Abstract
Elevated atmospheric CO2 (eCO2) can significantly enhance plant metabolism and improve their nutritional quality. Although several studies addressed the effect of eCO2 on plants, to our knowledge, there are no studies that have applied eCO2 to improve growth, chemical composition, and biological properties of ajwain (Trachyspermum ammi) during sprouting. Herein, eCO2 (620 µmol mol−1) was employed to enhance photosynthetic reactions. Improved photosynthesis induced primary and bioactive secondary metabolism, which led to improved biological activities of T. ammi sprouts in comparison with control sprouts and seeds. eCO2-treated sprouts showed significantly (p < 0.05) higher levels of most measured nutritional primary and secondary metabolites like soluble sugars, essential amino acids, organic acids, essential oils, phenolics, and flavonoids than control sprouts, which had significantly higher values than control seeds. eCO2 significantly improved the antimicrobial properties of T. ammi sprouts against 11 out of 13 microbial species than control sprouts, which had more potent antimicrobial activities than seeds. The significant increase in the antioxidant potential of treated sprouts was indicated by improved levels of ferric reducing antioxidant power (FRAP), DPPH, and oxygen radical absorbance capacity (ORAC). In addition, the anticancer activity against four different human tumor cell lines was significantly (p < 0.05) boosted by both sprouting and eCO2 exposure. Thus, the present study confirms the synergistic effect of sprouting with eCO2 exposure as promising approaches to produce ajwain sprouts with rich nutraceuticals, effective phytochemicals, and enhanced biological activities.
1. Introduction
Plant sprouts are valuable sources of protein, minerals, vitamins, and phenolic compounds []. They are the young seedlings produced through seed germination. They were globally used for thousands of years as vegetables and became more popular in western countries as a functional food []. Additionally, sprouts are considered to be healthy foods due to their content of several bioactive metabolites that have a positive impact on human health, such as the prohibition of cancer and cardiovascular diseases []. Furthermore, they have high levels of oligo- and monosaccharides, free fatty acids, oligopeptides, and amino acids, in addition to vitamins and valuable phytochemicals. They also contain low levels of antinutritive compounds compared to mature plants [,,].
Herbal plants have been used since the beginning of human civilization for the treatment of many illnesses. Many of these herbs, because of their pharmaceutical properties, became current drug candidates []. Out of these herbs, the family Apiaceae has been widely studied due to its countless biological activities []. One important species belonging to this family is Trachyspermum ammi (T. ammi), which is a highly important medicinal plant. Fruits of T. ammi are reported to have antiseptic, antifungal/antibacterial, and anthelmintic properties []. The seeds of this plant species contain 2–4.4% brown-colored oil known as ajwain oil. The main component of this oil is thymol, which is used for the treatment of gastrointestinal ailments, lack of appetite, and bronchial problems []. The oil exhibits fungicidal, [] antimicrobial [], and anti-aggregatory activities []. In addition, the fruit has antispasmodic and carminative activities and is used traditionally as a remedial agent for many gastrointestinal and respiratory disorders. Additionally, the roots are diuretic, and the seeds possess excellent aphrodisiac properties []. Therefore, numerous studies have been conducted to improve the levels of the phytochemical compounds in sprouts.
In this concern, the growth and environmental conditions, such as abiotic stress and climate change, were reported to influence the accumulation of phytochemicals in plant tissues []. Additionally, improving the secondary metabolite contents in plants could be achieved by the application of elicitors, which are growth factors that can induce the metabolic rhythm in plants []. As a response to environmental stress or elicitor, plants modify their metabolism and accumulate specific metabolites to survive the stress. The aggregation of these beneficial metabolites could increase the nutritional and health-promoting values of plants. In this regard, elevated atmospheric CO2 (eCO2) is considered a novel environmental factor to improve plant growth and to enhance their nutritive value []. Therefore, many pieces of research have examined the effect of eCO2 on plant growth and metabolism. It was reported that eCO2 is a way that assists in enhancing the photosynthetic C assimilation, to promote the aggregation and breakdown of carbohydrates through dark respiration [,]. This approach could provide the precursor and metabolic energy needed for the formation of different phytochemicals. Hence, eCO2 fertilization could be an effective way to improve the nutritional and health-promoting values of plants []. The effect of CO2 fertilization on the growth and accumulation of bioactive phytochemicals was reported in several plant species, such as broccoli sprouts [] and buckwheat sprouts []. However, to the best of our knowledge, no data are available about the effect of eCO2 on the growth, nutritive value, or pharmaceutical effects of T. ammi sprouts.
Therefore, we conducted this study to investigate the effect of eCO2 on the growth, photosynthesis, nutraceutical values in terms of the contents of sugars, amino acids, organic acids, and essential oils, phenolic and flavonoid profiles, and pharmaceutical effects including antioxidant, anticancer, and antimicrobial properties of T. ammi sprouts. The data were compared to control seeds and control sprouts grown under ambient CO2 level, and the mechanisms behind those effects were also explored. We assumed that both sprouting and eCO2 exposure might improve the growth and enhance the nutritional and phytochemical compounds in T. ammi, which can open the way for multiple promising applications of this herb in the functional food and pharmaceutical industries.
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
Seeds of Trachyspermum ammi were sterilized with sodium hypochlorite (5 g seeds /L) for 1 h then washed with distilled water. Two experiments in controlled conditions have been performed. A pilot experiment was set up to demonstrate the effect of elevated CO2 on Trachyspermum ammi sprouting. In this experiment, the seeds were germinated on vermiculite and watered with Milli-Q water twice per week. Seed germination and seedling growth occurred in a growth chamber and under controlled growth conditions (25 °C air temperature, 16-h light/8-h dark cycle, photosynthetically active radiation (PAR) of 380 µmol m−2 s−1 and 55% humidity). T. ammi seeds were subjected to two CO2 conditions: (1) Ambient CO2 (aCO2, 400 ± 27 µmol CO2 mol−1 air), and (2) elevated CO2 (eCO2, 620 ± 42 µmol CO2 mol−1 air) for the second group. Three trays each containing 10 plants were used for each treatment. The concentration of eCO2 was selected according to IPCC-SRES B2-scenario prediction of high CO2, which was continuously monitored by CO2 analyser system (WMA-4, PP Systems, Hitchin, UK). After 12 days, physiological analyses were performed; moreover, the sprouts from each group were taken to calculate the fresh and dry mass (Table S1). On the basis of the results obtained in the pilot experiment, we repeated this experiment on a large scale (6 trays per treatment) under the same growth conditions. Twelve-day-old sprouts were used for physiological and biomass analyses. Then, the sprouts were frozen in liquid nitrogen and stored (−80 °C) for biochemical and bioactivity analysis.
2.2. Physiological Analyses
Photosynthesis and dark respiration of treated T. ammi sprouts were measured by utilizing EGM-4 infrared gas analyzer (PP Systems, Hitchin, UK) and calculated as µmol CO2 m−2 s−1. The photosynthetic rate was determined from 3-min measurements of net exchange of CO2, in polymethyl pentene cuvette (13-cm high and 6-cm wide). The measurement conditions were controlled at 390 ppm CO2 and 24 °C at PAR (350 µmol m−2 s−1). Whole sprout photosynthetic and dark respiration were determined from the measurements of net CO2 exchange for 180 s using the same sprouts by darkling the polymethyl pentene cuvette of EGM-4 and the same CO2 and temperature conditions.
2.3. Determination of Primary Metabolises
Individual sugar levels in sprout samples were measured by high-performance liquid chromatography (HPLC, UPLC TQD device (Milford, Worcester County, MA, USA)) []. Sugars were extracted in acetonitrile/water (2 mL, 1:1, v/v). After boiling for 15 min at 55–60 °C, samples were filtered through filter paper (a Whatman No. 541). The column temperature was 30 °C, the injection volume was 20 µL, and the mobile phase (acetonitrile and HPLC-grade water, 75:25 (v/v)) was run at 1 mL min−1 rate. Identified sugars were quantified by using a calibration curve of the corresponding standards.
Organic acids were performed according to []. Frozen sprouts tissues were extracted in 0.1% phosphoric acid and then centrifuged for 30 min (14,000 g, 4 °C). The supernatants were filtered through 0.2-µm pore size Millipore microfilters and used to detect organic acids (HPLC, SUPELCOGELC-610H column and UV detector, LaChrom L-7455 diode array, Japan). The mobile phosphoric acid (0.1%, v/v) was run at a flow rate of 0.45 mL min−1.
The essential oils were analyzed [] using GC mass spectroscopy in T. ammi sprouts under air temperature. Then the dried sprouts were treated with steam distillation (2.5 h) using a Clevenger-type instrument and the essential oil content was then calculated as mg/g FW.
Amino acids were extracted in 150 mg of T. ammi sprouts using aqueous ethanol (80%, v/v). Centrifugation of extracted samples was done at 14,000 rpm for 30 min. The centrifuged spouts were re-extracted in water and mixed with the pellet suspended in chloroform. Then the extracts were centrifuged (10 min at 20,000 g) and supernatants were filtered (Millipore microfilters, 0.2 µm pore size). Amino acids were measured (Waters Acquity UPLC-tqd system (Milford, MA, USA) and separated by BEH amide 2.1 × 50 column []. Then 10 µL of each sample was injected and eluted with a gradient of solvent A (0.1% formic acid in H2O) and solvent B (0.1% FA in acetonitrile) at 0.5 mL/min. Norvaline was used as an internal standard.
2.4. Measurement of Secondary Metabolises
HPLC (Shimadzu HPLC system (SCL-10 AVP, Tokyo, Japan) was used to measure the levels of phenolic acids and flavonoids in T. ammi sprouts treated with ambient and high CO2 []. The metabolites were identified by comparing the peak areas of the corresponding standard. Approximately 100 mg of freeze-dried sprouts were homogenized in an acetone-water solution (4:1 v/v) for 20 h. Phenolic acids and flavonoids were quantified on an equipped with a Lichrosorb Si-60, 7 µm, 3 × 150 mm column, and a diode array detector (SPDM10AVP). Water/formic acid (90:10, v/v) and acetonitrile/water/formic acid (85:10:5, v/v/v) were used as mobile phase at a flow rate (0.8 mL min−1) and 3,5-dichloro-4-hydroxybenzoic acid was added to samples as an internal standard.
2.5. Evaluation of Biological Activities
The antioxidant capacity was measured by using ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), and DPPH (diphenyl picrylhydrazyl) methods []. About 0.25 g of the seed powder or 150 mg of T. ammi sprouts were extracted in 80% ethanol and centrifuged at 4 °C for 25 min at 14,000 rpm. Then 0.025 mL of extracts were used to determine the antioxidant capacity after mixing with 0.175 mL of FRAP reagent consisting of 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), FeCl3 (20 mM) in acetate buffer (0.25 M, pH 3.6) at room temperature. Regarding DPPH and ORAC, the antioxidant capacity was measured by mixing the sprout extracts (0.025) with 0.25 mL of the DPPH or ORAC (in presence of Cu2+ and H2O2) solution. After incubation at room temperature, the absorbance was measured at 517 nm for FRAP and DPPH and 600 mm for ORAC using the spectrometric method. The copper-initiated prooxidant activity was calculated using [(Area Blank—Area Sample)/Area Blank] × 100 and expressed as prooxidant units; one unit equals the prooxidant activity that reduces the area under the fluorescein decay curve by 1% in the ORAC assay. All values were expressed as Trolox equivalents.
To measure the in vitro cytotoxicity of T. ammi, about 0.2 g of T. ammi seeds and sprouts were grounded in liquid nitrogen and then extracted in ethanol and centrifuged at room temperature for 25 min at 800,000 rpm. Then, the supernatant was filtered (Whatmann No. 1 filter paper) and concentrated by using a rotary evaporator, the samples were stored at −80 °C until use. The in vitro cytotoxicity of T. ammi extracts against human tumor cell lines—namely cell lines hepatocellular carcinoma (HepG2), colon carcinoma (Colo205), embryonic kidney adenocarcinoma (293) and urinary bladder carcinoma (T24P)—was carried out following the protocol described in Almuhayawi et al. []. Dulbecco’s modified eagle medium (DMEM) (10% fetal calf serum, Na-pyruvate, 1mg/0.1 mL streptomycin, 1U/0.1 mL penicillin) was used as a medium which was supplied with the seeds and sprout extracts (0.02 mg/mL). Then, the cell cultures were incubated at 37 °C and 5% CO2 until a confluent growth was reached. The cells were collected by trypsinization (trypsin solution (0.25% trypsin w/v) and the vital cells were detected by CellTiter-Blue reagent (Promega, Madison, WI, USA).
The antibacterial activities of ethanolic extracts of T. ammi sprouts were investigated by the disc diffusion method (bacterial suspension of 106 CFU/mL of the bacterial test strain spread on Muller Hinton agar). The extracts loaded on sterilized filter paper discs (10 µg/disc) were used and those loaded with ethanol were used as a negative control. These discs were placed on the agar plates contained the tested bacteria and incubated for 24 h at 37 °C. The inhibition zones were measured by Vernier caliper.
2.6. Statistical Analysis
The statistical analysis was carried out using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). The experiment of seed germination and seedlings growth under ambient and elevated CO2 conditions was replicated twice. Both experiments showed the similar effect of eCO2 on sprouts growth. All growth and biochemical analyses were performed using plant samples from the second experiment. For all assays, 4 replicates were used, and each replicate corresponded to a group of sprouts harvested from a certain tray. One-way analysis of variance (ANOVA) was done. Tukey’s test was used as the post-hoc test for the separation of means (p < 0.05).
3. Results and Discussion
In addition to changes in plant phenology, climate change also affects plant growth and plant tissue chemical composition []. This effect of global change factors including high temperature and CO2 on plant chemical composition is plant species-specific and the type of environmental stressor. For instance, increased CO2 on plant affects chemical composition []. Thus, further research on chemical production efficiency of medicinal plants under climate changing scenario is important for developing agro-technologies for cultivation.
3.1. Effect of eCO2 on the Growth, Photosynthesis, and Respiration of T. ammi Sprouts
The effect of eCO2 exposure on the growth, photosynthesis, and respiration of T. ammi sprouts was evaluated (Table 1). Apparently, the exposure to the high level of CO2 induced the growth rates of T. ammi sprouts, which was clear in the differences between the fresh and dry weights of control and eCO2-treated sprouts (p < 0.05, Supplementary Table S1). In addition, eCO2 significantly improved (p < 0.0.5) the photosynthesis and slightly enhanced the respiration of this plant.

Table 1.
The effect of elevated CO2 (eCO2) treatment versus ambient CO2 (control) on fresh weight, dry weight, photosynthesis, and respiration of Trachyspermum ammi sprouts. Data are represented by means ± standard deviations of at least 8 replicates. Different a, b letters within a row indicate a significant difference between means at p < 0.05.
In context, CO2 is essential for plant photosynthesis, so its increase directly enhanced the photosynthesis of plants []. Accordingly, increasing the photosynthesis level induced the accumulation of soluble sugars, starch, and organic acids that increase plant growth. It is well documented that eCO2 could improve the photosynthetic rate by suppressing the oxygenation reaction of rubisco, leading to improved C gain []. For instance, high CO2 (620 ppm) significantly increased the biomass of two cultivars of broccoli sprouts by about 1.5 to 1.8-fold compared to ambient CO2 concentration []. As well as eCO2 exposure enhanced most measured physical parameters in fenugreek seeds, including seed length, width, mass, thickness, and pod length [].
3.2. Sprouting and eCO2 Improved the Tissue Quality of T. ammi
3.2.1. Improved Photosynthesis Induced the Primary Metabolism
Following the assessment of eCO2 effect on growth and photosynthesis of T. ammi sprouts, we evaluated this effect on the primary metabolism of T. ammi sprouts in comparison with control sprouts and control seeds: i.e., sugars, amino acids, organic acids, and essential oils (Table 2 and Table 3). The treatment with eCO2 significantly improved (p < 0.05, Supplementary Table S2 the contents of starch and total carbohydrates, as well as increasing the concentrations of glucose, sucrose, and fructose in T. ammi sprouts compared to those of control sprouts, which showed higher values than seeds (Table 2). In this regard, Al Jaouni et al. [] stated that the eCO2-induced plant growth of peppermint and basil was consequent with the improved rate of photosynthesis and accumulation of non-structural carbohydrates (NSC), i.e., sucrose, fructose, glucose, and starch. This elevation in the level of NSC could clarify the fertilizing effect of eCO2 as sugars well-known for their pleiotropic roles in the regulation of plant growth and metabolism.

Table 2.
The levels of sugars, amino acids, and organic acids in control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi. Data are represented by means ± standard deviations of at least 4 replicates. Different a, b, c letters within a row indicate a significant difference between means at p < 0.05.

Table 3.
The levels of essential oils in control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi. Data are represented by means ± standard deviations of at least 4 replicates. Different a, b, c letters within a row indicate a significant difference between means at p < 0.05.
Commonly, the nutritional and health-promoting values of plants could be related to their content of essential amino acids []. Free amino acids are also precursors for health-promoting metabolites such as glucosinolates. Accordingly, the improvement in the level of essential amino acids in ajwain sprouts by applying a promising approach such as high CO2 treatment might increase their nutritional values. In the current study, the most predominant amino acids in T. ammi seeds and sprouts grown under control air condition were glutamine [69.2 ± 2.6 and 76.2 ± 1.46 mg/g fresh weight (FW), respectively] and glutamic acid (24.1 ± 1.2 and 39.1 ± 1.2 mg/g FW, respectively) followed by alanine (5.8 ± 0.9 and 11.9 ± 0.39 mg/g FW, respectively). Interestingly, the treatment with eCO2 significantly enhanced (p < 0.05, Supplementary Table S2) the levels of most targeted amino acids in T. ammi sprouts (Table 2). Given that ajwain sprouts are rich in glutamine and asparagine, particularly eCO2-treated, they could be considered a significant functional food or medicinal herb for ensuring the proper function of our nervous system, with glutamine being a well-known important nutrient for immune cells, lymphocyte proliferation, and the production of cytokine, in addition to its role in controlling neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease []. As well as asparagine, an important amino acid for protecting the human nervous system and increasing stress resistance is abundant in T. ammi []. The accumulation of amino acids as a result of eCO2 exposure could be explained in the light of the concept that the coordination between N and C metabolisms is well documented at both biochemical and transcriptional levels, as the synthesis of organic N requires C skeleton and energy being provided by C metabolism []. Hence, high CO2 may promote the formation of amino acids by increasing the availability of required intermediates and energy through upregulation of C metabolism.
Additionally, eCO2-treated T. ammi sprouts showed significant (p < 0.05) higher levels of most measured organic acids than those of T. ammi sprouts grown under control air conditions, whereas control sprouts had significantly (p < 0.05, Supplementary Table S2)) higher levels of 5 out of 6 organic acids than control seeds. Oxalic and malic acids (2.2 ± 0.53 and 2.19 ± 0.44 mg/g FW, respectively) were the most predominant organic acids in control T. ammi sprouts. While they contained very negligible amounts of fumaric acid (0.36 ± 0.16 mg/g FW), these values were significantly raised by eCO2 treatment (Table 2).
The enhanced primary metabolism in eCO2-treated ajwain sprouts could be attributed to the significant improvement in photosynthesis. The eCO2-induced plant growth was consequent with the improved rate of photosynthesis and accumulation of NSC (sucrose, fructose, glucose, and starch). Such accumulation of NSC could explain the fertilization effect of eCO2 because sugars are known to have pleiotropic roles in the regulation of plant growth and metabolism. Al Jaouni et al. [] found that high CO2 did not only improve the levels of NSC in basil and peppermint, but also promoted their breakdown via dark respiration and, consequently, affected the concentrations of tricarboxylic acid (TCA) cycle intermediates. Moreover, under sufficient N, fumarate, malate, and total contents of TCA organic acids increased in Arabidopsis grown under eCO2 []. The upregulation of carbohydrate metabolism and dark respiration has been observed in plants grown under eCO2 []. Similarly, Li et al. [] reported that the elevation in the level of plant photosynthesis enhances the production of soluble sugars, starch, and organic acids. In addition, enrichment of atmospheric CO2 augmented the photochemical efficiency in broccoli leaves [] and increased the level of soluble carbohydrates in parsley and dill shoots [].
Furthermore, we investigated the effect of eCO2 treatment on the concentrations of essential oils in T. ammi sprouts versus control sprouts and seeds (Table 3). α-terpinene and cymene were the most prevalent essential oils in T. ammi seeds (15.5 ± 1.7 and 24.4 ± 2.13 mg/g FW, respectively) and control sprouts (26.5 ± 1.27 and 24.4 ± 2.11 mg/g FW, respectively). Interestingly, 5 out of 14 essential oils were significantly (p < 0.05) higher in control sprouts than seeds. Additionally, although eCO2 increased the concentrations of most examined essential oils, only 6 out of 14 essential oils were significantly elevated (p < 0.05, Supplementary Table S2) as a result of eCO2 treatment of sprouts. Excitingly, thymol, which is one of the most important antibacterial compounds in T. ammi, was among those essential oils which were significantly improved (p < 0.05) by eCO2 exposure (Table 3).
To conclude, the improvement in photosynthesis by eCO2 treatment was reflected by enhancement in the primary metabolism of T. ammi sprouts which led to elevations in the levels of sugars, amino acids, organic acids, and essential oils.
3.2.2. Improved Photosynthesis Induced the Secondary Metabolism
Given the dangerous effect of reactive oxygen species (ROS) and free radicals on human health, a crucial factor that determines the health-promoting values of plants is their content of antioxidant metabolites such as phenolic compounds, vitamins, and some minerals [,]. Thus, we investigated the effect of applying eCO2 on T. ammi sprouts on the levels of secondary metabolites in terms of individual and total phenolics and flavonoids in comparison with control sprouts and seeds (Table 4). Regarding individual phenolics, protocatechuic acid (3.62 ± 0.20 and 3.62 ± 0.26 mg/g FW, respectively) followed by p-coumaric acid (1.72 ± 0.4 and 1.52 ± 0.32 mg/g FW, respectively) were the most predominant phenolics in T. ammi seeds and sprouts under control air conditions. Control sprouts had slightly higher values of most measured phenolics and flavonoids than control sprouts. Then by exposure of sprouts to eCO2, the concentrations of most measured individual phenolics were significantly enhanced (p < 0.05, Supplementary Table S2). Additionally, total phenols and total flavonoids were significantly (p < 0.05, Supplementary Table S2) higher in control sprouts than control seeds. In addition, they were significantly (p < 0.05) higher in eCO2-treated sprouts than in control sprouts as they accounted for 4.2 ± 0.2 and 0.58 ± 0.01 mg/g FW in control seeds, 8.2 ± 0.89 and 1.15 ± 0 mg/g FW in control sprouts, and 17.24 ± 0.4 and 2.47 ± 0.15 mg/g FW in eCO2-treated sprouts, respectively. The improvement in the concentrations of phenolic and flavonoid compounds in ajwain, due to eCO2 exposure, could be explained in the light of the theory that the accumulation of antioxidant metabolites depends on intermediates of C and N metabolism []. Thus, by its positive effect on primary metabolism and nutrient uptake, eCO2 is expected to enhance the contents of phenolic and flavonoids [,,,,,,,,,,,].

Table 4.
The levels of phenolic and flavonoid compounds in control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi. Data are represented by means ± standard deviations of at least 4 replicates. Different a, b, c letters within a row indicate significant differences between means at p < 0.05.
3.3. Sprouting and eCO2 Enhanced the Biological Activities of T. ammi
Ajwain is well known for its antioxidant activity []. Therefore, we investigated the overall antioxidant capacity of eCO2-treated ajwain sprouts versus control sprouts and control seeds (Figure 1). The obtained results showed that control sprouts possess a significantly greater antioxidant capacity in terms of FRAP, ORAC, and DPPH than control seeds. In addition, the exposure to eCO2 significantly (p < 0.05, Supplementary Table S2) increased the antioxidant capacity in terms of FRAP and DPPH by about 35 % than control sprouts, while ORAC showed a slight elevation in eCO2-treated sprouts than control ones. The eCO2-induced improvement in the level of antioxidant metabolites in ajwain is responsible for the enhancement in their antioxidant capacity. In this context, it was reported that high eCO2 enriched the antioxidant capacities of many plants such as ginger, strawberry, and Labisia pumila [,,,].
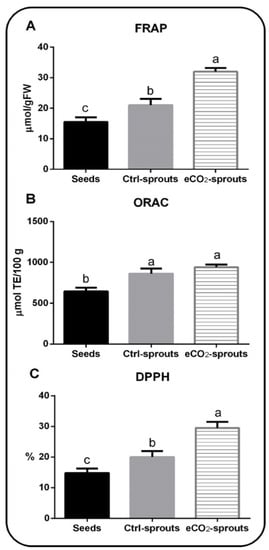
Figure 1.
The antioxidant capacity of control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi represented by (A) ferric reducing antioxidant power (FRAP), (B) oxygen radical absorbance capacity (ORAC), and (C) DPPH. Data are represented by means ± standard deviations of at least 4 replicates. Different a, b, c letters above bars indicate significant differences between means at p < 0.05.
As well as the anticancer activities of ajwain sprouts were examined using the following tumor cell lines: Hepatocellular carcinoma (HepG2), colon carcinoma (Colo205), embryonic kidney adenocarcinoma (Emb293), and urinary bladder carcinoma (T24P) (Figure 2). Interestingly, control sprouts presented significantly (p < 0.05, Supplementary Table S2) more potent anticancer activities against the four tumor cell lines than control seeds. Furthermore, eCO2-treated sprouts revealed significantly (p < 0.05, Supplementary Table S2) higher anticancer activities against HepG2, Colo205, and T24P cell lines by about 10, 12, and 14 % improvement rates compared with control sprouts, respectively.
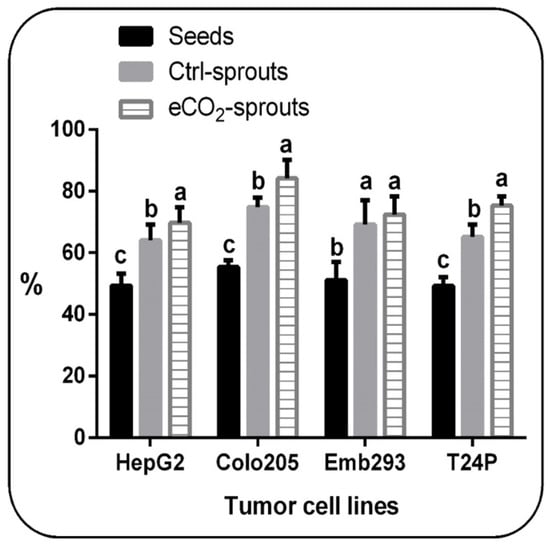
Figure 2.
In vitro cytotoxicity of control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi against human cancer cell lines including hepatocellular carcinoma (HepG2), colon carcinoma (Colo205), embryonic kidney adenocarcinoma (Emb293), and urinary bladder carcinoma (T24P). Data are represented by means ± standard deviations of at least 4 replicates. Different a, b, c letters above bars within each cell line indicate significant differences between means at p < 0.05.
In addition, the antimicrobial activity of ajwain has been well documented []. Thus, we additionally investigated the antimicrobial activity of control seeds, control sprouts, and eCO2-treated sprouts of ajwain using the disc diffusion method against 13 microbial species (Figure 3). Variable degrees of inhibition were recorded depending on the microbial species and sample (seeds, control sprouts, or eCO2-treated sprouts). The greatest microbial growth inhibition rate of ajwain was recorded against Aspergillus flavus. Interestingly, eCO2 treatment duplicated such ability (p < 0.05, Supplementary Table S2), while the lowest inhibition rate was reported against Candida glabrata, which also was significantly duplicated when treated with eCO2. In addition, the inhibition rate of ajwain against Serratia marcescens was not high enough, and the treatment with eCO2 did not induce a significant change. Likewise, Salmonella Typhimurium and Candida albicans showed considerable resistance to ajwain sprouts and eCO2 did not improve such a shortage. On the contrary, eCO2 treatment significantly (p < 0.05) enhanced the antimicrobial properties of ajwain sprouts against Staphylococcus saprophyticus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus salivarius, Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris, and Enterobacter aerogenes. In total, eCO2 treatment significantly improved the antimicrobial properties of ajwain sprouts against 11 out of 13 microbial species. Regarding ajwain seeds, we noticed that generally there was an approximate direct relationship between the antimicrobial activity of ajwain seeds and sprouts. Moreover, the sprouting under control conditions improved the antimicrobial properties of ajwain as compared to control seeds, and this improvement was significant (p < 0.05, Supplementary Table S1) in Aspergillus flavus, Enterobacter aerogenes, and Candida albicans.
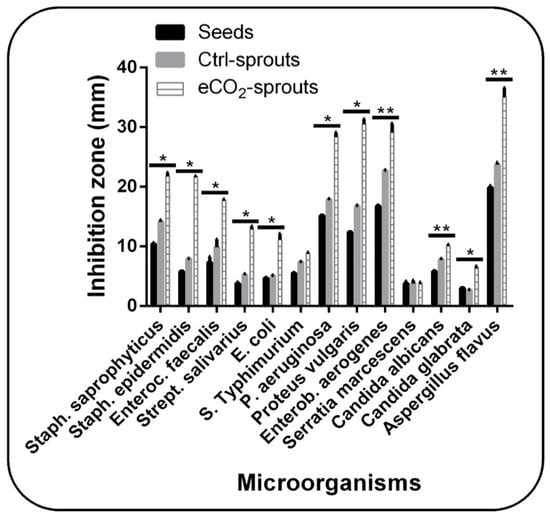
Figure 3.
The antimicrobial activity of control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi. Data are represented by means ± standard deviations of at least 4 replicates. Bars with (**) symbols within each microorganism indicate significant differences among the three sample types at p < 0.05. Bars with (*) symbols within each microorganism indicate significant differences among control and treated sprouts at p < 0.05.
Likewise, the antioxidant capacity, apparently, the improvement in the anticancer and antimicrobial properties of ajwain after exposure to eCO2, could be attributed to the promotion in the levels of phenolics and flavonoids in treated sprouts. Similarly, Al Jaouni et al. [] reported direct relationships among different biological activities (antioxidant, anticancer, antiprotozoal, and antibacterial properties) of control and eCO2-exposed peppermint and basil plants, as well as with their contents of phenolic and flavonoid compounds. They hypothesized that these different biological activities may share common underlying mechanisms and/or causative metabolites. Similarly, in the current study, we noticed a direct relationship between the levels of phenolics and flavonoids and the biological activities of ajwain. In agreement, Ghasemzadeh and Jaafar [] reported that eCO2 improved the anticancer activity of ginger against human cancer cell lines (MDA-MB-231 and MCF-7). They attributed such improvement to the paralleled increase in phenolic and flavonoid contents. Supporting this theory, phenolic and flavonoid compounds are well known for their antioxidant [], anticancer, and antimicrobial [] activities.
3.4. Hierarchical Analysis Findings
Clustering analysis of the obtained results of primary and secondary metabolites produced by control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi confirm the synergistic effect achieved by the coupling between sprouting and eCO2 treatment (Figure 4). The obtained values representing 62 measured parameters can be clustered into 3 main clusters. In the first cluster, representing 4 parameters (tyrosine, carvacrol, rutin, and naringenin), eCO2-treated sprouts had the lowest or similar values to control seeds and control sprouts. The remaining 2 clusters contained 58 parameters where their highest values were reported mostly in eCO2-treated sprouts, while control sprouts had moderate amounts and control seeds mostly had the lowest values.
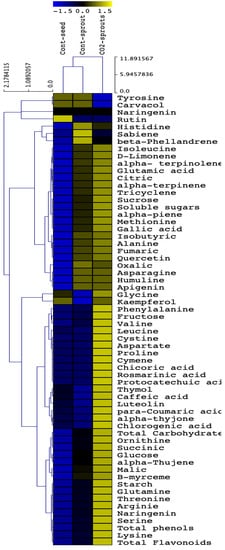
Figure 4.
Hierarchical analysis of primary and secondary metabolites in control seeds, control (ambient CO2-exposed) sprouts, and elevated CO2 (eCO2)-treated sprouts of Trachyspermum ammi. Faint olive and blue colors represent high and low values, respectively.
4. Conclusions
As our study comes to an end, it could be inferred that growing T. ammi sprouts under eCO2 could be a promising method not only to increase the biomass production of plant sprouts but also to improve the sprouting process that induced plant nutritive and medical impact against human diseases such as cancer. This was supported by the observed induction in a wide range of biological activities including antioxidant, antibacterial and anticancer activities. Hence, our study could suggest higher levels of CO2 as a promising strategy to enhance the food functionality and health-promoting values of T. ammi sprouts as a successful model for plant-based foods. The present work can serve as a basis for future large-scale replicated and/or more detailed research into the effects explored by this exploratory assessment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11050830/s1, Table S1. Results of a polit experiment to test the effect of elevated CO2 (eCO2) treatment versus ambient CO2 (control) on fresh weight, dry weight, photosynthesis and respiration of Trachyspermum ammi sprouts. Data are represented by means ± standard deviations of at least 3 replicates. Different a, b, c letters within a row indicate a significant difference between means at p < 0.05. Table S2. Sum of Squares, Degrees of freedom (df), F-values, p-values, and mean squares generated from analysis of variance of seed, control sprouts and elevated CO2 treated sprouts.
Author Contributions
Conceptualization, A.H.A.H., M.K.O., F.H.M.A., and H.A.; data curation, M.A.E.-T., M.A.A.-M., S.S., and F.H.M.A.; formal analysis, I.M.M., A.H.A.H., M.A.A.-M., F.H.M.A., S.A.A.-a., and H.A.; funding acquisition, H.A.; investigation, A.H.A.H., M.K.O., M.A.E.-T., S.A.A.-a., S.S., and H.A.; methodology, A.H.A.H., M.A.A.-M., and H.A.; project administration, I.M.M., M.K.O., K.S.A., and H.A.; resources, M.K.O., S.S., I.M.M., K.S.A. and M.A.E.-T.; supervision, M.K.O. and F.H.M.A.; validation, M.A.A.-M. and Y.B.E.; visualization, A.H.A.H.; writing—original draft, A.H.A.H.; writing—review and editing, M.K.O., M.A.E.-T., M.A.A.-M., I.M.M., F.H.M.A., Y.B.E., and H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University for funding through research group No. (RG-1441-485).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RG-1441-485).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marton, M.; Mandoki, Z.; Caspo, J.; Caspo-Kiss, Z. The role of sprouts in human nutrition. A review. Aliment. Hung. Univ. Transylvania 2010, 3, 81–117. [Google Scholar]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of heat- and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Martino, M.; Mattioli, S.; Marconi, O.; Sileoni, V.; Benincasa, P. The effect of dietary alfalfa and flax sprouts on rabbit meat antioxidant content, lipid oxidation and fatty acid composition. Meat Sci. 2015, 106, 31–37. [Google Scholar] [CrossRef]
- Silva, L.R.; Pereira, M.J.; Azevedo, J.; Gonçalves, R.F.; Valentão, P.; de Pinho, P.G.; Andrade, P.B. Glycine max (L.) Merr., Vigna radiata L. and Medicago sativa L. sprouts: A natural source of bioactive compounds. Food Res. Int. 2013, 50, 167–175. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Doniyor, R.; Sagdullaev, S.S. Medicinal Plants of the Apiaceae and Rutaceae Families from the Chimgan Mountains (Uzbekistan): Ethnopharmacology, Chemical Composition and Biological Activities. Curr. Tradit. Med. 2018, 4, 166–183. [Google Scholar] [CrossRef]
- Morsi, N.M. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Biochim. Pol. 2000, 49, 63–74. [Google Scholar]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical constituents, antifungal and antioxidative effects of ajwain essential oil and its acetone extract. J. Agric. Food Chem. 2004, 52, 3292–3296. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nilolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and cytotoxic activities of origanum essential oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Srivastava, K.C. Extract of a spice Omum (Trachyspermum ammi) shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent. Fat. Acids 1988, 33, 1–6. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J. Phytochemicals of Brassicaceae in plant protection and human health-Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation. A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Saleh, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. CO2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). Food Chem. 2018, 269, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Leakey, A.D.; Xu, F.; Gillespie, K.M.; McGrath, J.M.; Ainsworth, E.A.; Ort, D.R. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2009, 9, 3597–3602. [Google Scholar] [CrossRef]
- Noguchi, K.; Watanabe, C.K.; Terashima, I. Effects of elevated atmospheric CO2 on primary metabolite levels in Arabidopsis thaliana Col-0 leaves: An examination of metabolome data. Plant Cell Physiol. 2015, 56, 2069–2078. [Google Scholar]
- Almuhayawi, M.; AbdElgawad, H.; Al Jaouni, S.; Selim, S.; Hassan, A.H.; Khamis, G. Elevated CO2 improves glucosinolate metabolism and stimulates anticancer and anti-inflammatory properties of broccoli sprouts. Food Chem. 2020, 328, 127102. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Hassan, A.H.; Al Jaouni, S.K.; Alkhalifah, D.H.M.; Hozzein, W.N.; Selim, S.; Khamis, G. Influence of elevated CO2 on nutritive value and health-promoting prospective of three genotypes of Alfalfa sprouts (Medicago sativa). Food Chem. 2020, 340, 128–147. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Van den Ende, W.; Janssens, I.A.; Asard, H. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: A comparison of fructan and non-fructan accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Gaurav, Z.; Han, A.; Sherif, H.; Momtaz, H.; Nashwa, H.; Samy, S. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef]
- Hassanpour, H.; Khavari-Nejad, R.A.; Niknam, V.; Razavi, K.; Najafi, F. Effect of penconazole and drought stress on the essential oil composition and gene expression of Mentha pulegium L. (Lamiaceae) at flowering stage. Acta Physiol. Plant. 2014, 36, 1167–1175. [Google Scholar] [CrossRef]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiol. Plant. 2006, 126, 458–468. [Google Scholar] [CrossRef]
- Watanabe, C.K.; Sato, S.; Yanagisawa, S.; Uesono, Y.; Terashima, I.; Noguchi, K. Effects of elevated CO2 on levels of primary metabolites and transcripts of genes encoding respiratory enzymes and their diurnal patterns in Arabidopsis thaliana: Possible relationships with respiratory rates. Plant Cell Physiol. 2014, 55, 341–357. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Saleh, A.M.; Habeeb, T.H.; Wadaan, M.A.M.; AbdElgawad, H. CO2 treatment improves the hypocholesterolemic and antioxidant properties of fenugreek seeds. Food Chem. 2020, 308, 1256–1261. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Kanunnikova, N.P. Role of brain glutamic acid metabolism changes in neurodegenerative pathologies. J. Biol. Earth Sci. 2012, 2, 1–10. [Google Scholar]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 19952–19958. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar] [CrossRef]
- Li, P.; Li, H.; Zong, Y.; Li, F.Y.; Han, Y.; Hao, X. Photosynthesis and metabolite responses of Isatisindigotica Fortune to elevated [CO2]. Crop J. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Krumbein, A.; Kläring, H.P.; Schonhof, I.; Schreiner, M. Atmospheric carbon dioxide changes photochemical activity, soluble sugars and volatile levels in broccoli (Brassica oleracea var. italica). J. Agric. food Chem. 2010, 58, 3747–3752. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.; Rahmat, A. Improvement in flavonoids and phenolic acids production and pharmaceutical quality of sweet basil (Ocimum basilicum L.) by Ultraviolet-B irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef]
- Fedor, M.; Socha, K.; Urban, B.; Soroczyńska, J.; Matyskiela, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Serum concentration of zinc, copper, selenium, manganese, and Cu/Zn ratio in children and adolescents with myopia. Biol. Trace Elem. Res. 2017, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Fabado, M.A.; Munné-Bosch, S. Vitamins in plants: Occurrence, biosynthesis and antioxidant function. Trends Plant Sci. 2010, 15, 582–592. [Google Scholar] [CrossRef]
- Asif, M.; Yilmaz, O.; Ozturk, L. Elevated carbon dioxide ameliorates the effect of Zn deficiency and terminal drought on wheat grain yield but compromises nutritional quality. Plant Soil. 2017, 411, 57–67. [Google Scholar] [CrossRef]
- Goswami, N.; Chatterjee, S. Assessment of free radical scavenging potential and oxidative DNA damage preventive activity of Trachyspermum ammi L. (carom) and Foeniculum vulgare Mill. (fennel) seed extracts. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Bunce, J.A.; Maas, J.L. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef]
- Jaafar, H.Z.; Ibrahim, M.H.; Karimi, E. Phenolics and flavonoids compounds, phenylanine ammonia lyase and antioxidant activity responses to elevated CO2 in Labisia pumila (Myrisinaceae). Molecules 2012, 17, 6331–6347. [Google Scholar] [CrossRef]
- Moein, M.R.; Zomorodian, K.; Pakshir, K.; Yavari, F.; Motamedi, M.; Zarshenas, M.M. Trachyspermum ammi (L.) sprague: Chemical composition of essential oil and antimicrobial activities of respective fractions. J. Evid. Based Complement. Altern. Med. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z. Antioxidant potential and anticancer activity of young ginger (Zingiber officinale Roscoe) grown under different CO2 concentration. J. Med. Plants Res. 2011, 5, 3247–3255. [Google Scholar]
- Kumar, M.Y.; Tirpude, R.J.; Maheshwari, D.T.; Bansal, A.; Misra, K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves in vitro. Food Chem. 2013, 141, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).