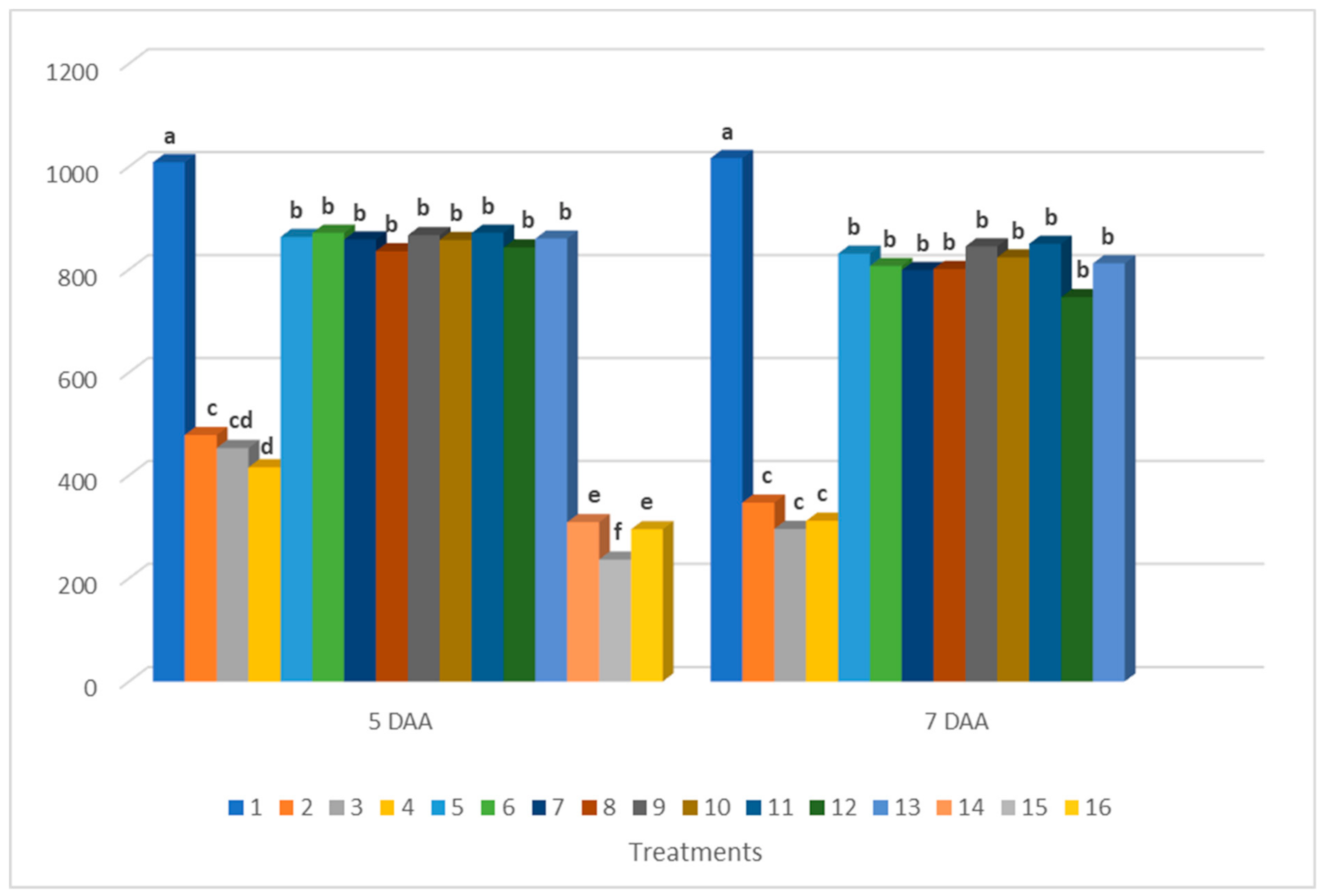
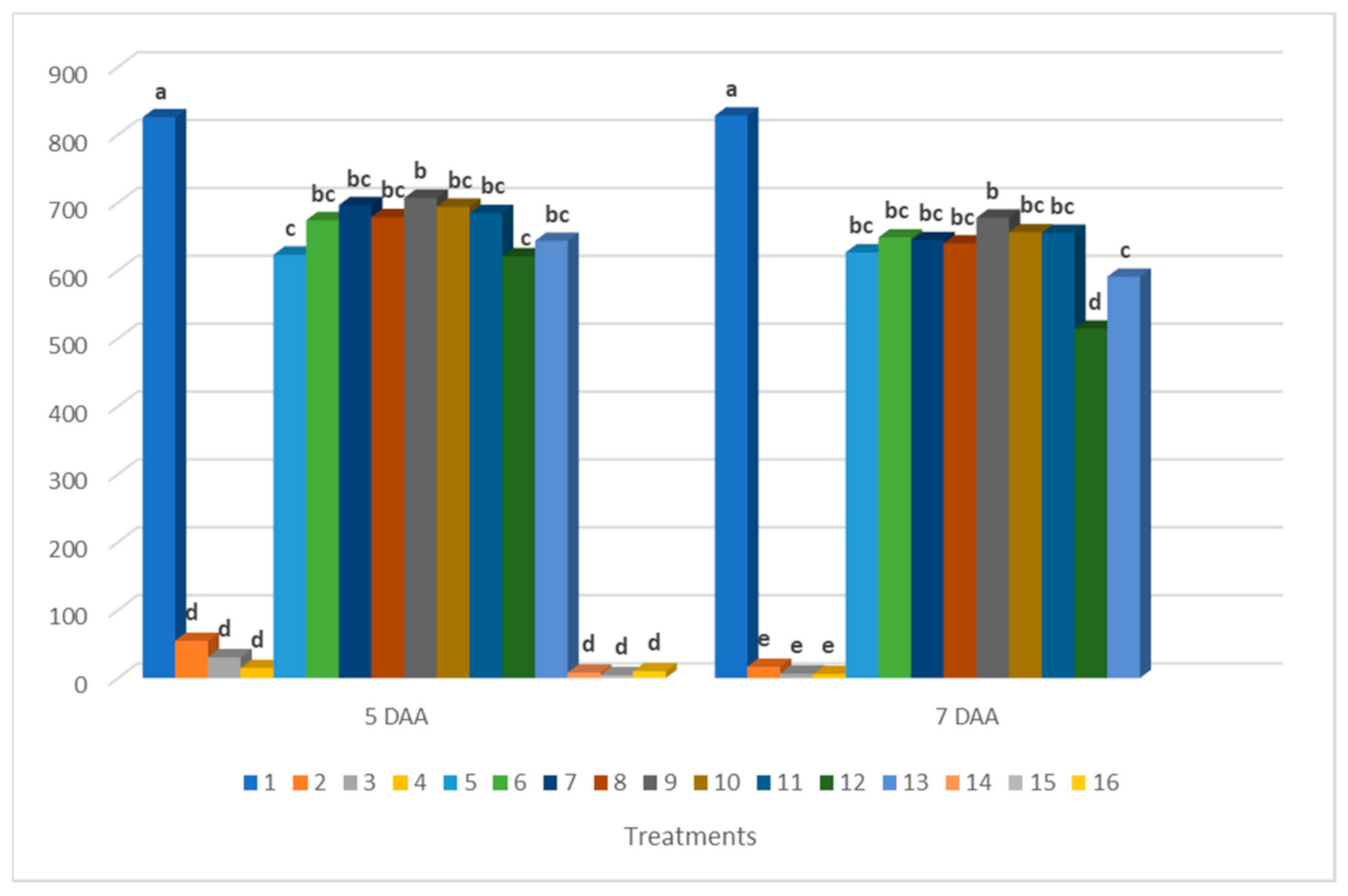
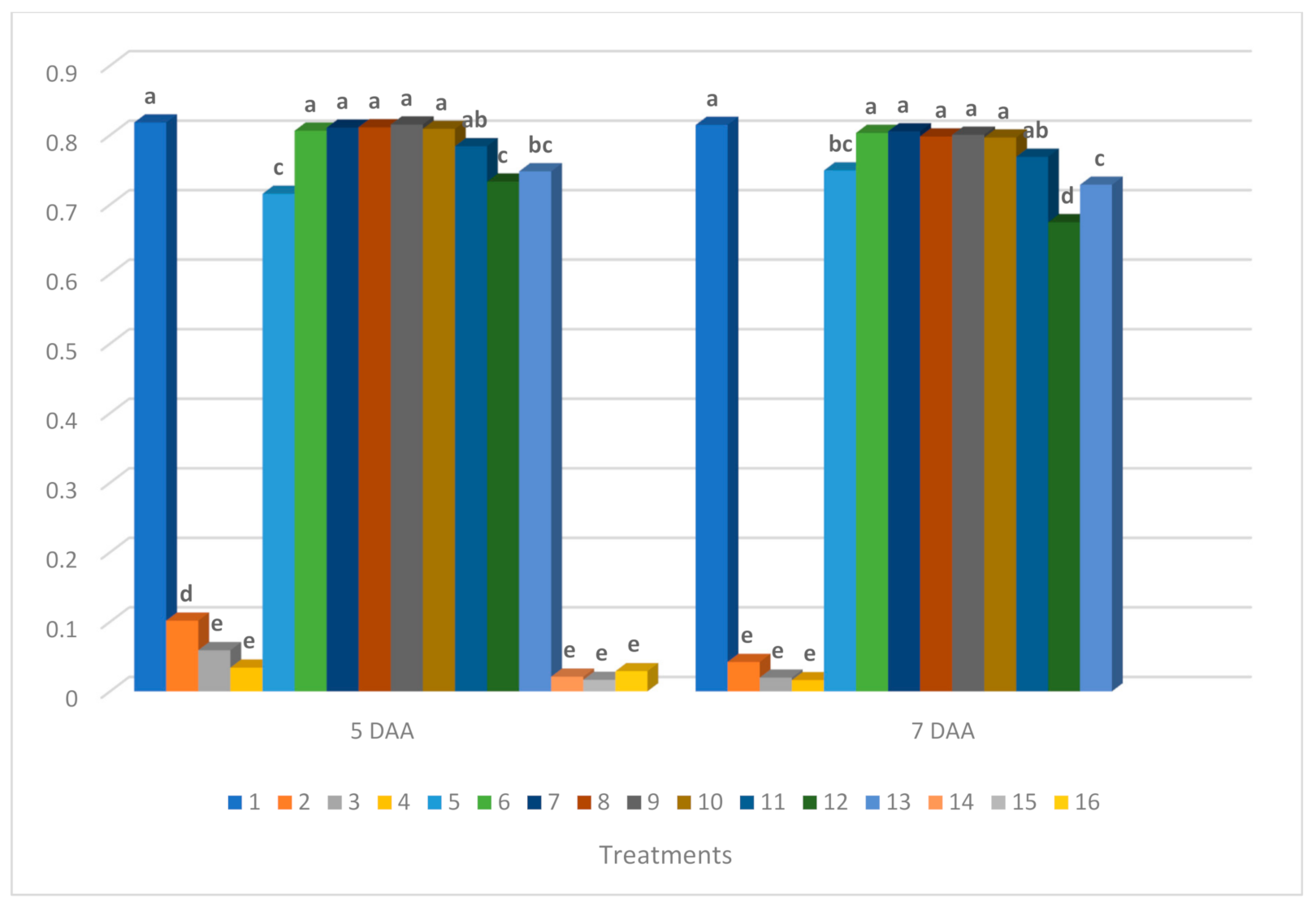
3.2. Results of Chlorophyll Fluorescence Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudsk, P. Optimising herbicide dose: A straightforward approach to reduce the risk of side effects of herbicides. Environmentalist 2008, 28, 49–55. [Google Scholar] [CrossRef]
- Matocha, M.A.; Krutz, L.J.; Senseman, S.A.; Koger, C.H.; Reddy, K.N.; Palmer, E.W. Spray carrier pH effect on absorption and translocation of trifloxysulfuron in Palmer amaranth (Amaranthus palmeri) and Texasweed (Caperonia palustris). Weed Sci. 2006, 54, 969–973. [Google Scholar] [CrossRef]
- Scrano, L.; Bufo, S.A.; Perucci, P.; Meallier, P.; Mansour, M. Photolysis and hydrolysis of rimsulfuron. Pestic. Sci. 1999, 55, 955–961. [Google Scholar] [CrossRef]
- McMullan, P.M. Utility Adjuvants. Weed Technol. 2000, 14, 792–797. [Google Scholar] [CrossRef]
- Tian, C.; Zhou, X.; Liu, Q.; Peng, J.; Zhang, Z.; Song, H.; Ding, Z.; Zhran, M.A.; Eissa, M.A.; Kheir, A.M.S.; et al. Increasing yield, quality and profitability of winter oilseed rape (Brassica napus) under combinations of nutrient levels in fertiliser and planting density. Crop. Pasture Sci. 2020, 71, 1010–1019. [Google Scholar] [CrossRef]
- Krato, C.H.; Petersen, J. Competitiveness and yield impact of volunteer oilseed rape (Brassica napus) in winter and spring wheat (Triticum aestivum). J. Plant Dis. Protect. 2012, 119, 74–82. [Google Scholar] [CrossRef]
- Weber, E.A.; Gruber, S.; Claupein, W. Emergence and performance of volunteer oilseed rape (Brassica napus) in different crops. Eur. J. Agron. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Streit, B.; Rieger, S.B.; Stamp, P.; Richner, W. The effect of tillage intensity and time of herbicide application on weed com-munities and populations in maize in central Europe. Agric. Ecosyst. Environ. 2002, 92, 211–224. [Google Scholar] [CrossRef]
- Lutman, P.J.W.; Freeman, S.E.; Pekrun, C. The long-term persistence of seeds of oilseed rape (Brassica napus) in arable fields. J. Agric. Sci. 2003, 141, 231–240. [Google Scholar] [CrossRef]
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dayan, F.E. Current Status and Future Prospects in Herbicide Discovery. Plants 2019, 8, 341. [Google Scholar] [CrossRef]
- Kudsk, P.; Streibig, J.C. Herbicides—A two-edged sword. Weed Res. 2003, 43, 90–102. [Google Scholar] [CrossRef]
- Sawinska, Z.; Świtek, S.; Głowicka-Wołoszyn, R.; Kowalczewski, P. Łukasz Agricultural Practice in Poland Before and After Mandatory IPM Implementation by the European Union. Sustainability 2020, 12, 1107. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Allemann, J.; Molomo, J.M. Sensitivity of selected dry bean (Phaseolus vulgaris L.) cultivars to mesotrione in a simulated carry-over trial. S. Afr. J. Plant Soil 2016, 33, 229–235. [Google Scholar] [CrossRef]
- García, P.S.; Moreau, A.L.D.; Ierich, J.C.M.; Vig, A.C.A.; Higa, A.M.; Oliveira, G.S.; Abdalla, F.C.; Hausen, M.; Leite, F.L. A Nanobiosensor Based on 4-Hydroxyphenylpyruvate Dioxygenase Enzyme for Mesotrione Detection. IEEE Sensors J. 2014, 15, 2106–2113. [Google Scholar] [CrossRef]
- van Almsick, A. New HPPD-inhibitors–A proven mode of action as a new hope to solve current weed problems. Outlooks Pest Manag. 2009, 20, 27–30. [Google Scholar] [CrossRef]
- Rosenbrock, P.; Munch, J.; Scheunert, I.; Dörfler, U. Biodegradation of the herbicide bromoxynil and its plant cell wall bound residues in an agricultural soil. Pestic. Biochem. Physiol. 2004, 78, 49–57. [Google Scholar] [CrossRef]
- Cutulle, M.A.; Armel, G.R.; Brosnan, J.T.; Best, M.D.; Kopsell, D.A.; Bruce, B.D.; Bostic, H.E.; Layton, D.S. Synthesis and Evaluation of Heterocyclic Analogues of Bromoxynil. J. Agric. Food Chem. 2013, 62, 329–336. [Google Scholar] [CrossRef]
- Hess, F.D. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Damalas, C.A.; Lithourgidis, A.S.; Eleftherohorinos, I.G. Echinochloa species control in maize (Zea mays L.) with sulfonylurea herbicides applied alone and in mixtures with broadleaf herbicides. Crop. Prot. 2012, 34, 70–75. [Google Scholar] [CrossRef]
- Anthimidou, E.; Ntoanidou, S.; Madesis, P.; Eleftherohorinos, I. Mechanisms of Lolium rigidum multiple resistance to ALS- and ACCase-inhibiting herbicides and their impact on plant fitness. Pestic. Biochem. Physiol. 2020, 164, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Baghestani, M.A.; Zand, E.; Soufizadeh, S.; Eskandari, A.; Pourazar, R.; Veysi, M.; Nassirzadeh, N. Efficacy evaluation of some dual purpose herbicides to control weeds in maize (Zea mays L.). Crop. Prot. 2007, 26, 936–942. [Google Scholar] [CrossRef]
- Peterson, R.B.; Oja, V.; Laisk, A. Chlorophyll fluorescence at 680 and 730 nm and leaf photosynthesis. Photosynth. Res. 2001, 70, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new appli-cations. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bosa, K.; Kościelniak, J.; Hossain, Z. Chlorophyll a Fluorescence—A Useful Tool for the EarlyDetection of Temperature Stress in Spring Barley (Hordeum vulgare L.). Omics A J. Integr. Biol. 2011, 15, 925–934. [Google Scholar] [CrossRef]
- Kowalczewski, P. Łukasz; Radzikowska, D.; Ivanišová, E.; Szwengiel, A.; Kačániová, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397. [Google Scholar] [CrossRef]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence re-sponses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef]
- Zai, X.M.; Zhu, S.N.; Qin, P.; Wang, X.Y.; Che, L.; Luo, F.X. Effect of Glomus mosseae on chlorophyll content, chlorophyll fluorescence parameters, and chloroplast ultrastructure of beach plum (Prunus maritima) under NaCl stress. Photosynthetica 2012, 50, 323–328. [Google Scholar] [CrossRef]
- Eppo, C. European and Mediterranean Plant Protection Organization. Bull. OEPP/EPPO Bull. 1997, 42, 367–381. [Google Scholar]
- Sulewska, H.; Koziara, W.; Śmiatacz, K.; Szymańska, G.; Panasiewicz, K. Efficacy of selected herbicides in weed control of maize. Fragm. Agron. 2012, 29, 144–151. [Google Scholar]
- Jursík, M.; Kolářová, M.; Soukup, J. Competition, reproduction ability, and control possibilities of conventional and Clear-field® volunteer oilseed rape in winter wheat. Crop Prot. 2019, 122, 30–34. [Google Scholar]
- Idziak, R.; Woznica, Z. Impact of tembotrione and flufenacet plus isoxaflutole application timings, rates, and adjuvant type on weeds and yield of maize. Chil. J. Agric. Res. 2014, 74, 129–134. [Google Scholar] [CrossRef]
- Colbach, N.; Clermont-Dauphin, C.; Meynard, J. GeneSys: A model of the influence of cropping system on gene escape from herbicide tolerant rapeseed crops to rape volunteers. Agric. Ecosyst. Environ. 2001, 83, 235–253. [Google Scholar] [CrossRef]
- Green, J.M.; Hale, T. Increasing and Decreasing pH to Enhance the Biological Activity of Nicosulfuron. Weed Technol. 2005, 19, 468–475. [Google Scholar] [CrossRef]
- Sobiech, Ł.; Idziak, R.; Skrzypczak, G. Wpływ pH i napięcia powierzchniowego cieczy opryskowej na skuteczność działania mezotrionu. Przem. Chem. 2018, 97, 1325–1327. [Google Scholar] [CrossRef]
- Sobiech, Ł.; Skrzypczak, G.; Grzanka, M. Wpływ pH cieczy opryskowej i adiuwantów należących do różnych grup chemicznych na skuteczność działania tembotrionu. Przem. Chem. 2019, 98, 1551–1554. [Google Scholar] [CrossRef]
- Dávid, I.; Máté, E. Efficacy of herbicides influenced by spray carrier water pH and hardness. J. Agric. Sci. 2009, 141–146. [Google Scholar]
- Matocha, M.A.; Senseman, S.A. Trifloxysulfuron Dissipation at Selected pH Levels and Efficacy on Palmer amaranth (Ama-ranthus palmeri). Weed Technol. 2007, 21, 674–677. [Google Scholar] [CrossRef]
- Pigatto, C.S.; Tarouco, C.P.; Nicoloso, F.T.; Berghetti, Á.L.P.; Leães, G.P.; Werle, I.S.; Ulguim, A.D.R. Barnyardgrass control using tank-mixed herbicides with saflufenacil and its influence in photosynthesis and chlorophyll fluorescence. Ciência Rural 2020, 50, e20190919. [Google Scholar] [CrossRef]
- Silva, F.B.; Costa, A.C.; Alves, R.R.P.; Megguer, C.A. Chlorophyll Fluorescence as an Indicator of Cellular Damage by Glyphosate Herbicide in Raphanus sativus L. Plants. Am. J. Plant Sci. 2014, 5, 2509–2519. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M. Effect of Adjuvants on Trifloxysulfuron Efficacy and Chlorophyll Fluorescence of Sicklepod, Guineagrass, Yellow Nutsedge and Cotton. Indian J. Weed Sci. 2007, 39, 1–12. [Google Scholar]
- Linn, A.I.; Zeller, A.K.; Pfündel, E.E.; Gerhards, R. Features and applications of a field imaging chlorophyll fluorometer to measure stress in agricultural plants. Precis. Agric. 2020, 1–17. [Google Scholar] [CrossRef]
- Sobiech, Ł.; Grzanka, M.; Kurasiak-Popowska, D.; Radzikowska, D. Phytotoxic Effect of Herbicides on Various Camelina [Camelina sativa (L.) Crantz] Genotypes and Plant Chlorophyll Fluorescence. Agriculture 2020, 10, 185. [Google Scholar] [CrossRef]
- Dayan, F.E.; Zaccaro, M.L.D.M. Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- Skórska, E.; Murkowski, A. Comparison of susceptibility of crop oat cv. Akt and wild oat leaves to Dicuran 80 WP in mixture with two adjuvants, measured by chlorophyll fluorescence. Acta Agrophysica 2009, 14, 463–468. [Google Scholar]
- Zhang, J.; Jäck, O.; Menegat, A.; Li, G.; Wang, X. Chlorophyll Fluorescence Measurement: A New Method to Test the Effect of Two Adjuvants on the Efficacy of Topramezone on Weeds. In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Jilin, China, 12–15 August 2017; pp. 206–216. [Google Scholar]
- Baker, N.R.; Rosenqvitz, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Gholamin, R.; Khayatnezhad, M. The effect of end season drought stress on the chlorophyll content, chlorophyll fluorescence parameters and yield in maize cultivars. Sci. Res. Essays 2011, 6, 5351–5357. [Google Scholar]
- Briantais, J.-M.; Dacosta, J.; Goulas, Y.; Ducruet, J.-M.; Moya, I. Heat stress induces in leaves an increase of the minimum level of chlorophyll fluorescence, Fo: A time-resolved analysis. Photosynth. Res. 1996, 48, 189–196. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Fernandez, R.T.; Perry, R.L.; Flore, J.A. Drought Response of Young Apple Trees on Three Rootstocks. II. Gas Exchange, Chlorophyll Fluorescence, Water Relations, and Leaf Abscisic Acid. J. Am. Soc. Hortic. Sci. 1997, 122, 841–848. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Misra, A.N.; Misra, M.; Singh, R. Chlorophyll Fluorescence in Plant Biology. In Biophysics; Misra, A.N., Ed.; InTech: London, UK, 2012; p. 220. ISBN 978-953-51-0376-9. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
| No. | Treatment | Solution for Each Herbicide | pH | Efficacy (%) Based On | |
|---|---|---|---|---|---|
| Visual Assessment | Fresh Weight Reduction | ||||
| 1 | untreated | - | - | 0 | 0 |
| 2 | mesotrione | neutral | 6.1 | 82.5 efg | 72.6 de |
| 3 | mesotrione | acidic | 4.0 | 87.5 bc | 80.3 cd |
| 4 | mesotrione | basic | 9.0 | 85 cde | 79.1 cd |
| 5 | nicosulfuron | neutral | 7.2 | 80 g | 84.1 abcd |
| 6 | nicosulfuron | acidic | 4.0 | 89.4 b | 88.9 abc |
| 7 | nicosulfuron | basic | 9.0 | 86.9 bcd | 87.5 abc |
| 8 | rimsulfuron | neutral | 7.5 | 85.6 cd | 83.7 abcd |
| 9 | rimsulfuron | acidic | 4.0 | 73.8 h | 55.4 f |
| 10 | rimsulfuron | basic | 9.0 | 71.3 h | 59.6 f |
| 11 | mesotrione + nicosulfuron + rimsulfuron + adjuvant | neutral | 6.3 | 71.9 h | 64.6 ef |
| 12 | mesotrione + nicosulfuron + rimsulfuron + adjuvant | acidic | 4.0 | 84.4 def | 82.3 bcd |
| 13 | mesotrione + nicosulfuron + rimsulfuron + adjuvant | basic | 9.0 | 81.9 fg | 80.9 cd |
| 14 | bromoxynil | neutral | 7.3 | 95.6 a | 95.4 a |
| 15 | bromoxynil | acidic | 4.0 | 96.6 a | 94.2 ab |
| 16 | bromoxynil | basic | 9.0 | 87.5 bc | 83.3 abcd |
| LSD (0.05) | 2.96 | 12.18 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzanka, M.; Sobiech, Ł.; Skrzypczak, G.; Piechota, T. Herbicides Efficacy against Volunteer Oilseed Rape as Influenced by Spray Solution pH. Agronomy 2021, 11, 887. https://doi.org/10.3390/agronomy11050887
Grzanka M, Sobiech Ł, Skrzypczak G, Piechota T. Herbicides Efficacy against Volunteer Oilseed Rape as Influenced by Spray Solution pH. Agronomy. 2021; 11(5):887. https://doi.org/10.3390/agronomy11050887
Chicago/Turabian StyleGrzanka, Monika, Łukasz Sobiech, Grzegorz Skrzypczak, and Tomasz Piechota. 2021. "Herbicides Efficacy against Volunteer Oilseed Rape as Influenced by Spray Solution pH" Agronomy 11, no. 5: 887. https://doi.org/10.3390/agronomy11050887
APA StyleGrzanka, M., Sobiech, Ł., Skrzypczak, G., & Piechota, T. (2021). Herbicides Efficacy against Volunteer Oilseed Rape as Influenced by Spray Solution pH. Agronomy, 11(5), 887. https://doi.org/10.3390/agronomy11050887







