Integrated Application of K and Zn as an Avenue to Promote Sugar Beet Yield, Industrial Sugar Quality, and K-Use Efficiency in a Salty Semi-Arid Agro-Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site and the Properties of the Tested Soil
2.2. Experimental Setup and Sugar Beet Husbandry
2.3. Sampling
2.4. Morpho-Physiological Responses
2.5. Industrial Sugar Quality
2.6. Sugar Beet Productivity and Potassium-Use Efficiencies (R-KUE and S-KUE)
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Morpho-Physiological Responses
3.2. Industrial Sugar Quality
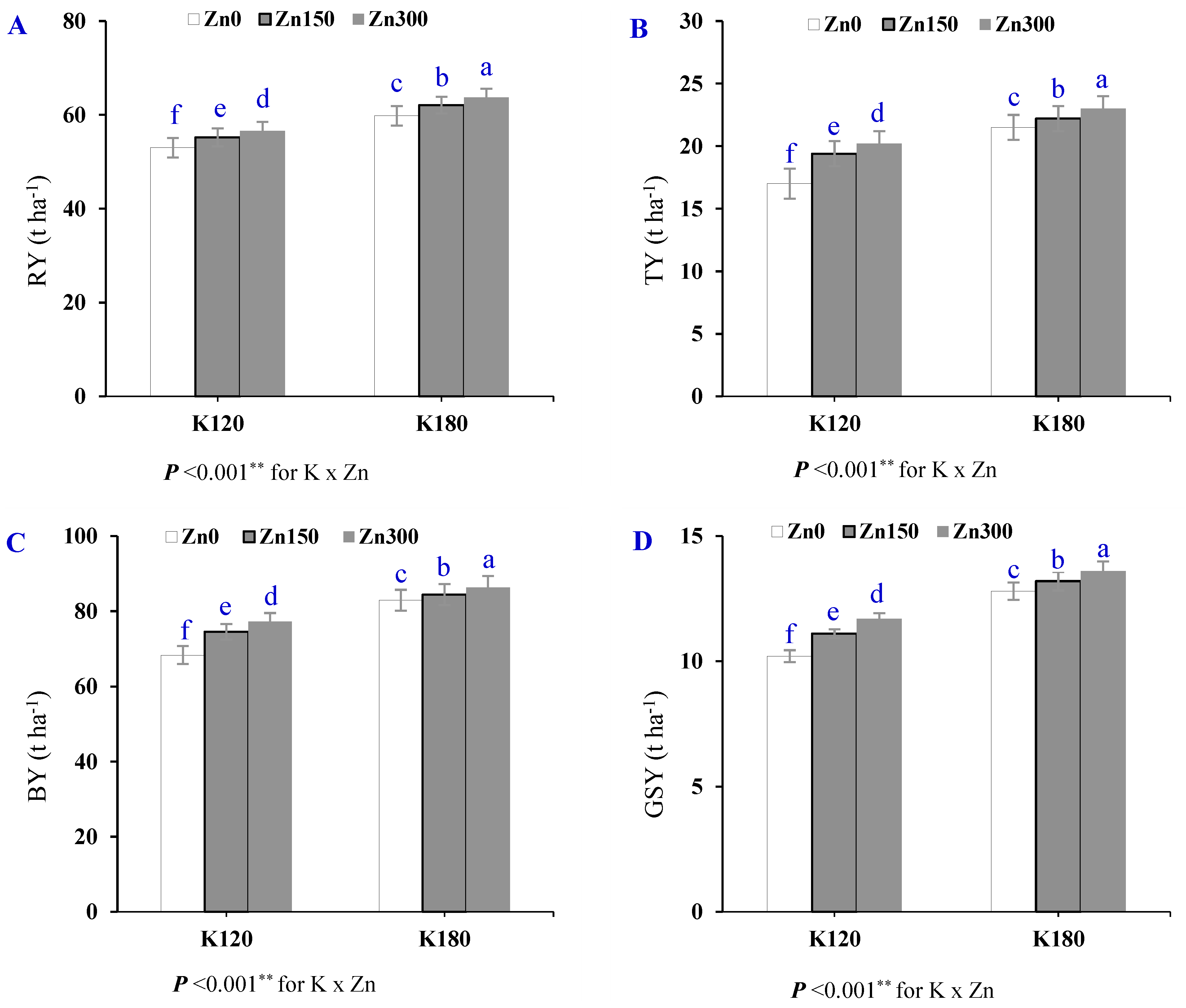
3.3. Sugar Beet Productivity
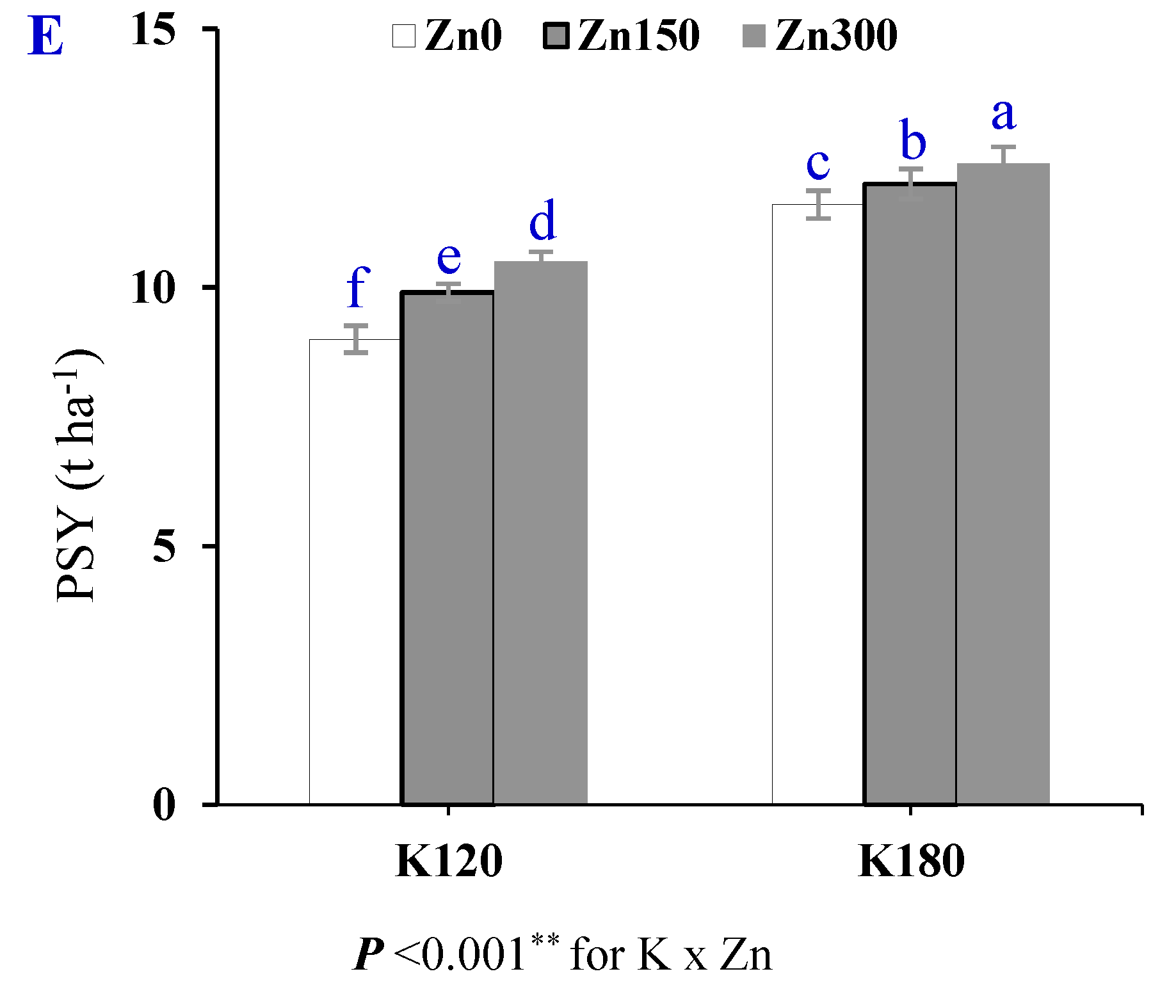
3.4. Potassium-Use Efficiencies (R-KUE and S-KUE)
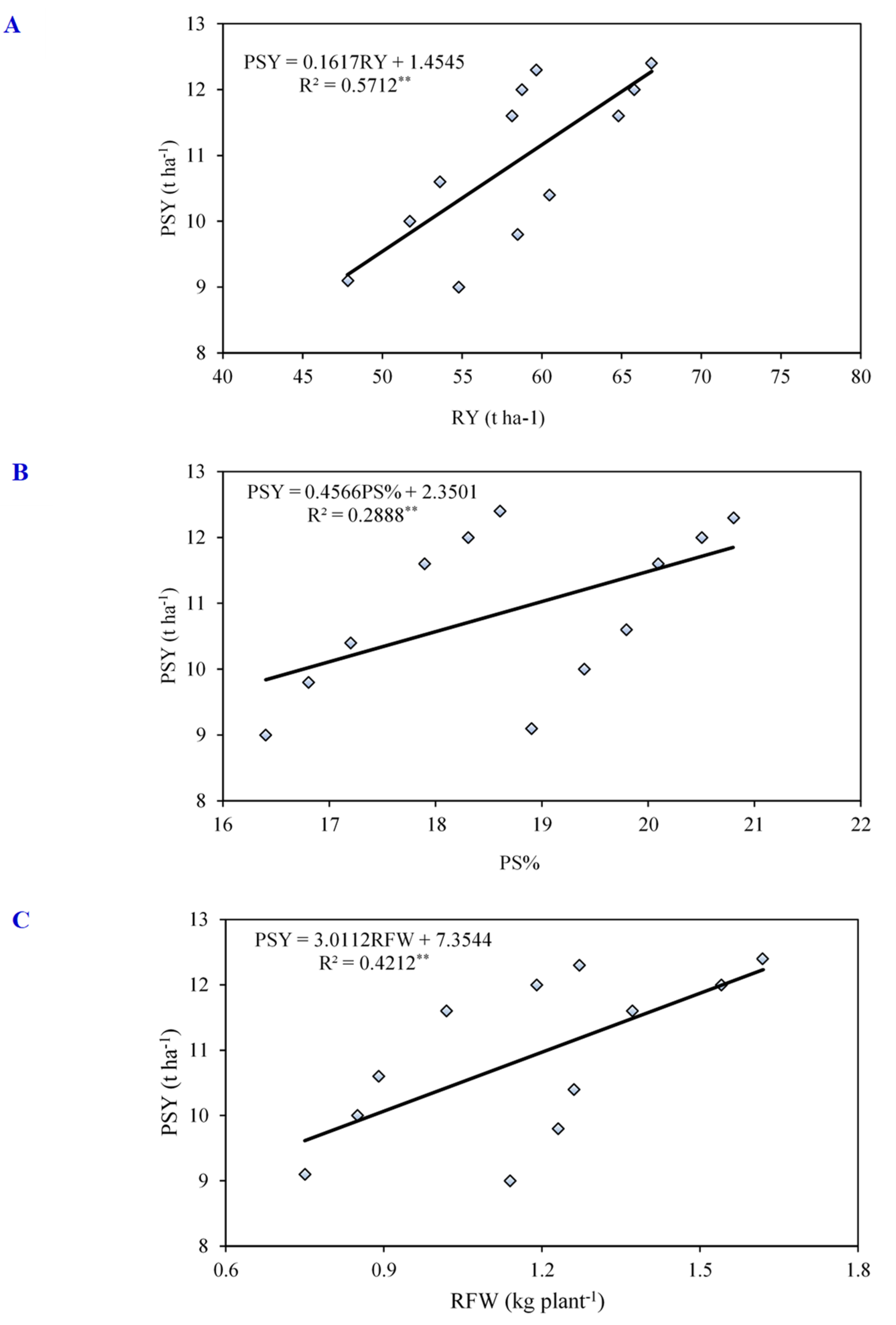
3.5. Correlation, Regression, and Path-Coefficient Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. World Food and Agriculture-Statistical Pocketbook; FAO: Rome, Italy, 2018; p. 254. Available online: http://www.fao.org/economic/ess/ess-publications/ess-yearbook/en/ (accessed on 15 July 2019).
- Gumienna, M.; Szwengiel, A.; Szczepańska-Alvarez, A.; Szambelan, K.; Lasik-Kurdyś, M.; Czarnecki, Z.; Sitarski, A. The impact of sugar beet varieties and cultivation conditions on ethanol productivity. Biomass Bioenerg. 2016, 85, 228–234. [Google Scholar] [CrossRef]
- Carr, M.K.V.; Knox, J.W. The water relations and irrigation requirements of sugar cane (Saccharum officinarum): A review. Exp. Agric. 2011, 47, 1–25. [Google Scholar] [CrossRef]
- CCSC. Central Council for Sugar Crops Annual Report; Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2018. [Google Scholar]
- Vaughan, M.; Block, A.; Christensen, S.A.; Allen, L.H.; Schmelz., E.A. The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytoch. Rev. 2018, 17, 37–49. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Metternicht, G.; Zinck, J. Remote sensing of soil salinity: Potentials and constraints. Remote Sens. Environ. 2003, 85, 1–20. [Google Scholar] [CrossRef]
- Cheeseman, J. Food Security in the Face of Salinity, Drought, Climate Change, and Population Growth. Halophytes for Food Security in Dry Lands; Elsevier: Amsterdam, The Netherland, 2016; pp. 111–123. [Google Scholar]
- FAOSTAT. AQUASTAT Country Profil–Egypt; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Hussain, Z.; Khattak, R.A.; Irshad, M.; Mahmood, Q. Sugar beet (Beta vulgaris L.) response to diammonium phosphate and potassium sulphate under saline–sodic conditions. Soil Use Manag. 2014, 30, 320–327. [Google Scholar] [CrossRef]
- El-Mageed, T.A.A.; El-Sherif, A.M.; El-Mageed, S.A.A.; Abdou, N.M. A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric. Water Manag. 2019, 226, 105831. [Google Scholar] [CrossRef]
- Merwad, A.M.A. Efficiency of potassium fertilization and salicylic acid on yield and nutrient accumulation of sugar beet grown on saline soil. Commun. Soil Sci. Plant Anal. 2016, 47, 1184–1192. [Google Scholar] [CrossRef]
- Abbasi, Z.; Golabadi, M.; Khayamim, S.; Pessarakli, M. The response of drought-tolerant sugar beet to salinity stress under field and controlled environmental conditions. J. Plant Nutr. 2018, 41, 2660–2672. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M. Effect of salt stress on growth and ion accumulation of alfalfa (Medicago sativa L.) cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK; Waltham, MA, USA; San Diego, CA, USA, 2012. [Google Scholar]
- Feizi, M.; Fallahzade, J.; Noorshargh, P. Sugar beet yield response to different levels of saline irrigation water and leaching in an arid region. J. Plant Nutr. 2018, 41, 654–663. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Steffens, D.; Schubert, S. Substitution of K+ by Na+ in sugar beet nutrition on K+-fixing soils. J. Plant Nutr. Soil Sci. 2010, 173, 127–134. [Google Scholar] [CrossRef]
- Wakeel, A.; Sümer, A.; Hanstein, S.; Yan, F.; Schubert, S. In vitro effect of different Na+/K+ ratios on plasma membrane H+-ATPase activity in maize and sugar beet shoot. Plant Physiol. Biochem. 2011, 49, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Bybordi, A. Interactive effects of silicon and potassium nitrate in improving salt tolerance of wheat. J. Integr. Agric. 2014, 13, 60345–60347. [Google Scholar]
- Hatam, Z.; Sabet, M.S.; Malakouti, M.J.; Mokhtassi-Bidgoli, A.; Homaee, M. Zinc and potassium fertilizer recommendation for cotton seedlings under salinity stress based on gas exchange and chlorophyll fluorescence responses. S. Afr. J. Bot. 2020, 130, 155–164. [Google Scholar] [CrossRef]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 13, 73–82. [Google Scholar] [CrossRef]
- Shin, R. Strategies for improving potassium use efficiency in plants. Mol. Cells 2014, 37, 575. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, M.; Li, S.; Alva, A.K.; Ashraf, M. Potassium fertilization mitigates the adverse effects of drought on selected Zea mays cultivars. Turk. J. Bot. 2014, 38, 713–723. [Google Scholar] [CrossRef]
- Piskin, A. Effect of Zinc applied together with compound fertilizer on yield and quality of sugar beet (Beta vulgaris L.). J. Plant Nutr. 2017, 40, 2521–2531. [Google Scholar] [CrossRef]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of zinc in plants. In Zinc Soils Plants; Robson, A.D., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 1993; pp. 90–106. [Google Scholar]
- Jan, A.U.; Hadi, F.; Nawaz, M.A.; Rahman, K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2017, 116, 139–149. [Google Scholar] [CrossRef]
- Afeez, B.; Khanif, Y.M.; Saleem, M. Role of zinc in plant nutrition—A review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar]
- Ashraf, M.Y.; Tariq, S.; Saleem, M.; Khan, M.A.; Hassan, S.W.U.; Sadef, Y. Calcium and zinc mediated growth and physio-biochemical changes in mungbean grown under saline conditions. J. Plant Nutr. 2020, 43, 512–525. [Google Scholar] [CrossRef]
- Christenson, D.R.; Draycott, A.P. Nutrition-phosphorus, Sulphur, Potassium, Sodium, Calcium, Magnesium And Micro- Nutrients-Liming and Nutrient Deficiencies. In Sugar Beet; Draycott, A.P., Ed.; Bury St. Edmunds, Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 185–219. [Google Scholar]
- Attia, K.K.; Abdel-Motagally, F.M.F. Influence of potassium fertilization and foliar application of zinc on sugar beet plants grown on a calcareous sandy soil. Assiut. J. Agric. Sci. 2015, 46, 1–14. [Google Scholar]
- Barłóg, P.; Nowacka, A.; Błaszyk, R. Effect of zinc band application on sugar beet yield, quality and nutrient uptake. Plant Soil Environ. 2016, 62, 30–35. [Google Scholar] [CrossRef]
- Finck, A. Plant Nutrition in Keywords; Verlag Ferdinand Hirt: Wroclaw, Poland, 1969; pp. 158–159. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015; pp. 537–564. [Google Scholar]
- Moradi, S.; Jahanban, L. Salinity stress alleviation by Zn as soil and foliar applications in two rice cultivars. Commun. Soil Sci. Plant Anal. 2018, 49, 2517–2526. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Zinc Association: Brussels, Belgium, 2008; pp. 42–46. [Google Scholar]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.D.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.E.; Knezek, B.D. Climatic and soil conditions promoting micronutrient deficiencies in plants. In Micronutrients in Agriculture; Mortvedt, J.J., Giordano, P.M., Lindsay, W.L., Madison, W.I., Eds.; Soil Science society of America: Madison, WI, USA, 1972; pp. 265–288. [Google Scholar]
- Ponce, V.; Pandey, R.; Ercan, S. Characterization of drought across climatic spectrum. J. Hydrol. Eng. 2000, 5, 222–224. [Google Scholar] [CrossRef]
- Klute, A. Methods of Soil Analysis: Part. 1-Physical and Mineralogical Methods, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1986. [Google Scholar]
- Page, A.I.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Part. 2-Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Dahnke, W.C.; Whitney, D.A. Measurement of soil salinity. In Recommended Chemical Soil Test Procedures for the North Central Region; Dahnke, W.C., Ed.; North Central Regional Publication: Orchard, CO, USA, 1988; pp. 32–34. [Google Scholar]
- Soil Survey Staff USDA. Soil Taxonomy. A Basic System of Soil Classification for Making Sand Interpreting Soil Surveys, 2nd ed.; Agriculture Handbook no. 466; USDA: Washington, WA, USA, 1999.
- CAAE (Central Administration for Agricultural Extension). Beta vulgaris (L.) Cultivation; Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2017. Available online: https://ar-ar.facebook.com/caae.eg/ (accessed on 9 February 2017).
- Watson, D.J. The physiological basis of variation in yield. Adv. Agron. 1952, 4, 101–145. [Google Scholar]
- McGinnus, R.A. Sugar Beet Technology, 2nd ed.; Sugar Beet Development Foundation: Denver, CO, USA, 1971. [Google Scholar]
- Harvey, G.W.; Dotton, J.V. Root quality and processing. In The Sugar Beet Crop Science into Practice; Cooke, D.A., Scott, R.K., Eds.; Chapman & Hall: London, UK, 1993; pp. 571–617. [Google Scholar]
- Dewey, J.R.; Lu, K.H. A correlation and path coefficient analysis of yield components of crested wheat grass seed production. Agron. J. 1959, 51, 515–518. [Google Scholar] [CrossRef]
- Yuncai, H.; Schmidhalter, U. Drought and salinity: A comparison of the effects of drought and salinity. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar]
- Zaki, N.M.; Hassanein, M.S.; Ahmed, A.G.; El-Housini, E.A.; Tawfik, M.M. Foliar application of potassium to mitigate the adverse impact of salinity on some sugar beet varieties. 2: Effect on yield and quality. Middle East J. Agric. Res. 2014, 3, 448–460. [Google Scholar]
- Mehrandish, M.; Moeini, M.M.; Armin, M. Sugar beet (Beta vulgaris L.) response to potassium application under full and deficit irrigation. Eur. J. Exp. Biol. 2013, 2, 2113–2119. [Google Scholar]
- Faust, F.; Schubert, S. Protein synthesis is the most sensitive process when potassium is substituted by sodium in the nutrition of sugar beet (Beta vulgaris). Plant Physiol. Biochem. 2016, 107, 237–247. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Chen, H.; Yang, X.; Geng, J. Effects of different potassium fertilizer types and dosages on cotton yield, soil available potassium and leaf photosynthesis. Arch. Agron. Soil Sci. 2020, 67, 275–287. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, J.A. An analysis of irreversible plant cell elongation. J. Theor. Biol. 1965, 8, 264–275. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494. [Google Scholar] [CrossRef]
- Hager, A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Dutton, J.; Huijbregts, T. Root Quality and Processing. In Sugar Beet; Draycott, A.P., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 409–442. [Google Scholar]
- Carter, J.N. Potassium and sodium uptake effects on sucrose concentration and quality of sugar beet roots. J. Am. Soc. Sugar Beet Technol. 1986, 23, 183–202. [Google Scholar] [CrossRef]
- Mubarak, M.U.; Zahir, M.; Ahmad, S.; Abdul, W. Sugar beet yield and industrial sugar contents improved by potassium fertilization under scarce and adequate moisture conditions. J. Integr. Agric. 2016, 15, 2620–2626. [Google Scholar] [CrossRef]
- Wang, X.G.; Hua, Z.X.; Ji, J.C.; Hong, L.C.; Shan, C.; Di, W.; Qiu, C.Y.; Qiu, Y.H.; Yan, W.C. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J. Integr. Agric. 2015, 14, 856–863. [Google Scholar] [CrossRef]
- Milford, G.F.J.; Armstrong, M.J.; Jarvis, P.J.; Houghton, B.J.; Bellett-Travers, D.M.; Jones, J.; Leigh, R.A. Effect of potassium fertilizer on the yield, quality and potassium offtake of sugar beet crops grown on soils of different potassium status. J. Agric. Sci. 2000, 135, 1–10. [Google Scholar] [CrossRef]
- Bhador, M.A.; Minoii, M.A.; Alizadeh, A.N. Effect of planting dates and NPK fertilization on growth and yield of sugar beet (Beta vulgaris L.). J. Agric. Sci. Miyane Univ. 2010, 20, 2683–2689. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Jafarzadeh, A.A.; Aliasgharza, N. Salinity and salt composition effects on seed germination and root length of four sugar beet cultivars. Biol. Bratisl. 2007, 62, 562–564. [Google Scholar] [CrossRef]
- Ćirić, M.Z. The effect of genotype x environment interaction on root yield and quality of sugar beet. Fac Agric. Univ. Belgrade. 2017, 9, 1041. [Google Scholar]
- Beringer, H.; Koch, K.; Lindhauer, M.G. Sucrose accumulation and osmotic potentials in sugar beet at increasing levels of potassium nutrition. J. Sci. Food Agric. 1986, 37, 211–218. [Google Scholar] [CrossRef]
- Schlumbach, K.; Scharfe, M.; Flöter, E. Color transfer into sucrose crystallized from blended beet and cane syrups. Sugar Ind. 2016, 141, 97–104. [Google Scholar] [CrossRef]
- Moustafa, M.S. Influence of sowing dates and foliar spraying of iron and zinc on sugar beet productivity in salt affected soil. J. Plant Prod. 2019, 10, 569–573. [Google Scholar] [CrossRef]
- Fischer, E.S. Moderate magnesium deficiency affected chlorophyll content of bean plat. Photosynthetica 1997, 33, 385–390. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Henriques, F.S. Loss of blade photosynthetic area and of chloroplasts’ photochemical capacity account for reduced CO2 assimilation rates in zinc-deficient sugar beet leaves. J. Plant Physiol. 2001, 158, 915–919. [Google Scholar] [CrossRef]
- Neamatollahi, E.; Khademosharieh, M.M.; Darban, A.S.; Jahansuz, M.R. Application of different amounts of ZnSO4 in five varieties of sugar beet. Adv. Environ. Biol. 2013, 7, 1113–1116. [Google Scholar]
- Abo-Steet, S.; El-Edfawy, Y.; Niazy, M. Mutual effect among compost and foliar spraying with zinc and boron on sugar beet (Beta vulgaris L.) grown on saline sandy loam soil. J. Soil Sci. Agric. Eng. 2015, 6, 1091–1105. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Çakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 191–248. [Google Scholar]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Badakhshan, H. Effects of zinc application on growth, absorption and distribution of mineral nutrients under salinity stress in soybean (Glycine max L.). J. Plant Nutr. 2014, 37, 2255–2269. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Boulila, M.; Rafudeen, M.S.; Sengupta, S.; Rady, M.M. Melatonin regulatory mechanisms and phylogenetic analyses implying new sequences of melatonin biosynthesis related genes extracted from peanut under salinity stress. Plants 2020, 9, 854. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequenced Antioxidants Application Rectifies Ion Imbalance and Strengthens Antioxidant Systems in Salt-stressed Cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Alotaibi, M.; Alhammad, B.A.; Rady, M.M.; Mahdi, A.H.A. Exogenous potassium treatments elevate salt tolerance and performances of Glycine max by boosting antioxidant defense system under actual saline field conditions. Agronomy 2020, 10, 1741. [Google Scholar] [CrossRef]
- Desoky, E.S.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
| Month | Air Temperatures (°C) | RH (%) | Wind Speed (m s−1) | Precipitation (mm d−1) | EP (mm d−1) | |
|---|---|---|---|---|---|---|
| Day | Night | |||||
| September | 35.40 | 20.45 | 42.70 | 3.55 | 0.00 | 5.50 |
| October | 30.65 | 17.05 | 46.90 | 3.10 | 0.10 | 4.50 |
| November | 25.05 | 12.55 | 56.65 | 2.35 | 1.30 | 2.30 |
| December | 20.45 | 9.10 | 63.35 | 2.25 | 0.14 | 1.51 |
| January | 18.65 | 5.80 | 56.40 | 2.55 | 0.19 | 1.50 |
| February | 21.75 | 8.50 | 49.85 | 2.25 | 0.13 | 2.70 |
| March | 26.20 | 10.40 | 41.30 | 2.85 | 0.07 | 4.00 |
| Characteristic | Unite | Soil (Before Sowing) | Soil (At Harvest) | Irrigation Water |
|---|---|---|---|---|
| Particle size analysis: | ||||
| Sand | (%) | 77.43 | 77.37 | --- |
| Silt | 11.20 | 11.23 | --- | |
| Clay | 11.37 | 11.40 | --- | |
| Soil textural class | ---- | Sandy loam | Sandy loam | --- |
| Dry bulk density | (g cm−3) | 1.53 | 1.55 | --- |
| Hydraulic conductivity | (cm3 h−1) | 1.97 | 1.98 | --- |
| Soil moisture content at: | ||||
| Field capacity | (%) | 22.26 | 22.34 | --- |
| Wilting point | 12.25 | 12.28 | --- | |
| Available water | 10.01 | 10.05 | --- | |
| pH * | 7.86 | 7.94 | 7.46 | |
| ECe ** | (dS m−1) | 8.60 | 8.65 | 1.63 |
| CaCO3 | (%) | 7.89 | 7.87 | --- |
| Organic matter | 0.95 | 0.94 | --- | |
| Soluble cations **: | ||||
| Ca2+ | mmolc L‒1 | 21.82 | 21.98 | 2.80 |
| Mg2+ | 15.74 | 15.67 | 2.00 | |
| Na+ | 67.60 | 67.71 | 8.10 | |
| K+ | 5.59 | 5.62 | 0.22 | |
| Soluble anions **: | ||||
| CO32− | mmolc L‒1 | Nil | Nil | Nil |
| HCO3− | 9.41 | 9.38 | 2.00 | |
| Cl− | 78.29 | 78.40 | 10.20 | |
| SO42− | 23.05 | 23.15 | 0.92 | |
| Available nutrients: | ||||
| Nitrogen (N) | mg kg−1 soil | 0.041 | 0.040 | --- |
| Phosphorus (P) | 3.54 | 3.61 | --- | |
| Potassium (K) | 39.12 | 39.27 | --- | |
| Zinc (Zn) | 1.55 | 1.52 | --- | |
| Manganese (Mn) | 4.90 | 4.84 | --- | |
| Iron (Fe) | 0.70 | 0.67 | --- | |
| Boron (B) | 0.32 | 0.30 | --- | |
| Treatment | RL | RD | RFW (kg Plant−1) | LAI | SPAD Index | |
|---|---|---|---|---|---|---|
| (cm) | ||||||
| Season (S) | SI | 26.6 ± 0.3 a | 11.9 ± 0.3 a | 1.18 ± 0.06 a | 3.8 ± 0.13 a | 49.7 ± 0.9 a |
| SII | 29.2 ± 0.5 a | 11.6 ± 0.4 a | 1.18 ± 0.04 a | 4.2 ± 0.17 a | 48.9 ± 0.9 a | |
| Cultivar (Cv) | Cv1 | 26.0 ± 0.2 b | 10.4 ± 0.2 b | 0.99 ± 0.04 b | 3.4 ± 0.07 b | 44.8 ± 0.4 b |
| Cv2 | 29.8 ± 0.4 a | 13.0 ± 0.2 a | 1.36 ± 0.04 a | 4.7 ± 0.13 a | 53.8 ± 0.4 a | |
| Potassium (K) | K120 | 26.9 ± 0.4 b | 10.8 ± 0.3 b | 1.02 ± 0.04 b | 3.6 ± 0.11 b | 47.6 ± 0.8 b |
| K180 | 28.9 ± 0.4 a | 12.6 ± 0.3 a | 1.33 ± 0.04 a | 4.4 ± 0.16 a | 51.1 ± 0.8 a | |
| Zinc (Zn) | Zn0 | 27.1 ± 0.6 c | 11.2 ± 0.4 c | 1.07 ± 0.06 c | 3.8 ± 0.17 c | 48.2 ± 1.1 c |
| Zn150 | 27.9 ± 0.6 b | 11.7 ± 0.4 b | 1.20 ± 0.06 b | 4.0 ± 0.18 b | 49.3 ± 1.0 b | |
| Zn300 | 28.6 ± 0.6 a | 12.2 ± 0.4 a | 1.26 ± 0.06 a | 4.3 ± 0.20 a | 50.4 ± 1.0 a | |
| p-value | S | 0.083 ns | 0.729 ns | 0.983 ns | 0.314 ns | 0.501 ns |
| Cv | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| K | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| Zn | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| C.V (%) | 1.00 | 0.90 | 0.60 | 3.40 | 0.90 | |
| Treatment | Sucrose | PS | LS | Purity | |
|---|---|---|---|---|---|
| (%) | |||||
| Season (S) | SI | 20.2 ± 0.1 b | 17.9 ± 0.2 b | 2.35 ± 0.08 a | 88.3 ± 0.5 b |
| SII | 21.3 ± 0.2 a | 19.6 ± 0.3 a | 1.74 ± 0.07 b | 91.7 ± 0.4 a | |
| Cultivar (Cv) | Cv1 | 20.0 ± 0.1 b | 17.5 ± 0.2 b | 2.43 ± 0.07 a | 87.7 ± 0.4 b |
| Cv2 | 21.6 ± 0.2 a | 19.9 ± 0.2 a | 1.65 ± 0.05 b | 92.3 ± 0.3 a | |
| Potassium (K) | K120 | 20.3 ± 0.2 b | 18.1 ± 0.2 b | 2.19 ± 0.09 a | 89.0 ± 0.5 b |
| K180 | 21.3 ± 0.2 a | 19.4 ± 0.3 a | 1.90 ± 0.08 b | 91.0 ± 0.5 a | |
| Zinc (Zn) | Zn0 | 20.5 ± 0.3 c | 18.3 ± 0.4 c | 2.12 ± 0.11 a | 89.5 ± 0.7 c |
| Zn150 | 20.8 ± 0.3 b | 18.7 ± 0.4 b | 2.05 ± 0.11 b | 90.0 ± 0.6 b | |
| Zn300 | 21.0 ± 0.3 a | 19.1 ± 0.4 a | 1.96 ± 0.11 c | 90.5 ± 0.6 a | |
| p-value | S | 0.049 * | 0.046 * | 0.049 * | 0.046 * |
| Cv | 0.007 ** | 0.001 ** | <0.001 ** | <0.001 ** | |
| K | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| Zn | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| C.V (%) | 0.40 | 0.60 | 2.20 | 0.30 | |
| Treatment | Na | K | α-Amino N | Alkalinity Index | |
|---|---|---|---|---|---|
| (mmol kg−1) | |||||
| Season (S) | SI | 21.2 ± 0.1 a | 35.6 ± 0.1 a | 11.9 ± 0.04 a | 4.78 ± 0.1 a |
| SII | 17.1 ± 0.1 a | 21.6 ± 0.1 b | 13.0 ± 0.04 a | 2.94 ± 0.1 b | |
| Cultivar (Cv) | Cv1 | 24.7 ± 0.1 a | 33.9 ± 0.1 a | 14.4 ± 0.02 a | 3.61 ± 0.2 b |
| Cv2 | 13.6 ± 0.1 b | 23.2 ± 0.1 b | 10.4 ± 0.03 b | 4.12 ± 0.2 a | |
| Potassium (K) | K120 | 20.7 ± 0.1 a | 31.1 ± 0.2 a | 13.4 ± 0.03 a | 3.85 ± 0.2 a |
| K180 | 17.6 ± 0.1 b | 26.1 ± 0.1 b | 11.5 ± 0.05 b | 3.88 ± 0.2 a | |
| Zinc (Zn) | Zn0 | 19.8 ± 0.1 a | 30.0 ± 0.2 a | 12.8 ± 0.05 a | 3.92 ± 0.2 a |
| Zn150 | 19.3 ± 0.1 b | 28.7 ± 0.2 b | 12.4 ± 0.05 b | 3.88 ± 0.2 b | |
| Zn300 | 18.3 ± 0.1 c | 27.0 ± 0.2 c | 12.1 ± 0.05 c | 3.79 ± 0.2 b | |
| p-value | S | 0.123 ns | 0.028 * | 0.425 ns | 0.014 ** |
| Cv | <0.001 ** | <0.001 ** | <0.001 ** | 0.011 ** | |
| K | <0.001 ** | <0.001 ** | <0.001 ** | 0.815 ns | |
| Zn | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| C.V (%) | 0.90 | 4.70 | 1.20 | 2.6 | |
| Treatment | RY | TY | BY | GSY | |
|---|---|---|---|---|---|
| (t ha−1) | |||||
| Season (S) | SI | 61.9 ± 1.3 a | 20.0 ± 0.5 a | 81.7 ± 1.8 a | 12.5 ± 0.31 a |
| SII | 54.9 ± 0.9 a | 21.1 ± 0.8 a | 76.0 ± 1.6 a | 11.7 ± 0.18 a | |
| Cultivar (Cv) | Cv1 | 54.9 ± 1.1 b | 17.9 ± 0.4 b | 72.8 ± 1.5 b | 11.8 ± 0.24 a |
| Cv2 | 61.9 ± 1.1 a | 23.2 ± 0.6 a | 85.1 ± 1.4 a | 12.4 ± 0.27 a | |
| Potassium (K) | K120 | 54.5 ± 0.9 b | 18.9 ± 0.6 b | 73.4 ± 1.4 b | 11.0 ± 0.16 b |
| K180 | 62.3 ± 1.3 a | 22.2 ± 0.6 a | 84.5 ± 1.6 a | 13.2 ± 0.21 a | |
| Zinc (Zn) | Zn0 | 56.4 ± 1.6 c | 19.3 ± 0.9 c | 75.6 ± 2.4 c | 11.5 ± 0.34 c |
| Zn150 | 58.7 ± 1.5 b | 20.8 ± 0.7 b | 79.5 ± 2.0 b | 12.2 ± 0.29 b | |
| Zn300 | 60.1 ± 1.5 a | 21.6 ± 0.8 a | 81.7 ± 2.1 a | 12.6 ± 0.29 a | |
| p-value | S | 0.076 ns | 0.310 ns | 0.157 ns | 0.222 ns |
| Cv | 0.013 * | 0.001 ** | 0.004 ** | 0.162 ns | |
| K | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| Zn | <0.001 ** | <0.001 ** | <0.001 ** | <0.001 ** | |
| C.V (%) | 0.60 | 0.90 | 0.40 | 0.70 | |
| Treatment | PSY (t ha−1) | R-KUE (kg Roots kg k−1) | S-KUE (kg Sugar kg k−1) | |
|---|---|---|---|---|
| Season (S) | SI | 11.1 ± 0.30 a | 0.42 ± 0.009 a | 0.075 ± 0.001 a |
| SII | 10.7 ± 0.18 a | 0.38 ± 0.013 a | 0.074 ± 0.002 a | |
| Cultivar (Cv) | Cv1 | 10.9 ± 0.23 a | 0.38 ± 0.010 b | 0.074 ± 0.002 a |
| Cv2 | 10.9 ± 0.26 a | 0.42 ± 0.012 a | 0.074 ± 0.002 a | |
| Potassium (K) | K120 | 9.8 ± 0.15 b | 0.45 ± 0.007 a | 0.082 ± 0.001 a |
| K180 | 12.0 ± 0.17 a | 0.35 ± 0.007 b | 0.067 ± 0.001 b | |
| Zinc (Zn) | Zn0 | 10.3 ± 0.32 c | 0.38 ± 0.012 c | 0.070 ± 0.002 c |
| Zn150 | 11.0 ± 0.27 b | 0.40 ± 0.014 b | 0.075 ± 0.002 b | |
| Zn300 | 11.4 ± 0.27 a | 0.41 ± 0.016 a | 0.078 ± 0.002 a | |
| p-value | S | 0.568 ns | 0.096 ns | 0.764 ns |
| Cv | 0.872 ns | 0.012 * | 0.824 ns | |
| K | <0.001 ** | <0.001 ** | <0.001 ** | |
| Zn | <0.001 ** | <0.001 ** | <0.001 ** | |
| C.V (%) | 0.90 | 0.60 | 1.00 | |
| Treatment | R-KUE (kg Roots kg k−1) | S-KUE (kg Sugar kg k−1) | |
|---|---|---|---|
| K120 | Zn0 | 0.43 ± 0.011 c | 0.075 ± 0.002 b |
| Zn150 | 0.46 ± 0.011 b | 0.082 ± 0.001 b | |
| Zn300 | 0.48 ± 0.013 a | 0.087 ± 0.002 a | |
| K180 | Zn0 | 0.34 ± 0.012 e | 0.064 ± 0.002 d |
| Zn150 | 0.35 ± 0.012 d | 0.067 ± 0.002 c | |
| Zn300 | 0.35 ± 0.013 d | 0.069 ± 0.002 c | |
| p-value | K × Z | <0.001 ** | <0.001 ** |
| Cv × K × Zn | 0.277 ns | 0.122 ns | |
| C | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RL | 1 | 0.967 ** | 0.935 ** | 0.872 ** | 0.970 ** | −0.420 * | 0.579 ** | 0.869 ** | 0.903 ** | 0.457 ** |
| 2 | RD | 1 | 0.961 ** | 0.896 ** | 0.959 ** | −0.367 * | 0.504 ** | 0.894 ** | 0.929 ** | 0.513 ** | |
| 3 | RFW | 1 | 0.922 ** | 0.890 ** | −0.234 ns | 0.394 * | 0.926 ** | 0.939 ** | 0.629 ** | ||
| 4 | LAI | 1 | 0.832 ** | −0.333 * | 0.500 ** | 0.840 ** | 0.931 ** | 0.492 ** | |||
| 5 | SPADindex | 1 | −0.575 ** | 0.675 ** | 0.808 ** | 0.868 ** | 0.309 ns | ||||
| 6 | PS | 1 | −0.939 ** | −0.099 ns | −0.300 ns | 0.549 ** | |||||
| 7 | LS | 1 | 0.273 ns | 0.486 ** | −0.366 * | ||||||
| 8 | RY | 1 | 0.946 ** | 0.776 ** | |||||||
| 9 | TY | 1 | 0.602 ** | ||||||||
| 10 | PSY | 1 | |||||||||
| r | R2 | SEE | Significance | Fitted Equation |
|---|---|---|---|---|
| 0.999 | 0.998 | 0.043 | *** | PSY = −9.413 + 0.19 RY + 0.54 PS% − 0.028 RL + 0.488 RFW − 0.059 RD |
| Character | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Total r | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RL | −0.08 | −0.05 | 0.12 | −0.01 | −0.01 | −0.26 | 0.02 | 0.78 | −0.05 | 0.46 ** |
| 2 | RD | −0.07 | −0.05 | 0.12 | −0.01 | −0.01 | −0.23 | 0.02 | 0.80 | −0.05 | 0.51 ** |
| 3 | RFW | −0.07 | −0.05 | 0.12 | −0.01 | −0.01 | −0.14 | 0.01 | 0.83 | −0.05 | 0.63 ** |
| 4 | LAI | −0.07 | −0.04 | 0.11 | −0.01 | −0.01 | −0.21 | 0.02 | 0.75 | −0.05 | 0.49 ** |
| 5 | SPAD index | 0.07 | −0.05 | 0.11 | −0.01 | −0.01 | −0.36 | 0.02 | 0.73 | −0.05 | 0.31 ns |
| 6 | PS | 0.03 | 0.02 | −0.03 | 0.004 | 0.01 | 0.62 | −0.03 | −0.09 | 0.02 | 0.55 ** |
| 7 | LS | −0.04 | −0.02 | 0.05 | −0.01 | −0.01 | −0.85 | 0.03 | 0.25 | −0.03 | −0.37 * |
| 8 | RY | −0.07 | −0.04 | 0.12 | −0.01 | −0.01 | −0.06 | 0.01 | 0.09 | −0.05 | 0.78 ** |
| 9 | TY | −0.07 | −0.04 | 0.12 | −0.01 | −0.01 | −0.19 | 0.01 | 0.85 | −0.06 | 0.60 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekdad, A.A.A.; Shaaban, A.; Rady, M.M.; Ali, E.F.; Hassan, F.A.S. Integrated Application of K and Zn as an Avenue to Promote Sugar Beet Yield, Industrial Sugar Quality, and K-Use Efficiency in a Salty Semi-Arid Agro-Ecosystem. Agronomy 2021, 11, 780. https://doi.org/10.3390/agronomy11040780
Mekdad AAA, Shaaban A, Rady MM, Ali EF, Hassan FAS. Integrated Application of K and Zn as an Avenue to Promote Sugar Beet Yield, Industrial Sugar Quality, and K-Use Efficiency in a Salty Semi-Arid Agro-Ecosystem. Agronomy. 2021; 11(4):780. https://doi.org/10.3390/agronomy11040780
Chicago/Turabian StyleMekdad, Ali A. A., Ahmed Shaaban, Mostafa M. Rady, Esmat F. Ali, and Fahmy A. S. Hassan. 2021. "Integrated Application of K and Zn as an Avenue to Promote Sugar Beet Yield, Industrial Sugar Quality, and K-Use Efficiency in a Salty Semi-Arid Agro-Ecosystem" Agronomy 11, no. 4: 780. https://doi.org/10.3390/agronomy11040780
APA StyleMekdad, A. A. A., Shaaban, A., Rady, M. M., Ali, E. F., & Hassan, F. A. S. (2021). Integrated Application of K and Zn as an Avenue to Promote Sugar Beet Yield, Industrial Sugar Quality, and K-Use Efficiency in a Salty Semi-Arid Agro-Ecosystem. Agronomy, 11(4), 780. https://doi.org/10.3390/agronomy11040780








