An Improved Vermicomposting System Provides More Efficient Wastewater Use of Dairy Farms Using Eisenia fetida
Abstract
1. Introduction
2. Materials and Methods
2.1. Dairy Farm Waste Collection and Worm Rearing
2.2. Vermicomposting Experimental Set-Up
2.3. The Biological Parameters of Earthworms
2.4. Physicochemical Parameter Analysis of the Vermicomposting Process and Germination tests
2.5. Microbial Diversity Analysis
2.5.1. Sample Collection
2.5.2. DNA Extraction and PCR Amplification
2.5.3. Illumina MiSeq Sequencing
2.5.4. Processing of Sequencing Data
2.6. Statistical Analysis
3. Results and Discussion
3.1. Consumption of Dairy Farm Wastewater during Vermicomposting
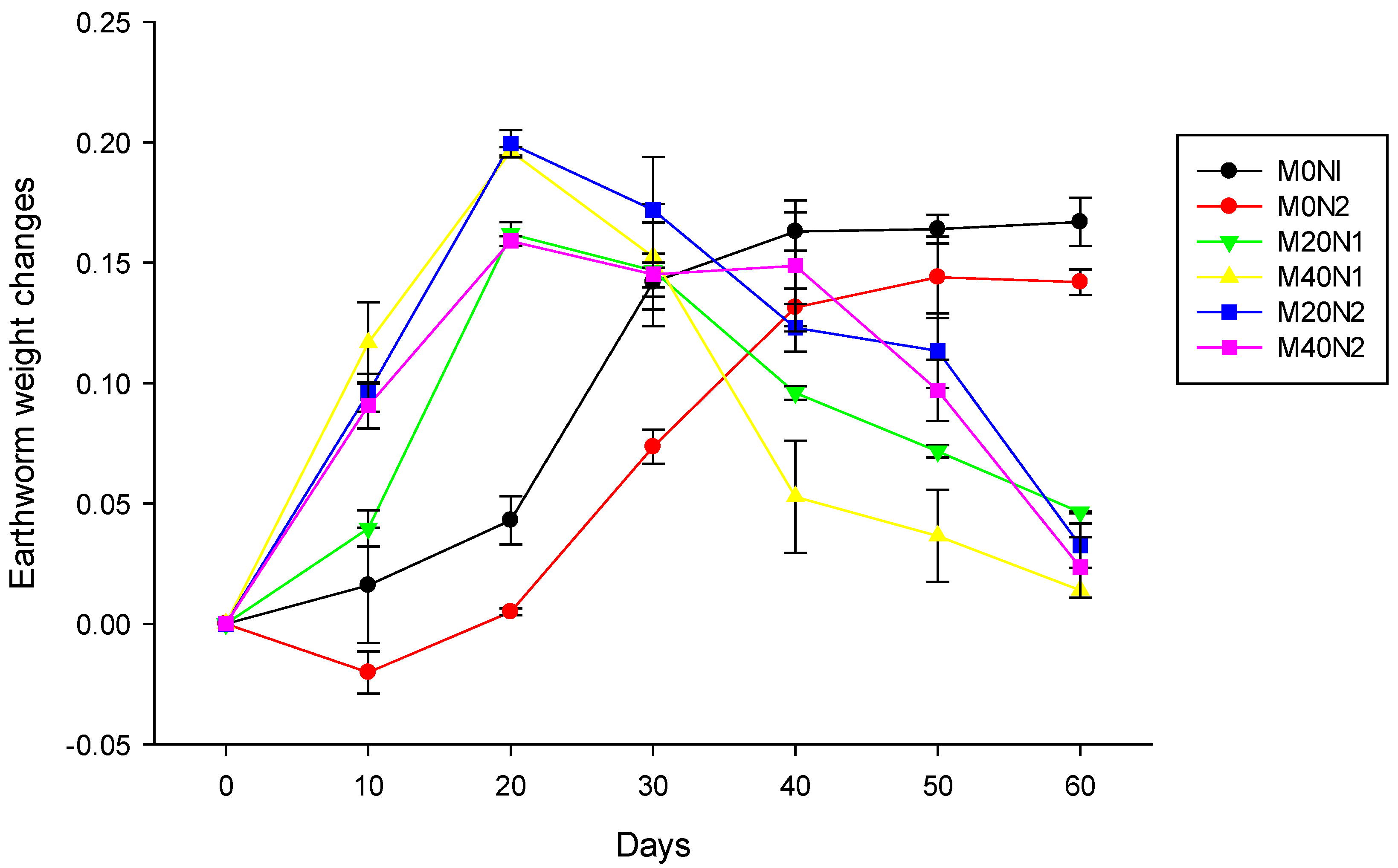
3.2. Earthworm Weight Changes during Vermicomposting
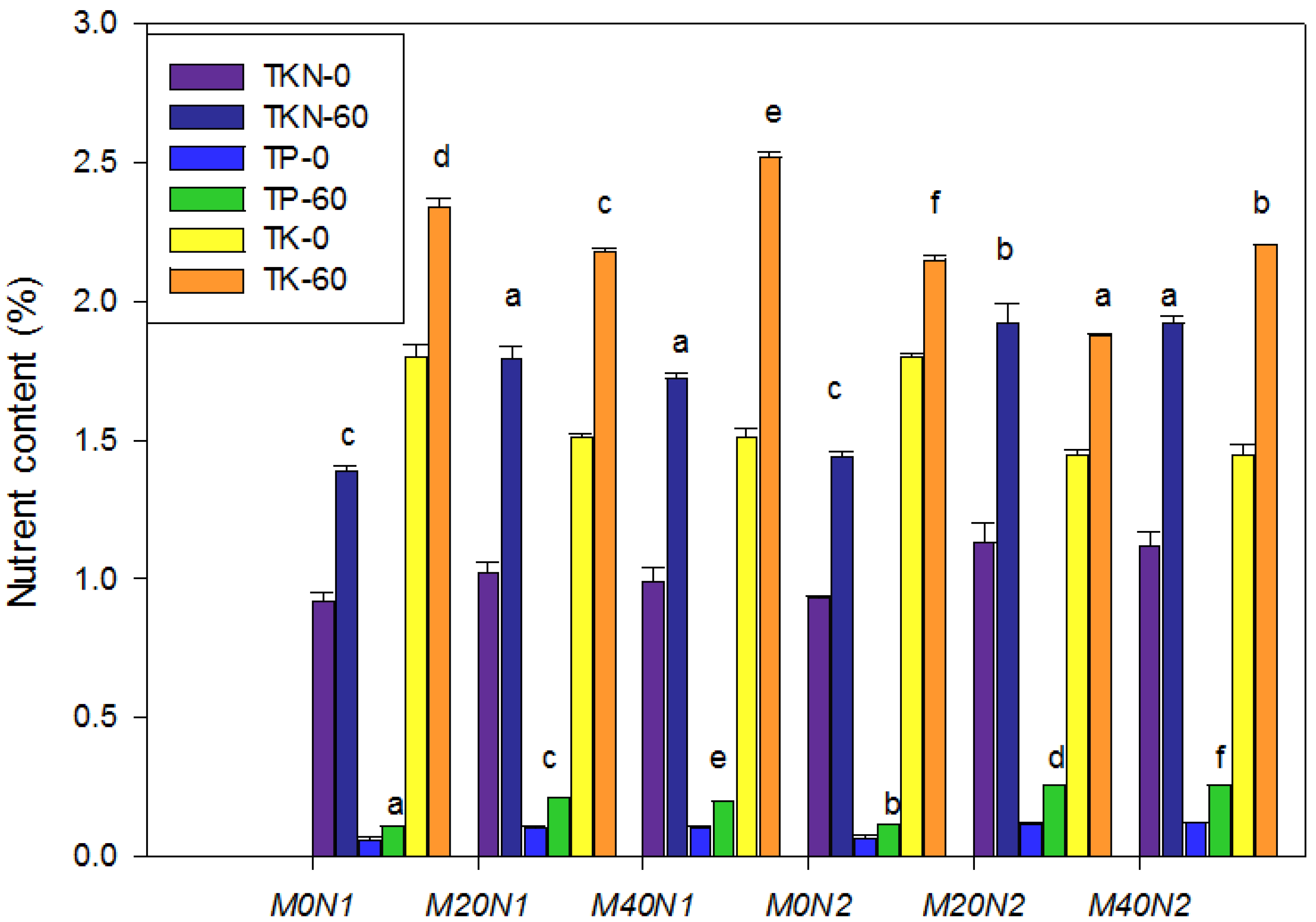
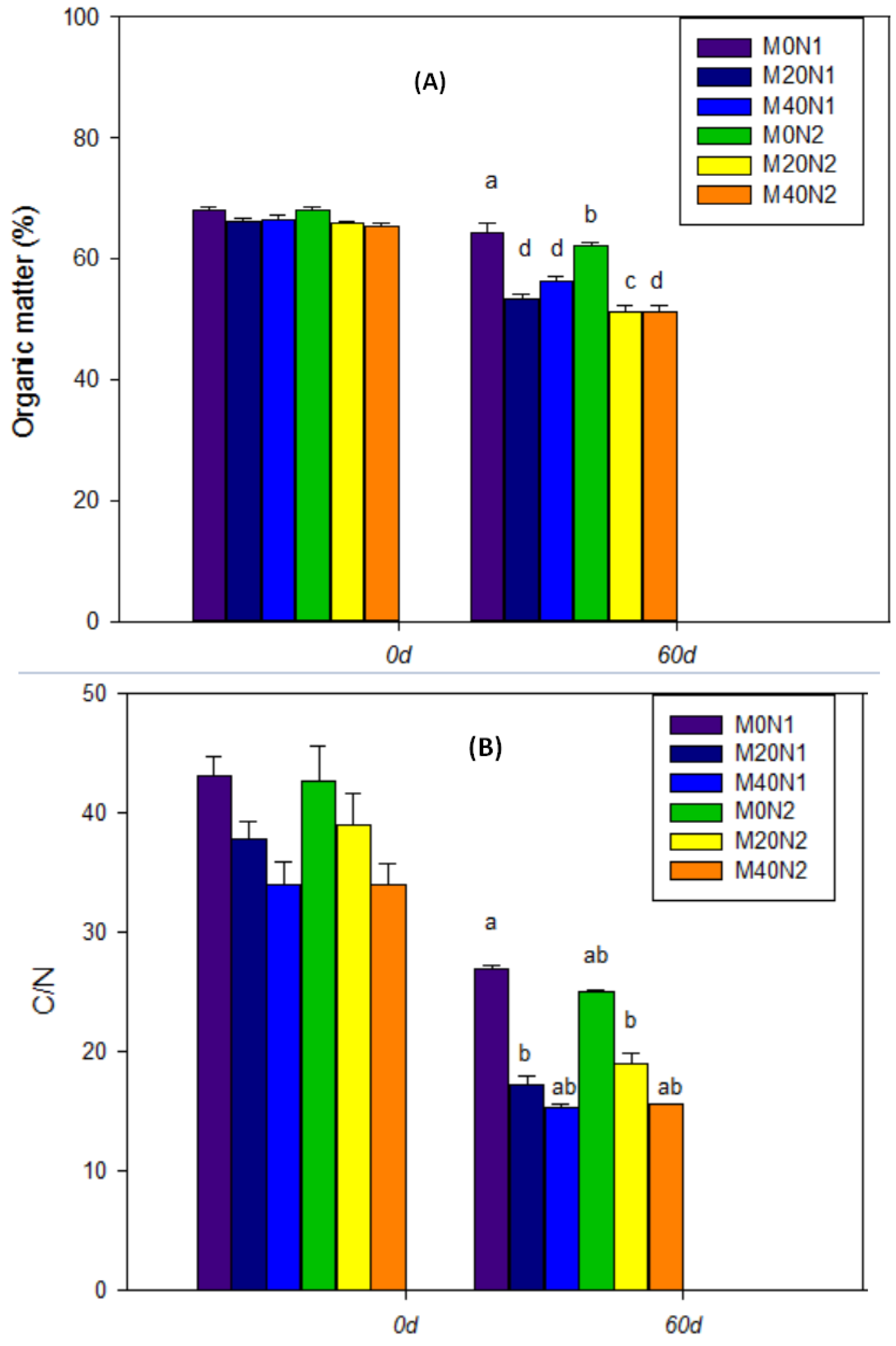
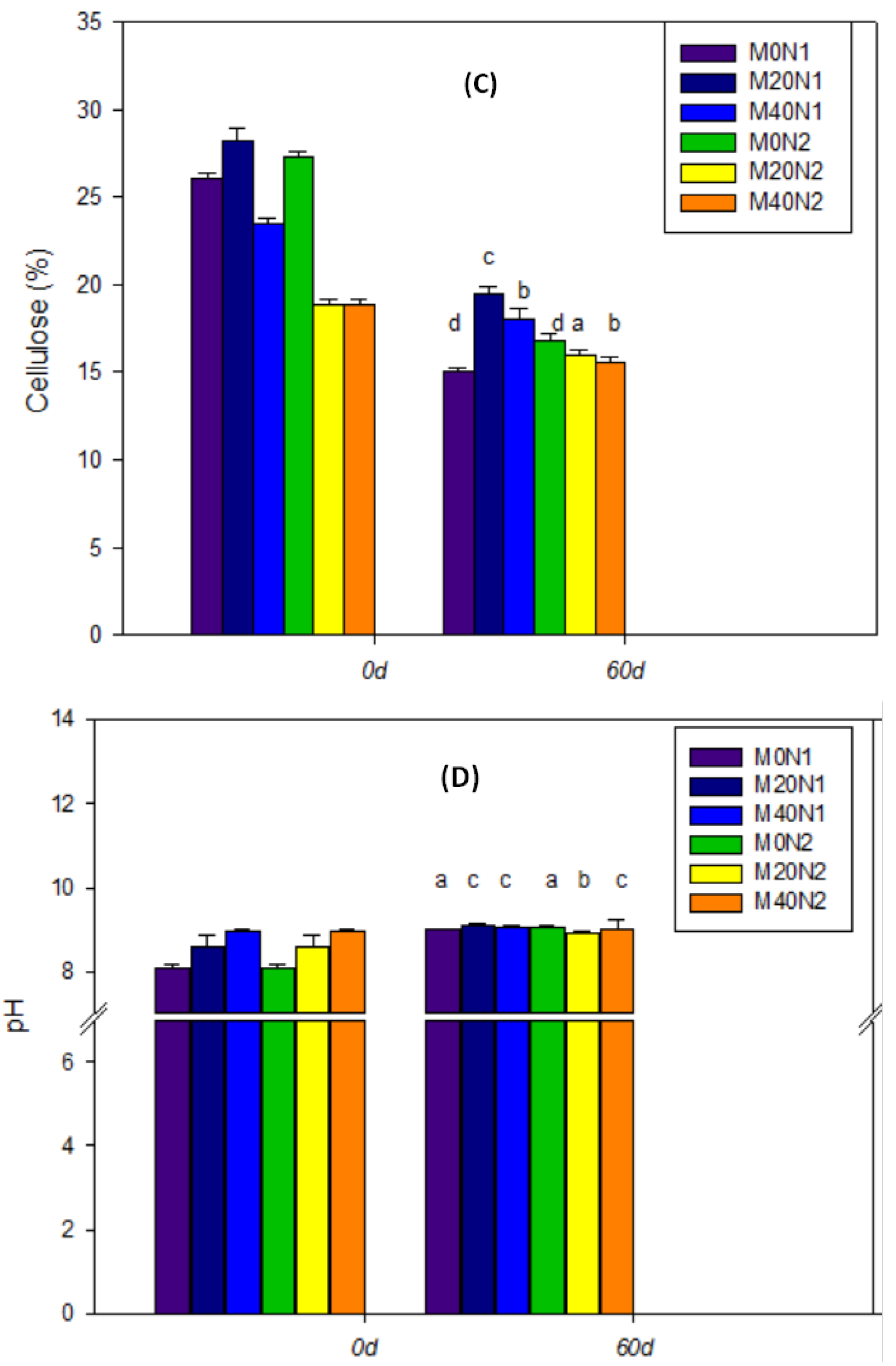
3.3. The Response of Physicochemical Parameters during the Vermicomposting Period
3.4. Phytotoxicity Test
3.5. Correlation of Biological and Chemical Indexes
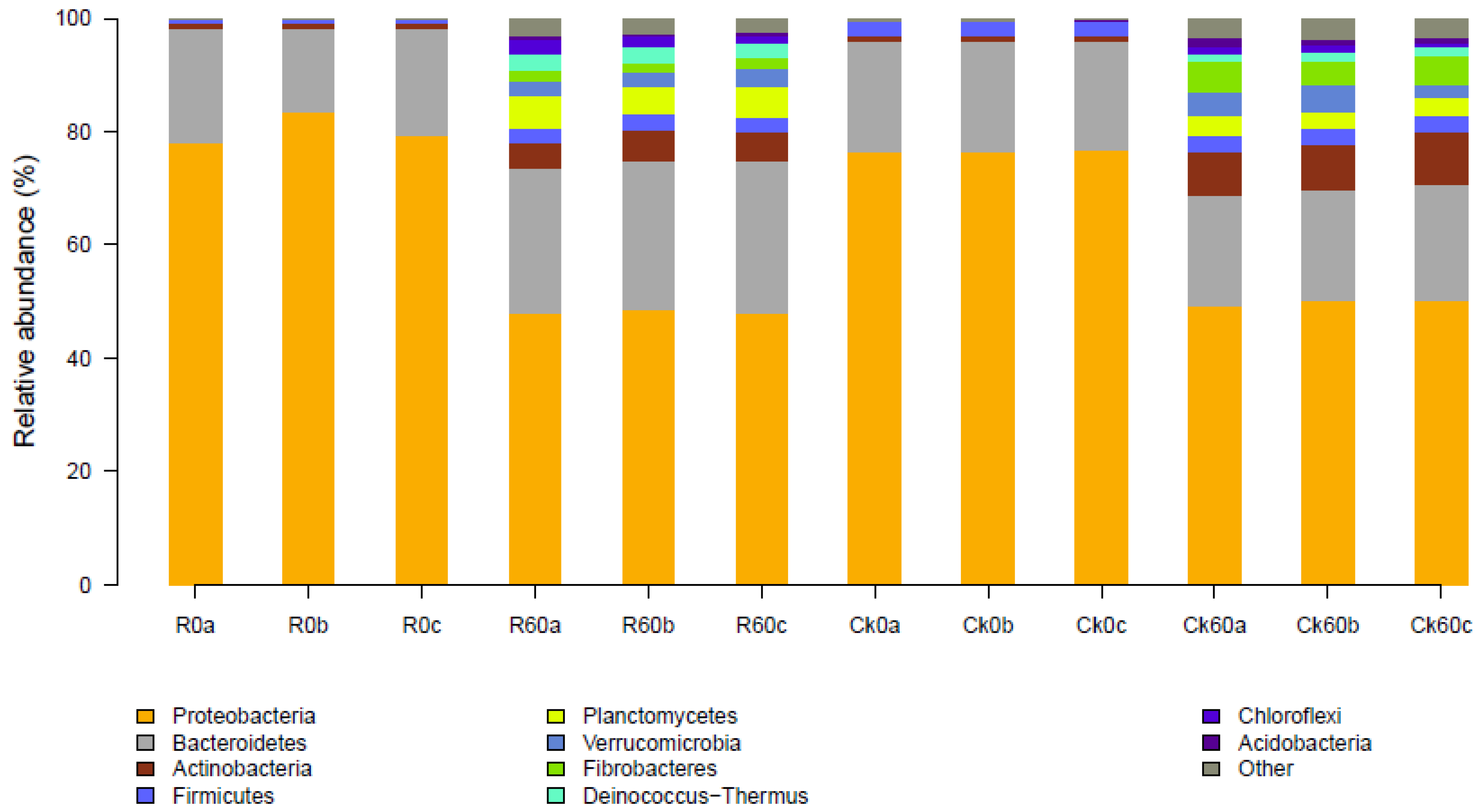
3.6. Composition of the Microbial Community
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NDCC. The First National Census Atlas of Pollution Sources; China Surveying and Mapping Publishing House: Beijing, China, 2011; ISBN 978750302485. The first National Data Compilation Committee on pollution sources census. [Google Scholar]
- Goncalves, M.R.; Costa, J.C.; Marques, I.P.; Alves, M.M. Strategies for lipids and phenolics degradation in the anaerobic treatment of olive mill wastewater. Water. Res. 2012, 46, 1684–1692. [Google Scholar] [CrossRef]
- Haugen, F.; Bakke, R.; Lie, B.; Hovland, J.; Vasdal, K. Optimal design and operation of a UASB reactor for dairy cattle manure. Comput. Electron. Agr. 2015, 111, 203–213. [Google Scholar] [CrossRef]
- Sergey, K.; Vyacheslav, F.; Alla, N. Anaerobic treatment of liquid fraction of hen manure in UASB reactors. Bioresour. Technol. 1998, 65, 221–225. [Google Scholar]
- Daud, M.K.; Rizvi, H.; Akram, M.F.; Ali, S.; Rizwan, M.; Nafees, M.; Jin, Z.S. Review of upflow anaerobic sludge blanket reactor technology: Effect of different parameters and developments for domestic wastewater treatment. J. Chem. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Sebastian, B.; Jarosław, D.; Laurence, W. Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Wast. Manag. 2014, 34, 513–521. [Google Scholar]
- Zhang, W.Q.; Wei, Q.Y.; Wu, S.B.; Qi, D.D.; Li, W.; Zuo, Z.; Dong, R. Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl. Energ. 2014, 128, 175–183. [Google Scholar] [CrossRef]
- Su, L.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar]
- Zziwa, A.; Jjagwea, J.; Kizitob, S.; Kabengea, I.; Komakecha, A.J.; Kayondoa, H. Nutrient recovery from pineapple waste through controlled batch and continuous vermicomposting systems. J. Environ. Manag. 2021, 279, 111784. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hernandez, J.C.; Domínguez, J. Dual Role of Vermicomposting in Relation to Environmental Pollution. In Bioremediation of Agricultural Soils; CRC Press: Boca Raton, FL, USA, 2019; p. 217. [Google Scholar]
- Rola, M.; Atiyeh, R.M.; Domínguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during processing by earthworms (Eiseniaandrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 44, 709–724. [Google Scholar]
- Lv, M.; Li, J.; Zhang, W.X.; Zhou, B.; Dai, J.; Zhang, C. Microbial activity was greater in soils added with herb residue vermicompost than chemical fertilizer. Ecol. Lett. 2020, 2, 209–219. [Google Scholar] [CrossRef]
- Sinha, R.K.; Agarwal, S.; Chauhan, K.; Valani, D. The wonders of earthworms & its vermicompost in farm production: Charles Darwin’s friends of farmers, with potential to replace destructive chemical fertilizers from agriculture. Agr. Sci. 2010, 1, 76–97. [Google Scholar]
- Patwa, A.; Parde, D.; Dohare, D.; Vijay, R.; Kumar, R. Solid waste characterization and treatment technologies in rural areas: An Indian and international review. Environ. Technol. Innov. 2020, 20, 101066. [Google Scholar] [CrossRef]
- Taylor, M.; Clarke, W.P.; Greenfield, P.F. The treatment of domestic wastewater using small scale vermicompost filter beds. Ecol. Eng. 2003, 21, 197–203. [Google Scholar] [CrossRef]
- Haimi, J.; Huhta, V. Comparison of composts produced from identical wastes by “vermistabilization” and conventional composting. Pedobiologia 1987, 30, 137–144. [Google Scholar]
- Kalembasa, S.J.; Jenkinson, D.S. A Comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. Sci. Food. Agri. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Crosland, A.R.; Zhao, F.J.; McGrath, S.P.; Lane, P.W. Comparison of aqua regia digestion with sodium carbonate fusion for the determination of total phosphorus in soil by inductively coupled plasma atomic emission spectroscopy (ICP). Commun. Soil Sci. Plan. 1995, 26, 1357–1368. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Khan, J.; Bundela, P.S.; Wong, J.W.C.; Selvam, A. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X.Y. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Datta, S.; Singh, J.; Singh, S.; Singh, J. Earthworms, pesticides and sustainable agriculture: A review. Environ. Sci. Pollut. R. 2016, 23, 8227–8243. [Google Scholar] [CrossRef]
- Deka, R.; Kumar, R.; Tamuli, R. Neurospora crassa homologue of neuronal calcium sensor-1 has a role in growth, calcium stress tolerance, and ultraviolet survival. Genetica 2011, 139, 885–894. [Google Scholar] [CrossRef]
- Partanen, P.; Hultman, J.; Paulin, L.; Auvinen, P.; Romantschuk, M. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Gu, W.J.; Zhang, F.B.; Xu, P.Z.; Tang, S.H.; Xie, K.Z.; Huang, X.; Huang, Q.Y. Effects of sulphur and Thiobacillus thioparus on cow manure aerobic composting. Bioresour. Technol. 2011, 102, 6529–6535. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, X.L.; Gao, W.; Qiu, J.P.; Li, Y.S. Comparative study of vermicomposting of garden waste and cow dung using Eisenia fetida. Environ. Sci. Pollut. Res. 2020, 27, 9646–9657. [Google Scholar] [CrossRef]
- Elvira, C.; Sampedro, L.; Benitez, E.; Nogales, R. Bioconversion of solid paper-pulp mill sludge by earthworms. Bioresour. Technol. 1998, 57, 173–177. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Thompson, S.A. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef]
- Bernal, M.P.; Paredes, C.; Sanchez-Monedero, M.A.; Cegarra, J. Maturity and stability parameters of composts prepared with a wide range of organic waste. Bioresour. Technol. 1998, 63, 91–99. [Google Scholar] [CrossRef]
- Bordna Mona. Compost Testing and Analysis Service—Interpretation of Results; Bordna Mona, Newbridge, Co.: Kildare, Ireland, 2003. [Google Scholar]
- NY 525-2012. People’s Republic of China Agricultural Industry Standard; China Agricultural Publishing House: Beijing, China.
- El-Haddad, M.E.; Zayed, M.S.; El-Sayed, G.A.M.; Hassanein, M.K.; El-Satar, A.M.A. Evaluation of compost, vermicompost and their teas produced from rice straw as affected by addition of different supplements. Ann. Ggr. Sci. 2014, 59, 243–251. [Google Scholar] [CrossRef]
- Aira, M.; Monroy, F.; Domínguez, J. C to N ratio strongly affects population structure of Eisenia fetida in vermicomposting systems. Eur. J. Soil Biol. 2006, 42, S127–S131. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparatives study. Bioresour. Technol. 2006, 97, 391–395. [Google Scholar] [CrossRef]
- Venkatesh, R.M.; Eevera, T. Mass reduction and recovery of nutrients through vermicomposting of fly ash. Appl. Ecol. Env. Res. 2008, 6, 77–84. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M.D. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Bajsa, O.; Nair, J.; Mathew, K.; Ho, G. Vermiculture as a tool for domestic wastewater management. Water. Sci. Technol. 2003, 48, 125–132. [Google Scholar] [CrossRef]
- Ravindran, B.; Contreras-Ramos, S.M.; Wong, J.W.C.; Selvam, A.; Sekaran, G. Nutrient and enzymatic changes of hydrolysed tannery solid waste treated with epigeic earthworm Eudrilus eugeniae and phytotoxicity assessment on selected commercial crops. Environ. Sci. Pollut. Res. 2014, 21, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Pan, S.Q.; Zhang, Z.Z.; Lin, X.Y.; Zhang, Y.Z.; Chen, S.H. Effects of the feeding ratio of food waste on fed-batch aerobic composting and its microbial community. Bioresour. Technol. 2017, 224, 397–404. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotech. 2011, 22, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Manz, W.; Amann, R.; Ludwig, W.; Vancanneyt, M.; Schleifer, K.-H. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 1996, 142, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Chiam, J.A.; Wang, J.Y. Microbial community structure reveals how micro aeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Zwart, G.; Crump, B.C.; Kamst-van Agterveld, M.P.; Hagen, F.; Han, S.K. Typical freshwater bacteria: An analysis of avail-able 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 2002, 28, 141–155. [Google Scholar] [CrossRef]
- Villar, I.; Alves, D.; Pérez-Díaz, D.; Mato, S. Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Wast. Manag. 2016, 48, 409–417. [Google Scholar] [CrossRef]
- Danon, M.; Franke-Whittle, I.H.; Insam, H.; Chen, Y.; Hadar, Y. Molecular analysis of bacterial community succession during prolonged compost curing. Fems. Microbiol. Ecol. 2008, 65, 133–144. [Google Scholar] [CrossRef]

| Parameter a | Treatments | |||||
|---|---|---|---|---|---|---|
| M0N1 | M0N2 | M20N1 | M40N1 | M20N2 | M40N2 | |
| Volume (mL) b | 980a | 980a | 895b | 895b | 770c | 770c |
| Total nitrogen (mg) c | 98d | 196a | 89.5e | 89.5e | 144c | 154b |
| Parameter a | Treatments | |||||
|---|---|---|---|---|---|---|
| M0N1 | M0N2 | M20N1 | M40N1 | M20N2 | M40N2 | |
| Weight changes of per worm (g) b | 0.16 ± 0.02a | 0.11 ± 0.02ab | 0.06 ± 0.02c | 0.02 ± 0.003d | 0.05 ± 0.05bc | 0.06 ± 0.03bc |
| Germination index (%) | 81.07 ± 1.35a | 81.13 ± 4.95a | 75.64 ± 7.74a | 70.16 ± 5.63a | 71.86 ± 4.20a | 68.66 ± 8. 97a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Geng, B.; Zhu, C.; Li, L.; Francis, F. An Improved Vermicomposting System Provides More Efficient Wastewater Use of Dairy Farms Using Eisenia fetida. Agronomy 2021, 11, 833. https://doi.org/10.3390/agronomy11050833
Liu X, Geng B, Zhu C, Li L, Francis F. An Improved Vermicomposting System Provides More Efficient Wastewater Use of Dairy Farms Using Eisenia fetida. Agronomy. 2021; 11(5):833. https://doi.org/10.3390/agronomy11050833
Chicago/Turabian StyleLiu, Xue, Bing Geng, Changxiong Zhu, Lianfang Li, and Frédéric Francis. 2021. "An Improved Vermicomposting System Provides More Efficient Wastewater Use of Dairy Farms Using Eisenia fetida" Agronomy 11, no. 5: 833. https://doi.org/10.3390/agronomy11050833
APA StyleLiu, X., Geng, B., Zhu, C., Li, L., & Francis, F. (2021). An Improved Vermicomposting System Provides More Efficient Wastewater Use of Dairy Farms Using Eisenia fetida. Agronomy, 11(5), 833. https://doi.org/10.3390/agronomy11050833







