Abstract
Selenium (Se) has been recently reported to play a crucial role in ameliorating the negative impact of abiotic stress, including salinity, on several plant species. Two field experiments (2016/17 and 2017/18) were carried out to investigate the possible effect of exogenous Se application at two levels (25, 50 mg L−1) on growth, bulb yield, physio-biochemical attributes, and antioxidant activities of onion grown under saline (5.25 dS m−1) soil condition. Se (25 or 50 mg L−1) foliar application enhanced growth characteristics, as well as membrane stability index (MSI) and relative water content (RWC) as a result of the osmotic adjustment by accumulating more osmoprotectants and enhancing the activity of antioxidants defense system, thus improving photosynthetic efficiency and bulb yield. Proline, glycine betaine, choline, and total soluble sugars content were higher in leaves and bulbs of Se-treated plants under salt stress. Se (25 or 50 mg L−1) significantly increased the activities of enzymatic (ascorbate peroxidase, superoxide dismutase, and catalase) and non-enzymatic (ascorbic acid and glutathione) antioxidants in both leaves and bulbs of salt-stressed onion. Se application at 25–50 mg L−1 may find, in the future, a potential application as anti-abiotic stresses for improving plant growth and productivity under saline soil condition.
1. Introduction
Onion (Allium cepa L.) is the second most broadly cultivated vegetable crop in the world for their economic importance, nutritional value, flavor, and medicinal properties []. In 2019, worldwide bulb onion production was approximately 100 million tons harvested from an area of approximately 5.2 million ha. Onion cultivated area in Egypt was 87,948 ha and produced 3.08 million tons. Among the main countries exporting onion, Egypt ranked fourth after China, India, and United States of America []. Onion is deemed to be a salt-sensitive crop, with a salinity threshold level of around 1.2 dS m−1 for bulb yield []. For every increase in ECe unit, onion bulb yield proportionally reduces [,].
Salinity is a major abiotic stress threatening crop production especially in arid/semi-arid areas including Egypt. Salt-affected soils are estimated to be 20–33% of the world’s cultivated and irrigated soils and, with lower rainfall, higher evaporation, and poor cultural practices, the salinized areas are predicted to reach 50% by 2050 [,,].
Besides its impact on the imbalance of nutrients, salinity contributes to the reduction of plant osmotic potential leading to water stress and a buildup of excessive amounts of ions in plant tissues to a potentially toxic level [,,]. Therefore, salinity induces various morphological, physiological, and biochemical alterations in plant tissues, including ion homeostasis disturbance, downregulation of photosynthetic pigments, and diminish the photosynthesis efficiency, due to minimizing the activity of photosystem II (PSII) [,], and decreasing stomatal conductance and CO2 uptake [], which ultimately hinder plant growth and productivity. A common response to abiotic stresses including salinity is the hyper production of reactive oxygen species (ROS) in plant tissues, such as hydrogen peroxide (H2O2), superoxide (O2−), singlet oxygen (1O2), hydroxyl radicals (OH), and alkoxy radicals (RO). Salinity-induced ROS provoke oxidative damages at the cellular level by increasing lipid peroxidation, protein degradation, and decreasing membrane stability [,,,].
In response to salt stress, plants have evolved various adaptive mechanisms to withstand that stress including the accumulation of osmoprotectants and anti-oxidative compounds []. Supporting plant defense system by exogenous application of adjuvants, such as selenium (Se2−), contributes to the alleviation of the adverse effects of salt stress; regulates several processes for increasing plant tolerance and protects cellular and subcellular tissues from the cytotoxic effects of ROS [,].
Egypt belongs to Se-deficient areas, where the daily intake is less than 55 μg per day []. Se is counted as important microelements for plants, having a beneficial role for enhancing growth and development, due to its physiological and anti-oxidative properties [,]. However, requirement of Se depends on plant species and also different concentrations []. At low concentration, Se stimulates the antioxidant machinery and protects plant tissues from oxidative stress, but at high concentration, it behaves like heavy metals and acts as a pro-oxidant []. Selenium application intensifies the phloem transport through phloem and xylem []. Se is involved in the structural construction of some selenoproteins, which has an important role in scavenging ROS [].
Previous literature indicated that exogenous Se prompts abiotic stress tolerance including salinity [,,]. Se has markedly alleviated salinity stress in maize plants via the improvements of photosynthetic capacity and the antioxidant defense system by increasing the activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX) enzymes []. Maintaining cell turgor and membrane integrity and increasing fruit yield of drought-stressed tomato plants were achieved by exogenous Se application, either to soil or by foliar spraying []. Exogenous Se spray could regenerate membrane enzymes and reactivate the important metabolites transport for chloroplast []. Recently, some other evidence has shown that lower concentrations of selenium might protect plants against abiotic stresses. According to [], Se stimulated photosynthetic capacity and biomass accumulation of cadmium-stressed Oryza sativa. Spraying Se at 20 mg L−1 helped in maintaining homeostasis between Na+ and K+, as it led to an increase of K+ and decline of Na+ in leaves and roots of Zea mays plants grown under 12 dS m−1 soil salinity [].
To the best of our knowledge, very limited information is available on the effect of Se at a field study and its prospective role in ameliorating salinity stress in widely cultivated and consumed crops like onion. In our endeavor to investigate the potential effect of Se foliar application in alleviating salt-induced damages on onion, we hypothesized that Se might induce a higher concentration of osmoprotectants and improve growth and productivity of salt-stressed onion, highlighting Se effects on, photosynthetic efficiency, stomatal conductance, plant water status, bulb yield, and the activities of enzymatic and non-enzymatic antioxidants.
2. Materials and Methods
2.1. Experimental Layout and Growth Condition
In the experimental station of the Faculty of Agriculture, Fayoum University (29.2938° N; 30.9144° E), southeast Fayoum, Egypt, field trials were conducted during two seasons of 2016/17 (SI) and 2017/18 (SII). Following the climatic characterization, the climate in this region is hyper-arid as reported by []. Healthy seeds of the most widely cultivated onion cultivar (Giza 20) in Egypt were sown on 28 September 2016 and 2017. Seedlings were then transplanted to the open field on 8 December of both 2016 and 2017, in four rows of 0.10 m apart per bed (1.1 m wide), with a distance of 0.15 m between plants. All plants were fertilized with 150 kg N ha−1, 62 kg P2O5 ha–1, and 72 kg K2O ha−1. Furthermore, all the cultural procedures and pest management were performed following the recommendation of the Ministry of Agriculture and Land Reclamation in Egypt.
The daily reference evapotranspiration (ETo) was computed according to FAO penman Monteith method [], using meteorological data recorded near the experimental site. The irrigation was applied by surface irrigation at 15 days intervals equally to all onion plants. The required water was supplied via plastic pipe (50 mm diameter) for each plot and the irrigation water transferred to each plot was controlled using the following equation []:
where Q is the discharge of irrigation water (L s−1), C is the coefficient of discharge (=0.62), A is the cross-section area of irrigation pipe (=19.62 cm2), g is the gravity acceleration, (=986 cm s−2), and h is the average effective head of water (cm).
2.2. Soil Analyses, Treatments, and Experimental Design
A representative soil sample was collected from 10 soil profiles from the experimental site to determine the soil’s physical and chemical properties. Table 1 presents the physical and chemical soil analysis, which was quantified as depicted by [,]. In the study area, the soil is coarse in texture (Sandy loam), moderately deep—classified as Typic Torripsamments—siliceous, and hyperthermic, according to the USDA soil taxonomy []. The soil analyses indicated that the ECe value is 5.27 dS m−1, which was rated as moderate saline soil []. In a parcel site with similar characteristics, Se concentrations (mg kg−1) was monitored, as illustrated in Table 2. 5 mL of HNO3 were added to 0.5 g of sample in a 250 mL dry flask and stirred. Thus, all the material was wet. Then, 4 mL of 33% H2O2 were added in a well-ventilated hood and slightly stirred after the addition. It was heated on a hot plate and a strong effervescence was produced. When the brown fumes were less dense (7–8 min), the solution was allowed to cool. A slightly yellow dissolution and a small white solid quantity in suspension still remained. The solution was filtered, washed with 5 mL of (1:1) HCI (density 1.18 g mL−1), and diluted up to 25 mL with distilled H2O. The readings of digested samples were performed using an Induction Plasma Spectrometer (ICP), Thermo Jarell Ash brand, IRIS Advantage model, following the 984.27 []. The quantification of Se (mg kg−1 DW) was performed.

Table 1.
Some initial physical and chemical properties of the experimental soil.

Table 2.
Selenium concentrations (mg kg−1) monitoring in the studied area.
Se (Na2SeO4; Sigma-Aldrich, St. Louis, MO, USA) was foliarly-applied to onion plants grown under 5.27 dS m−1 soil salinity with three concentrations: 0 mg L−1 (spraying tap water +0.1%, v/v Tween-20 as a control), 25 mg L−1, and 50 mg L−1. Spraying Se was started from 20 days after transplanting at three times with 20 days intervals. Spraying was carried out using hand atomizer to run off. Tween-20 (0.1%, v/v) was added to the sprays with the Se solution as a surfactant to facilitate penetration of the solution into the leaf tissues.
The experimental layout was a randomized complete block design with three replications. The total experimental area was 275 m−2, included 9 plots; each one was approximately 16.5 m−2 (1.1 m wide × 15 m length). Each experimental plot contained 12 planting rows set 10 cm apart, and 15 cm was a distance between plants to achieve the typical practice of onion producers. Treatments were randomly distributed within each block (Replica). Each of the three replicates contains all treatments. From each experimental unit (plot) three plants were collected for the analysis (n = 9) for growth measurements, and n = 5 for physiological measurements.
2.3. Onion Growth, Biomass, and Bulb Yield
At 91 days after transplanting, three plants per plot were chosen randomly to determine shoot length and number of leaves. Thereafter, leaf area was measured using a graph sheet and counting, as described by []. Shoot dry weight plant−1 was recorded after oven-drying at 70 °C until a constant weight.
At 8 may of both 2017 and 2018 all plants of each plot area were harvested then total bulb yield was determined, which was sorted to three bulb sizes; <50 mm, 50–75 mm, and >75 mm. Water use efficiency (WUE; kg m−3) was calculated as a ratio of total bulb yield to irrigation water applied [], according to the following formula:
2.4. Photosynthetic Efficiency, Plant Water Status, and Stomatal Conductance (Gs)
Fully developed leaves were collected in two different sunny days to measure relative chlorophyll content (SPAD value; n = 5), using a chlorophyll meter (SPAD-502, Minolta, Japan). Afterwards, chlorophyll a fluorescence was measured using a portable fluorometer (n = 5) (Handy PEA, Hansatech Instruments Ltd., Kings Lynn, UK). Chlorophyll fluorescence parameters in terms of the maximum quantum yield PSII (Fv/Fm) was calculated considering the equation outlined by [], the activity of PSII reaction centers (Fv/F0) was calculated [] and the photosynthetic performance index (PI) was quantified []. Leaf membrane stability index (MSI), using the methodology of [], modified by [], and leaf relative water content (RWC), using the method of [], modified by [] were then conducted. 2 cm diameter discs (10 discs) from the fully-expanded leaf were weighed (fresh mass; FM) and immediately floated on distilled water in Petri dishes for 6 h, in a dark room, the turgid mass (TM) was then measured. Discs dry mass (DM) was recorded after dehydration at 70 °C until the constant weight. The RWC was then calculated according to the following formula:
RWC (%) = [(FM − DM)/(TM − DM)] × 100
On the other hand, 0.2 g Leaf sample was placed in a test-tube containing 10 mL of distilled water. Tubes were then heated at 40 °C in a water bath for half an hour, and the electrical conductivity (C1) of the solution was recorded, using a conductivity bridge. A second sample was boiled at 100 °C for 10 min, and the conductivity was measured (C2). The MSI was calculated using the formula:
MSI (%) = [1 − (C1/C2)] × 100
35 days after transplanting, stomatal conductance (Gs) (mmol−2 S−1) was measured (n = 5) in field using leaf porometer (Decagon Devices Inc., Pullman, WA, USA) (from 9:00 a.m. to 12:00 p.m.).
2.5. Osmoprotectants and Selenium (Se) Contents Determinations
Osmoprotectants content i.e., total soluble sugar, free proline, choline, and glycine betaine were assessed in onion leaves and bulbs. Leaf surface was thoroughly washed in running tap water, followed by washing with double distilled water. Thereafter, dried samples of 0.5 g were used to determine free proline (mg g−1 DW), following the method described by []. Acid ninhydrin reagent was prepared by warming 1.25 g ninhydrin in 30 mL glacial acetic acid and 20 mL 6 M phosphoric acid with agitation until complete solubility; kept cool and stored at 4 °C for 24 h until the reagent remains stable. Two ml of the filtrate were mixed with 2 mL of glacial acetic acid and 2 mL of the acid ninhydrin reagent in a test tube. At the same time heat the samples at 100 °C for 60 min, with caps in place. The reaction mixture was extracted with 4 mL toluene, mixed vigorously in a test tube for 15–20 s. The chromophore containing toluene was aspired from the aqueous phase and warmed to room temperature. The absorbance measured at 520 nm using toluene as blank. The concentration of proline in the extract samples was calculated using the calibration curve was calculated on a dry matter basis. For the determination of total soluble sugars content (mg g−1 DW), 0.2 g dried samples were used, as outlined by []. 0.2 g dried leaf sample was homogenized in 5 mL of 96% (v/v) ethanol, and then washed with 5 mL 70% (v/v) ethanol. The extract was centrifuged at 3500× g for 10 min, and the supernatant was stored at 4 °C prior to determination. The reaction mixture of 0.1 mL of the ethanolic extract and 3 mL of freshly-prepared anthrone reagent (150 mg anthrone plus 100 mL of 72% (v/v) sulphuric acid) was placed in a boiling water bath for 10 min, and was then cooled. The absorbance of the mixture was recorded at 625 nm. Leaf and bulb content of glycine betaine (mmol g−1 DW) and choline (mmol g−1 DW) were determined based on the analytical procedures depicted by [] and [], respectively, using Shimadzu-V HPLC. Liquid N-frozen samples (0.5 g) were homogenized in 4 mL 3:5:12 (v/v/v) of a water, chloroform, methanol mixture, and were then incubated at 4 °C over- night. Using BioRad AG1-X8 ion exchange resin, purification of upper methanolic phase (1 mL) taken from the extract was done. Thereafter, centrifugation (5000× g for 10 min) was applied to remove the ion exchange resin, and supernatant was then filtered through a 0.45 mL membrane filter before loading to the HPLC system. With using nu-cleogel RP column (RP-S 100-8, 300 × 7.7 mm) proceeded by a guard column, mobile phase (15 mM KH2PO4) was delivered by an analytical isocratic pump at a flow rate of 0.8 mL min−1 at 70 °C. Sample contents of choline and GB were detected by UV detector at 230 nm, and were then quantified by comparing the peak surface areas (2.5 min and 3.5 min) with those obtained with pure choline and GB standards, respectively. Se content (mg g−1 DW) quantification in both onion leaves and bulbs was conducted using the analytical procedures of ICP-MS [].
2.6. Assays of Antioxidants Activities: Non-Enzymatic and Enzymatic
In both leaves and bulbs, ascorbic acid (AsA) and reduced glutathione (GSH) were determined according to the methods of [] and [], respectively, and both expressed as mmol g−1 fresh weight. For AsA contents determination, extraction was performed in 10 mL of 6% (w/v) trichloroacetic acid, and the extract was then mixed with 2% (w/v) dinitrophenylhydrazine, then one drop of 10% (w/v) thiourea in 70% (v/v) ethanol was added. After boiling the mixture for 15 min and cooling, 5 mL of 80% (v/v) H2SO4 was added and absorbances were read at 530 nm to calculate the contents of AsA from a standard curve. For GSH contents determination, homogenization of fresh leaf tissue (50 mg) was exercised in 2 mL of 2% (v/v) metaphosphoric acid, and centrifugation was then applied at 17,000× g for 10 min. Neutralization of supernatant (0.9 mL) was done with 0.6 mL of 10% (w/v) sodium citrate. Assessments of 3 replicates were made for each sample. A composition of 700 μL of 0.3 mM NADPH, 100 μL of 6 mM 5,5′-dithio-bis-2-nitrobenzoicacid, 100 μL distilled water, and 100 μL of extract was of each assay (1.0 mL) that was stabilized at 25 °C for 3–4 min, and GSH reductase (10 μL of 50 Units mL−1) was then added and absorbances were read at 412 nm to calculate GSH contents from a standard curve.
For SOD, CAT, GR, and APX extraction, samples (0.5 g fresh sample) were homogenized in ice cold 0.1 M phosphate buffer (pH = 7.5) containing 0.5 mM EDTA with pre-chilled pestle and mortar. Each homogenate was transferred to centrifuge tubes and was centrifuged at 4 °C in Beckman refrigerated centrifuge for 15 min at 15,000× g. The supernatant was used for enzyme activity assay []. The enzymes activities determinations were performed, and all expressed as μmol mg−1 protein. The activity of superoxide dismutase (SOD; EC 1.15.1.1) was assessed from recording an inhibition of cytochrome reduction of nitroblue tetrazolium (NBT) at 540 nm []. About 3 mL of reaction mixture, containing 0.1 mL of 200 mM methionine, 0.1 mL of 2.25 mM nitro-blue tetrazolium (NBT), 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 1 mL distilled water, and 0.05 mL of enzyme extraction, were taken in test tubes in duplicate from each enzyme sample. Two tubes without enzyme extract were taken as control. The reaction was started by adding 0.1 mL riboflavin (60 µM) and placing the tubes below a light source of two florescent lamps (15 W) for 15 min. Reaction was stopped by switching off the light and covering the tubes with black cloth. Tubes without enzyme developed maximal color. A non-irradiated complete reaction mixture, which did not develop color, served as blank. Absorbance was recorded at 560 nm and one unit of enzyme activity was taken as the quantity of enzyme, which reduced the absorbance reading of samples to 50% in comparison with tubes lacking enzymes. Catalase (CAT; EC 1.11.1.6) activity was performed by measuring the decomposition rate of H2O2 at 240 nm []. About 3 mL reaction mixture containing 1.5 mL of 100 mM potassium phosphate buffer (pH = 7), 0.5 mL of 75 mM H2O2, 0.05 mL enzyme extraction, and distilled water to make up the volume to 3 mL. Reaction started by adding H2O2 and decrease in absorbance recorded at 240 nm for 1 min. Enzyme activity was computed by calculating the amount of H2O2 decomposed. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was measured, according to [] by monitoring the rate of ascorbate oxidation at 290 nm (E = 2.8 mM−1cm−1). The reaction mixture contained 25 mM phosphate buffer (pH = 7), 0.1 mM EDTA, 1 mM H2O2, 0.25 mM AsA, and the enzyme sample. No change in absorption found in the absence of AsA in the test medium. Glutathione reductase (GR; EC 1.6.4.1) activity was determined by measuring the oxidation of NADPH at 340 nm []. Glutathione reductase (GR; EC 1.6.4.1) activity was assayed by recording the increase in absorbance in the presence of oxidized glutathione (GSSG) and 5,5-dithiobis-2-nitrbenzoic acid (DTNB) []. The reaction mixture contained 1 mL of 0.2 M potassium phosphate buffer (pH = 7.5) containing 0.1 mM EDTA, 0.5 mL of 3 mM DTNB in 0.01 M potassium phosphate buffer (pH = 7.5), 0.1 mL of 2 mM NADPH, 0.1 mL enzyme extract, and distilled water to make up a final volume of 2.9 mL. Reaction initiated by adding 0.1 mL of 2 mM GSSG. The increase in absorbance at 412 nm recorded at 25 °C over a period of 5 min on a spectrophotometer.
2.7. Statistical Analysis
Data of both field experimental seasons were analyzed using Genstat statistical software (version 11; VSN International Ltd., Oxford, UK). Differences between the treatment’s means were separated by Student Newman Keuls test at p ≤ 0.05. The results are presented as means ± standard error.
3. Results
3.1. Onion Growth and Biomass under Selenium and Salinity Stress
Onion growth characteristics and biomass results are presented in Table 3. Se-treated onions with 25 mg L−1 or 50 mg L−1 showed enhanced growth (e.g., shoot length, number of leaves, and leaf area) and biomass (shoot fresh and dry weight) compared to the non-treated plants. These improvements were more pronounced at 50 mg L−1 Se, which caused an increase of shoot length by 20% and 39%, the number of leaves by 45% and 30%, leaf area by 134% and 184%, shoot fresh weight by 165% and 134%, and shoot dry weight by 183% and 131% in SI and SII, respectively, compared to the control.

Table 3.
Effects of foliar applications with Selenium (Se) on vegetative growth characteristics of onion under saline soil (EC = 5.2 dS m−1) condition in 2016/17 (SI) and 2017/18 (SII) seasons.
3.2. Plant Water Status, Water Use Efficiency (WUE), Stomatal Conductance (Gs), and Photosynthetic Efficiency, in Response to Selenium under Salinity Stress
As presented in Table 4, in both seasons, salt-stressed onion plants sprayed with 25 mg L−1 or 50 mg L−1 Se markedly increased MSI by 9% and RWC by 19% (on average of SI and SII), compared to those unsprayed plants, which recorded the lowest values. Results exhibited that, 50 mg L−1 Se-treated plants showed enhanced WUE by 79%, relative to the control (Table 4). Application of Se (25 mg L−1 or 50 mg L−1) significantly increased relative chlorophyll content (SPAD value) and chlorophyll fluorescence apparatus as Fv/Fm, Fv/F0 and PI in comparison of the control (Figure 1 and Figure 2). Under saline soil condition, 50 mg L−1 Se-treated plants showed higher stomatal conductance (Gs) by 61% compared to the control and was more effective than 25 mg L−1 Se (Figure 2).

Table 4.
Effects of foliar applications with Selenium (Se) on RWC%, MSI% and water use efficiency (WUE) of onion plants under saline soil (EC = 5.2 dS m−1) condition in 2016/17 (SI) and 2017/18 (SII) seasons.
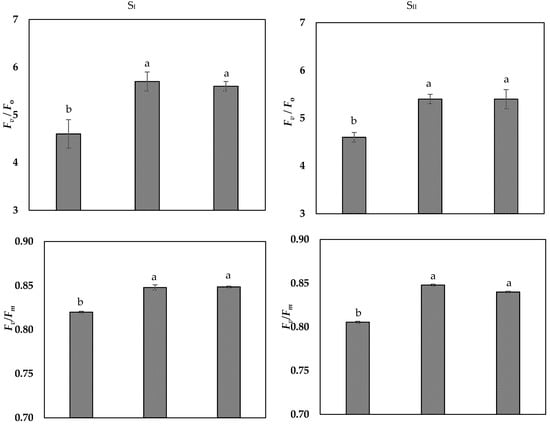
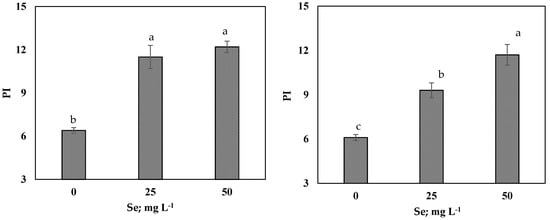
Figure 1.
Effects of selenium (Se; mg L−1) foliar applications on Chlorophyll a fluorescence i.e., (Fv/Fm and Fv/F0) and photosynthetic performance index (PI) of onion under saline soil (EC = 5.2 dS m−1) conditions in SI and SII seasons. Vertical bars represent means ± S.E (p ≤ 0.05). Columns marked by different letters are significantly different.
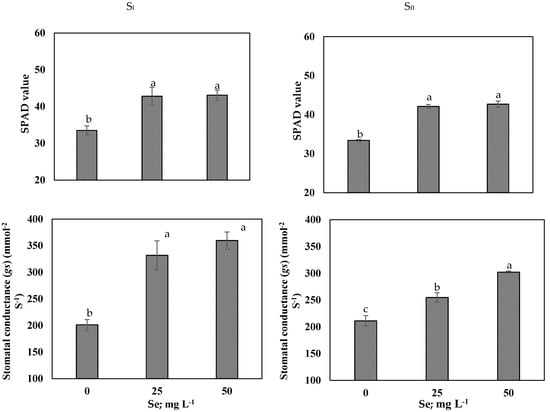
Figure 2.
Effects of selenium (Se; mg L−1) foliar applications on SPAD value and stomatal conductance (gs) of onion under saline soil (EC = 5.2 dS m−1) conditions in SI and SII seasons. Vertical bars represent means ± S.E (p ≤ 0.05). Columns marked by different letters are significantly different.
3.3. Bulb Yields and Water Use Efficiency under Selenium and Salinity Stress
Salinity noticeably decreased total bulb yield as well as large bulb grades (50–75 mm and >75 mm) yield, however, it increased the yield of small bulbs (<50 mm) (Table 5). Large bulb yield progressively increased with increasing Se level from 25 mg L−1 to 50 mg L−1. This increase in total bulb yield as a result of Se application, synchronized with increasing WUE. Results exhibited that, 50 mg L−1 Se-treated plants showed enhanced productivity of different bulb sizes (50–75 mm and >75 mm), and total bulb yield by 96%, 128%, and 78%, respectively, relative to the control (Table 5).

Table 5.
Effects of foliar applications with selenium (Se) on yield (t/ha) of different sizes of onion under saline soil (EC = 5.2 dS m−1) conditions in 2016/17 (SI) and 2017/18 (SII) seasons.
3.4. Osmoprotectants and Selenium Contents in Response to Selenium under Salinity Stress
In both onion leaves and bulbs, choline and total soluble sugars contents were increased by Se supplementation. However, 50 mg L−1 Se-treated plants exhibited higher contents of choline (by 36% in leaves and 14% in bulbs) and total soluble sugars (by 21% in leaves and 6% in bulbs) than the control (Table 6). However, significant increase in glycine betaine content in leaves of Se-treated plants was observed, whereas application of 25 mg L−1 and 50 mg L−1 Se decreased glycine betaine content in bulbs, when compared to control. Although, free proline content was increased with the application of 25 mg L−1 and 50 mg L−1 Se in onion bulbs by 65% and 82%, respectively, 25 mg L−1 Se application decreased free proline content in leaves by 35%, relative to non-treated plants (Table 6). Onion plants grown under salt stress without Se application recorded the lowest values of Se content in their leaves and bulbs. Whilst, Se contents were increased in leaves and bulbs of Se-treated plants, highlighting those supplemented with 50 mg L−1, which increased their Se content by 3 folds in leaves and 28 folds in bulbs (Table 6).

Table 6.
Effects of foliar applications with selenium (Se) on the leaf and bulb contents of osmoprotectants: choline, glycine betaine (GB), total soluble sugars (TSS), and proline of onion under saline soil (EC = 5.2 dS m−1) conditions.
3.5. Enzymatic and Non-Enzymatic Antioxidant Activities under Selenium and Salinity Stress
Exogenous Se application significantly influenced the activity of non-enzymatic and enzymatic antioxidants in onion leaves and bulbs (Table 7). Non-enzymatic antioxidant (i.e., ascorbic acid (AsA) and glutathione (GSH)) activities were elevated in bulbs by both 25 mg L−1 (by 61% and 126% for AsA and GSH, respectively) and 50 mg L−1 (by 70% and 147% for AsA and GSH, respectively), nevertheless, in onion leaves these activities were only elevated with 50 mg L−1 Se application by 63% for AsA and 134% for GSH. Compared to salt-stressed plants grown without Se application, Se-treated plants showed higher activities of SOD and CAT, particularly at 50 mg L−1 concentration by 76% and 50% (in leaves), respectively, and 99% and 68% (in bulbs), respectively. Externally applied 25 mg L−1 or 50 mg L−1 Se up-regulated APX activity particularly in bulbs by 122% (on average), but both Se levels were significantly or non-significantly reduced by GR activity.

Table 7.
Effects of foliar applications with selenium (Se) on the activity of leaf and bulb non-enzymatic; ascorbic acid (AsA) and glutathione (GSH) and enzymatic; ascorbate peroxidase (APX), superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR) antioxidants of onion under saline soil (EC = 5.2 dS m−1) conditions.
3.6. Correlation Analysis
Table 8 presents the correlation coefficient between onion yield and other important traits for both growing seasons. This type of analysis can be used as a suitable tool to regulate any trait of them is positive and closely related to the bulb’s yield obtained. The correlation coefficient indicated a strong positive correlation (r between 0.674–0.972) between bulb’s yield and other traits. Results of Table 9 exhibited that SPAD, PI, and leaves area in SI and Fresh weight, SPAD, and leaves number in SII, strongly contributed to variations in bulb’s yield.

Table 8.
A matrix of simple correlation coefficient between onion yield and other important traits estimated in 2016/17 (SI) and 2017/18 (SII) seasons.

Table 9.
Correlation coefficient (r), coefficient of determination (R2), and standard error of the estimates (SEE) for predicting onion yield for SI (2016/17) and SII (2017/18) seasons.
4. Discussion
At present, soil salinity has stood out as an important problem threatening agriculture production worldwide. In different plant species, salinity stress has been shown to extensively hinder plant growth and production [,,,,].
In the present study, the tested soil has a salinity of 5.27 dS m−1 and led to the exposure of onion plants to salt stress. This salt-stressed onion induces not only reduction of plant water status (in terms of MSI and RWC), but also decreased stomatal conductance and the photosynthetic efficiency (Table 4; Figure 1 and Figure 2), which reflected negatively in onion growth, namely shoot length, leaf number, leaf area, and plant biomass (Table 3), and consequently declines of onion yields (Table 5). Saline soil may upset the nutrients balance in plant and interfere uptake of necessary nutrients leading to nutrient deficiency [], that inhibits or delay development of onion roots, shoots (Table 3), and bulb enlargement, thence negatively affected bulb yields (Table 5). At the cellular level, salt stress arrest cell cycle through downregulation of the activity/expression of some protein kinase enzymes, precisely cyclins and cyclin-dependent kinases, resulting in fewer meristematic cells, in which diminish plant growth traits []. However, our study exhibited that the negative effects on growth traits of salt-stressed onion were alleviated by foliar-applied Se in particular, 50 mg L−1, thereby enhanced shoot length, leaf area, and number of leaves per plant; additionally, Se increased onion biomass production grown under salinity stress. The enhancement of the onion dry biomass by exogenous Se may be a positive criterion for salt tolerance correlates with an increase of the yields [], hence plant produces more metabolites that required for onion growth.
Salinity stress indirectly suppresses onion growth and productivity via induced reductions in cell division and elongation leading to a decrease of the leaf area (Table 3), concurrently with reduction of stomatal conductance and decreases of photosynthetic efficiency of PSII (Figure 1 and Figure 2) [,]. Gas exchange control is the responsibility of stomatal conductance, it responds quickly when the plant is subjected to salinity stress []. In this research, salt-stressed plants experienced a reduction of stomatal conductance (Figure 2), consequently this may reduce the intercellular CO2 and net photosynthesis, according to []. Along with the reduction of leaf relative chlorophyll content (SPAD value), salinity stress decreased the maximum quantum yield of PSII (Fv/Fm), PSII Fv/F0 ratio, and PI (Figure 1). The reduction of the Fv/Fm was obtained as a decrease of Fm, that represent photo inhibition of PSII [], while the activity of PSII reaction centers (Fv/F0) decreased due to increase of F0, indicating the damage occurred in the photosynthetic apparatus and the electron transport chain [,], all collectively indicating the damage in the light-harvesting complex of PSII in salt-stressed onion plants. Otherwise, the Se application improved the tolerance of onion to salinity stress, showing that Se increased relative chlorophyll content (SPAD value) and the efficiency of chlorophyll fluorescence synchronized with increase stomatal conductance of onion under salt stress (Figure 1). These improvements in relative chlorophyll content (SPAD value) and photosynthetic efficiency by Se supplementation was also observed in onion [], maize [], and tomato [] under salt stress. These findings may be linked with maintaining cell membrane integrity and increase tissues RWC by foliar spraying Se salt-stressed plants that restore structure of the damaged chloroplasts. Further, Se could stimulate the chlorophyll biosynthesis and increase chlorophyll content, hence increase the photosynthetic capacity. Furthermore, restoration of photosynthetic efficiency in stressed plants by exogenous Se application could be attributed to increase in osmoprotectants contents (Table 6) and antioxidant activity (Table 7). These plants’ defense system components involved in detoxifying of ROS generated by salinity stress and prevents chlorophyll degradation [,].
RWC is closely related to cell turgor, the process in which driving cell division and expansion, whereas MSI is used to estimate the degree of injuries induced by stressors []. In this research, the reductions in both MSI and RWC obtained in salt-affected plants (Table 4), indicating the detrimental effects of salinity stress on onion plants. It’s well documented that salinity stress-induced ROS formation causes lipid peroxidation, owing to decreases of membrane integrity and loss of cell turgor []. However, these negative responses of increased cell membrane injuries, and reduction of RWC triggered by salt stress, were alleviated by exogenously-applied Se (Table 4), in line with the findings obtained by []. Tissue water status promoting in Se-treated plants may be related to stimulated root growth and its capacity for water uptake []. Therefore, our results confirm that Se plays a role in stabilizing membrane integrity and maintains cell turgor under salinity stress. In this regard, increases of MSI and tissue RWC as metabolically available water, helping in maintaining tissue health and maybe reflect on the metabolic processes in onion plants [].
Our data showed that externally-applied Se markedly enhanced tissue water status, relative chlorophyll content (SPAD value), photosynthetic efficiency, growth traits, and biomass production of onion cultivated in salt-affected soil (Table 4; Figure 1 and Figure 2), which reflected consequently on a considerable increase of bulb yield and WUE (Table 4). Similar effects of Se on onion bulb yield have been reported by [], who noticed that foliar application of Se to onion plants grown under both salt-affected soil and irrigated with saline water of 4 dS m−1 increased onion bulb yield and large bulb percentage. It was interestingly found in this study that Se improved WUE by approximately twofold compared to non-Se-treated plants, due to the increase of bulb yield. Saline soil affects WUE, owing to the ion toxicity and the decrease water availability, as well as RWC, photosynthetic efficiency, and onion yield [], thereby increased as a result of exogenous Se application, as observed in this study. Correlation analysis indicated the Bulb yield was positively correlated with the other traits. This type of analysis can be used as a suitable tool to regulate any trait of them is positive and closely related to the Bulbs yield obtained []. In this study, significant correlations were observed among traits revealing the link between vegetative growth, photosynthetic capacity, plant water status, and the bulb yield of onion.
Endogenous Se contents increased by foliar-applied Se in leaves and bulbs of salt-stressed onion plants, which might be due to improved activities of the antioxidant defense molecules. Our results are concordant with that reported by [], which showed that Se supplementation increased Se content in shoots of maize plants under salinity stress.
Onion plants in this study produced more osmoprotectants like choline, soluble sugars, proline, and glycine betaine with the application of 50 mg L−1 Se (Table 6). In response to salt stress, plants react by accumulating more osmotically active solutes/osmoprotectants, such as proline, choline, soluble sugars, and glycine betaine. These osmoprotectants jointly, with endogenous Se, contribute in salt stress tolerance by osmotic regulation role for protection of the thylakoid membrane [], maintenance turgor pressure, thus preventing oxidative damage and photoinhibition, hence improved photosynthetic efficiency []. Refs. [,,] has noticed an accumulation of osmoprotectants by Se application for maintaining tissues water status in stressed plants, increasing RWC, as described by []. Osmotically-stressed plants sprayed with Se significantly increased its content from choline and glycine betaine (Table 6), this might be related to how Se up-regulates biosynthesis of choline/choline monooxygenase enzyme, which catalyzes the synthesis of glycine betaine [].
Under salt stress in this study, clear increases in AsA and GSH contents were observed in the Se-treated plant compared to the control (Table 7). Se mediated increase of AsA and GSH were also observed in tomato under drought [] and salinity [,] stress and wheat under cadmium stress [], indicating an improvement in the AsA-GSH cycle as an effective mechanism in scavenging ROS to alleviates the oxidative damages in cellular organelles under abiotic stress [,,,]. AsA is viewed as the most remarkable ROS scavenger, in light of its capacity to denote electrons in various enzymatic and non-enzymatic responses []. The activity of AsA and GSH largely correlated with regeneration and synthesis of the four enzymes DHAR, GR and MDHAR, and APX []. Thus, the balance of the AsA and GSH pool is linked with the activity of APX, that increased by Se application under this study (Table 7). Besides GSH directly detoxifying the ROS and maintaining the redox state in the AsA-GSH cycle, it plays a central role in the antioxidant defense system because of its capacity to regenerate AsA by reducing of DHA in the AsA-GSH cycle [,]. Se may also participate in the regeneration of AsA by up-regulating the associated enzymes, i.e., MDHAR and DHAR [].
Our results exhibited that exogenous Se application markedly elevated the activity of the antioxidant enzymes like APX, SOD, and CAT in onion leaves and bulbs, and GR only increased in onion leaves under salinity stress (Table 7). The activity of these enzymes is important for the detoxification of ROS in plant tissues, which helps to reduce the biomarkers of oxidative stress under salt stress. All the more explicitly, the activity levels and the balance between SOD and APX, or CAT activities in cells, are viewed as basic for deciding the steady-state level of O2− and H2O2 to prevent the formation of the highly toxic OH− []. In the enzymatic antioxidant defense system, SOD found in the frontline to scavenge ROS by dismuting O2− to H2O2 in the water–water cycle, the AsA–GSH cycle, and the glutathione–peroxidase cycle. Posteriorly, APX utilizes AsA as an electron donor in converting H2O2 to H2O as a part of the AsA–GSH cycle and the water–water cycle, also CAT scavenge H2O2 to H2O for ROS detoxification in peroxisomes during stress [,]. Results of the present study exhibited that under salinity stress, the activities of enzymatic and non-enzymatic antioxidants increased by Se application (Table 7), in coincidence with a reduction in cell membrane injury, increases in RWC, stomatal conductance, and photosynthetic efficiency (Table 4; Figure 1 and Figure 2). Thus, our findings suggest that Se alleviates salt stress on onion by enhancing the antioxidant activities. On the other hand, in a cytotoxicity study of Nano-Se (Se NP) on two types of human cell line, Se NP had the ability to increase percentage of growth inhibition of the tested cell lines via the induction of low molecular weight proteins, associated with total antioxidant value in seed of Nano-Se treated plants and vice versa, evidencing safety of the yield for human health []. Similar findings were also reported by [,].
5. Conclusions
Based on these results, it could be concluded that exogenous Se application showed a considerable potential positive effect to mitigate salt stress on onion plants, in the view of enhancing growth and productivity and improving water use efficiency via Se-mediated cell integrity (MSI) and maintained cell turgor (RWC). The protective role of Se could be also associated with the enhancement of enzymatic (APX, SOD, and CAT), and non-enzymatic (AsA and GSH) antioxidants, which together, increased osmoprotectants (proline, glycine betaine, choline, and soluble sugars) up-regulation and enhanced the photosynthetic efficiency. Se foliar application may therefore find in future a potential application as anti-abiotic stresses for minimizing the deleterious effects of salinity.
Author Contributions
Conceptualization, W.M.S., M.O.A.R. and T.A.A.E.-M.; methodology, W.M.S., S.M.H. and M.O.A.R.; formal analysis, T.A.A.E.-M., A.A. and W.M.S.; investigation, W.M.S., T.A.A.E.-M., H.A.A., K.A.H. and A.A.A.L.; resources W.M.S., T.A.A.E.-M., H.A.A., K.A.H., A.A.A.L., S.M.H. and M.O.A.R.; data curation, W.M.S., A.A., A.A.A.L. and T.A.A.E.-M.; writing—original draft preparation, A.A., W.M.S. and T.A.A.E.-M.; writing—review and editing, A.A., W.M.S., A.A.A.L. and T.A.A.E.-M.; visualization, W.M.S., A.A., S.M.H. and T.A.A.E.-M.; supervision, W.M.S., M.O.A.R., A.A. and T.A.A.E.-M.; project administration, W.M.S., M.O.A.R. and T.A.A.E.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data generated or analyzed during the current study are included in the published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ricciardi, L.; Mazzeo, R.; Marcotrigiano, A.R.; Rainaldi, G.; Iovieno, P.; Zonno, V.; Pavan, S.; Lotti, C. Assessment of genetic diversity of the “acquaviva red onion” (Allium cepa L.) apulian landrace. Plants 2020, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Data; Food and Agriculture Organization: Roma, Italy, 2019. [Google Scholar]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. Agronomy 1999, 38, 55–110. [Google Scholar]
- Rady, M.O.A.; Semida, W.M.; El-mageed, T.A.A.; Hemida, K.A.; Rady, M.M. Up-regulation of antioxidative defense systems by glycine betaine foliar application in onion plants confer tolerance to salinity stress. Sci. Hortic. 2018, 240, 614–622. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; El-Mageed, T.A.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z. Salinity: A major agricultural problem—Causes, impacts on crop productivity and management strategies. In Plant Abiotic Stress Tolerance Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer Nature: Cham, Switzerland, 2019; p. 490. ISBN 9783030061173. [Google Scholar]
- Desoky, E.M.; El-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Semida, W.M.; El-Mageed, T.A.A.; Mohamed, S.E.; El-Sawah, N.A. Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 1227–1239. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Mohamed, G.F.; Rady, M.M. Response of Solanum melongena L. seedlings grown under saline calcareous soil conditions to a new organo-mineral fertilizer. J. Anim. Plant Sci. 2015, 25, 485–493. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, R.S.; Semida, W.M.; Alharby, H.F. Modulation of salt stress effects on vicia faba l. plants grown on a reclaimed-saline soil by salicylic acid application. Rom. Agric. Res. 2017, 34, 175–185. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Moustafa, E.S.A.; El-Sobky, E.-S.E.A.; Farag, H.I.A.; Yasin, M.A.T.; Attia, A.; Rady, M.O.A.; Awad, M.F.; Mansour, E. Sowing Date and Genotype Influence on Yield and Quality of Dual-Purpose Barley in a Salt-Affected Arid Region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-mageed, T.A.; Howladar, S.M.; Rady, M.M. Foliar-applied α -tocopherol enhances salt-tolerance in onion plants by improving antioxidant defence system. Aust. J. Crop Sci. 2016, 10, 1030–1039. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Hemida, K.A.; Rady, M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018, 154, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Wassie, M.; Chen, L. Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ. Sci. Pollut. Res. 2020, 27, 9490–9502. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Kápolna, E.; Laursen, K.H.; Husted, S.; Larsen, E.H. Bio-fortification and isotopic labelling of Se metabolites in onions and carrots following foliar application of Se and 77 Se. Food Chem. 2012, 133, 650–657. [Google Scholar] [CrossRef]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Jalal, G.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H. Effect of exogenous selenium supply on photosynthesis, Na + accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I. Plant physiology and biochemistry phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Ponce, V.M.; Pandey, R.P.; Ercan, S. Characterization of drought across climatic spectrum. J. Hydrol. Eng. 2000, 5, 222–224. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Requirements, Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Israelsen, O.W.; Hansen, V.C. Irrigation Principles and Practices; John Wiley & Sons Inc.: New York, NY, USA, 1962. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity. Laboratory methods. Methods Soil Anal. 1986, 9, 687–734. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-NRCS: Washington, DC, USA, 2014; ISBN 0926487221.
- Dahnke, W.C.; Whitney, D.A. Measurement of soil salinity. In Recommended Chemical Soil Test Procedures for the North Central Region; Dahnke, W.C., Ed.; North Central Regional Publication 221; North Dakota Agricultural Experiment Station: St. Bull, ND, USA, 1988; Volume 499, pp. 32–34. [Google Scholar]
- Horwitz, W. (Ed.) AOAC Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995; Volume II, pp. 1058–1059. ISBN 0935584544. [Google Scholar]
- Jensen, M.E. Design and Operation of Farm Irrigation Systems; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1983; p. 827. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Spoustová, P.; Synková, H.; Valcke, R.; Čevrovská, N. Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic Pssu-ipt tobacco. Photosynthetica 2013, 51, 191–201. [Google Scholar] [CrossRef]
- Clark, A.J.J.; Landolt, W.; Bucher, J.B.B.; Strasser, R.J.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of cotton. 1. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Effect of humic acid as an additive to growing media to enhance the production of eggplant and tomato transplants. J. Hortic. Sci. Biotechnol. 2014, 89, 237–244. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wheeler, R.M.; Stutte, G.W.; Levine, L.H. How far can sodium substitute for potassium in red beet? J. Plant Nutr. 1999, 22, 1745–1761. [Google Scholar] [CrossRef]
- Bessieres, M.A.; Gibon, Y.; Lefeuvre, J.C.; Larher, F. A single-step purification for glycine betaine determination in plant extracts by isocratic HPLC. J. Agric. Food Chem. 1999, 47, 3718–3722. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; ISBN 0935584676.
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Abd El-Wahed, M.H. Effect of mulching on plant water status, soil salinity and yield of squash under summer-fall deficit irrigation in salt affected soil. Agric. Water Manag. 2016, 173, 1–12. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.B.A.A.; Hassan, M.M.; Shami, A. Sequential Application of Antioxidants Rectifies Ion Imbalance and Strengthens Antioxidant Systems in Salt-Stressed Cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Semida, W.M.; Rady, M.O.A. Silicon Defensive Role in Maize (Zea mays L.) against Drought Stress and Metals-Contaminated Irrigation Water. Silicon 2020, 1–12. [Google Scholar] [CrossRef]
- Melo, H.F.; de Souza, E.R.; Cunha, J.C. Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 232–237. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; El-mageed, T.A.A.; Howladar, S.M.; Shaaban, A. Foliage Applied Selenium Improves Photosynthetic Efficiency, Antioxidant Potential and Wheat Productivity under Drought Stress. Int. J. Agric. Biol. 2020, 24, 1293–1300. [Google Scholar] [CrossRef]
- Bybordi, A.; Saadat, S.; Zargaripour, P. The effect of zeolite, selenium and silicon on qualitative and quantitative traits of onion grown under salinity conditions. Arch. Agron. Soil Sci. 2018, 64, 520–530. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; Howladar, S.M.; Abd El-Mageed, T.A. Raised beds modulate physiological responses, yield and water use efficiency of wheat (Triticum aestivum L) under deficit irrigation. Agric. Water Manag. 2021, 106629. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Pascual, B.; Nájera, I.; Domene, M.A.; Baixauli, C.; Pascual-Seva, N. Effects of deficit irrigation on the yield and irrigation water use efficiency of drip-irrigated sweet pepper (Capsicum annuum L.) under Mediterranean conditions. Irrig. Sci. 2020, 38, 89–104. [Google Scholar] [CrossRef]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef]
- Proietti, P.; Nasini, L.; Del, D.; Amato, R.D.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hortic. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 2016, 6, 20917. [Google Scholar] [CrossRef] [PubMed]
- Khataar, M.; Mohhamadi, M.H.; Shabani, F. Soil salinity and matric potential interaction on water use, water use efficiency and yield response factor of bean and wheat. Sci. Rep. 2018, 8, 2679. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; El-Sherif, A.M.A.; Abd El-Mageed, S.A.; Abdou, N.M. A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric. Water Manag. 2019, 226, 105831. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Khattab, H.I.; Emam, M.A.; Emam, M.M.; Helal, N.M.; Mohamed, M.R. Effect of selenium and silicon on transcription factors NAC5 and DREB2A involved in drought-responsive gene expression in rice. Biol. Plant 2014, 58, 265–273. [Google Scholar] [CrossRef]
- Owusu-Sekyere, A.; Kontturi, J.; Hajiboland, R.; Rahmat, S.; Aliasgharzad, N.; Hartikainen, H.; Seppänen, M.M. Influence of selenium (Se) on carbohydrate metabolism, nodulation and growth in alfalfa (Medicago sativa L.). Plant Soil 2013, 373, 541–552. [Google Scholar] [CrossRef]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.-Y. Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1704–1721. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B.; El-Hallouty, S.M. Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol. Rep. 2019, 24, e00377. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B Biointerfaces 2012, 94, 304–308. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Faizy, S.E.D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.D.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium biofortification for human health: Opportunities and challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).