Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Composting Procedure
2.3. Greenhouse Assay
2.4. Chemical Analyses
2.5. Plant Growth
2.6. Statistical Analyses
3. Results
3.1. Influence of Factors in Soil and Plant Growth
3.2. Chemical Analysis of Soils
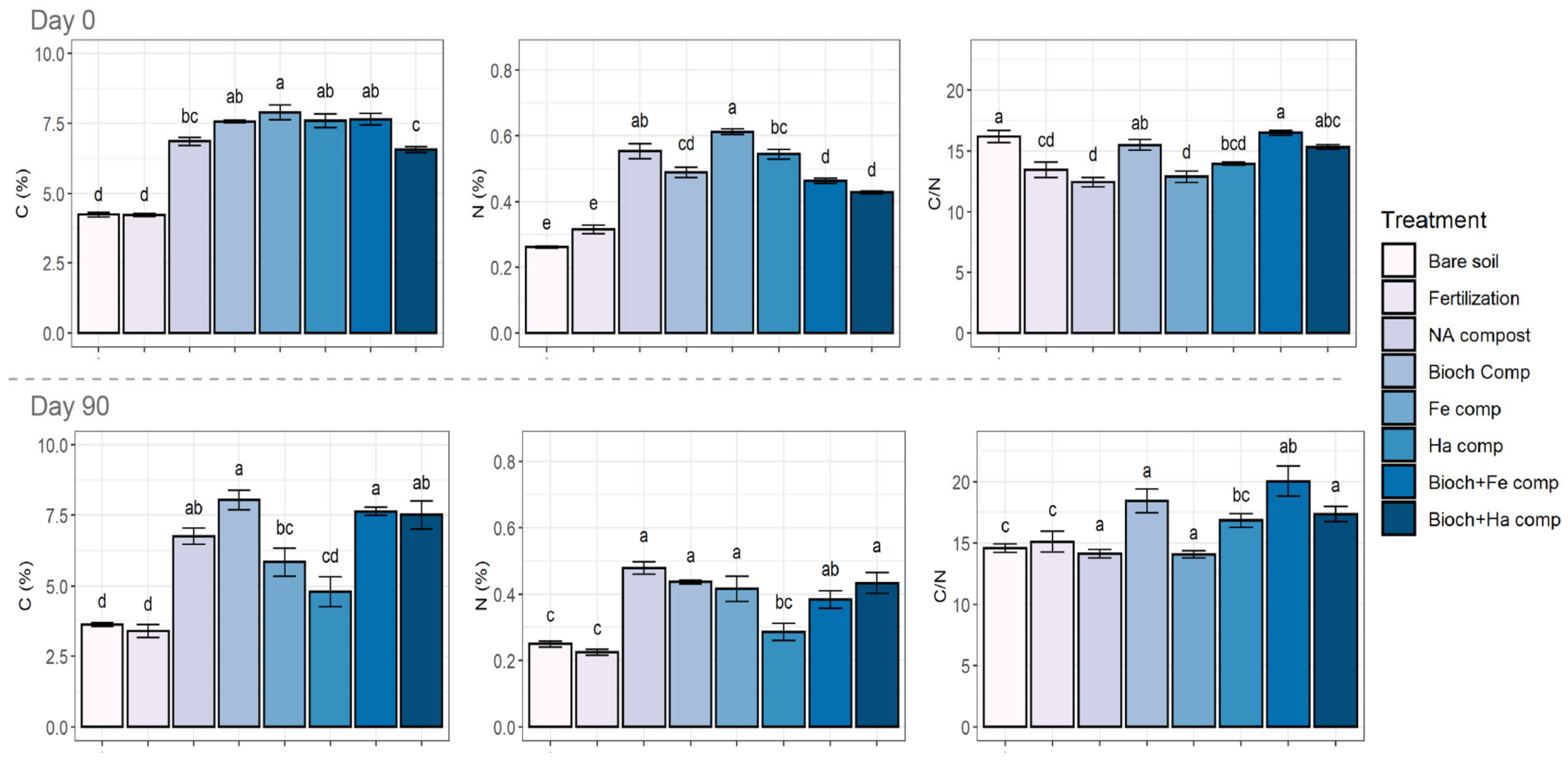
3.2.1. Elemental Analysis and C/N Atomic Ratio
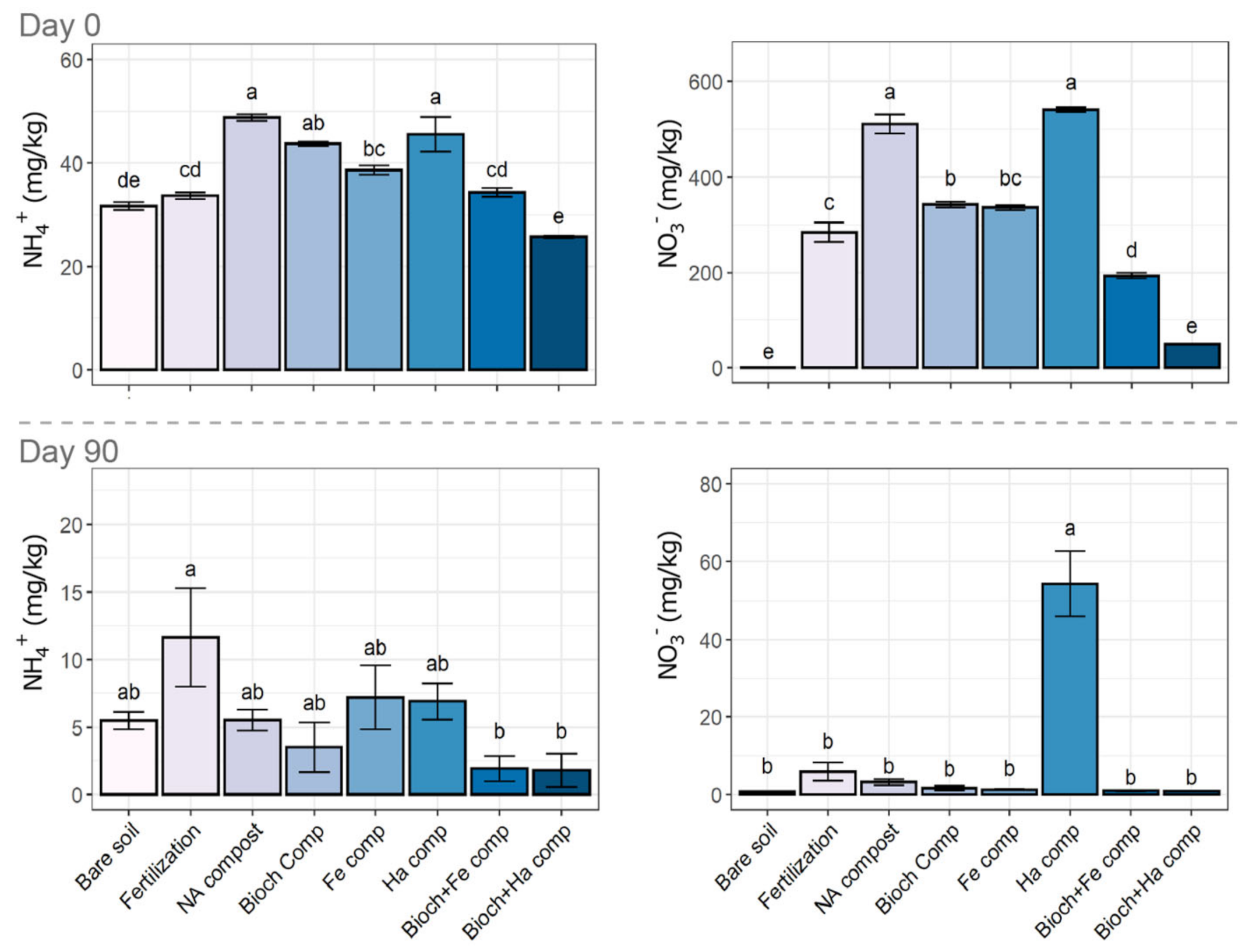
3.2.2. NH4+ and NO3− Concentrations in Soil
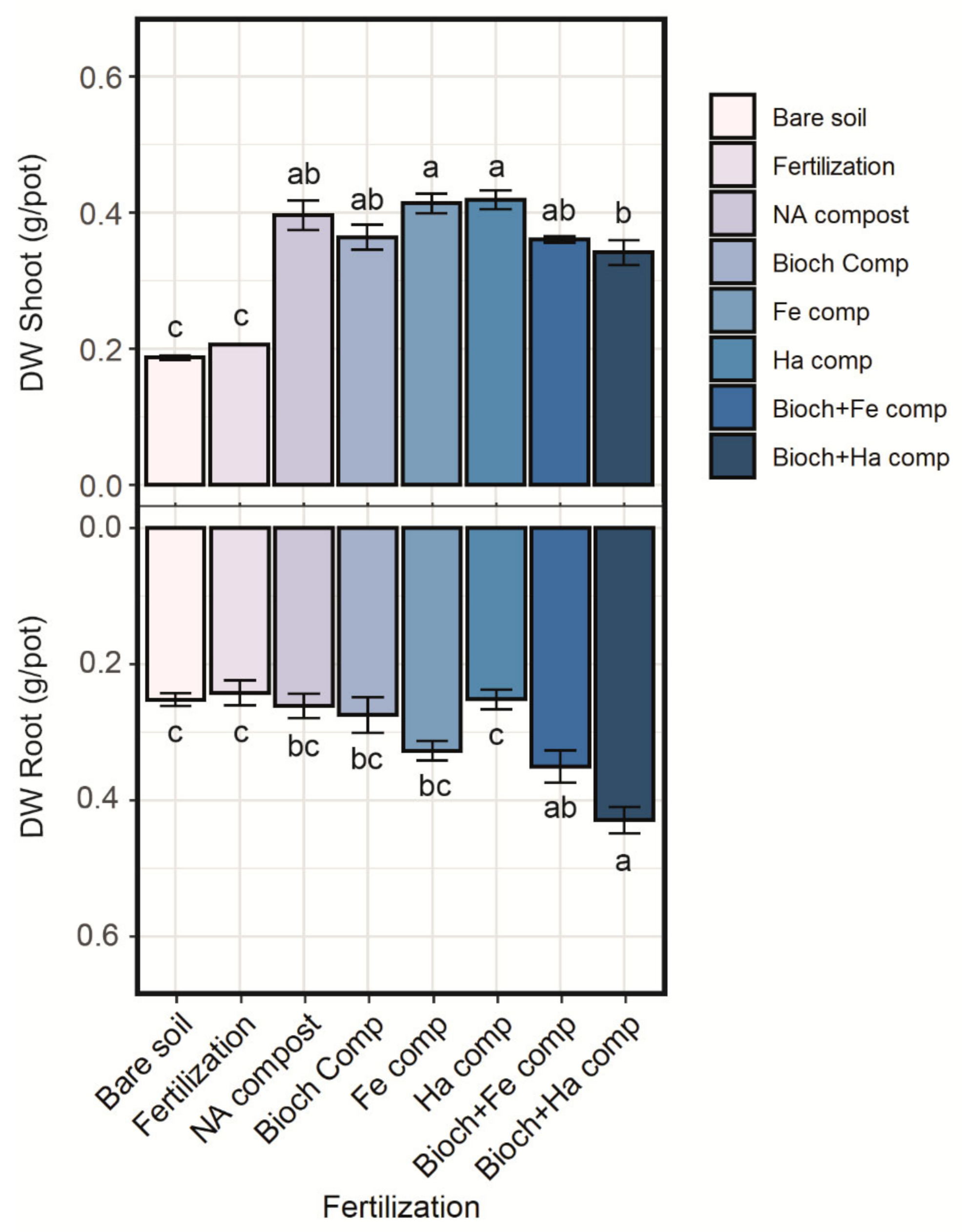
3.3. Shoot and Root Biomass Production
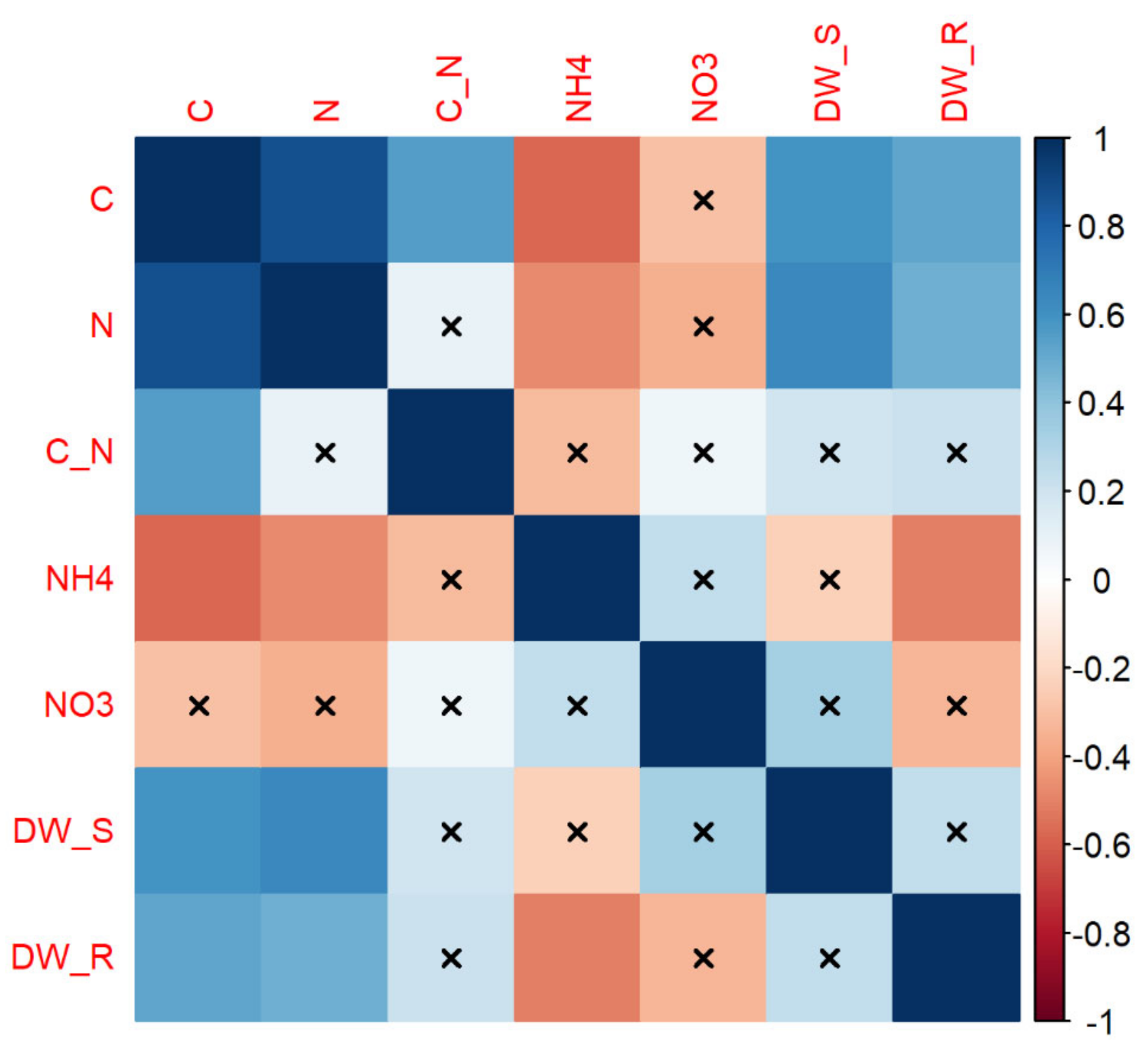
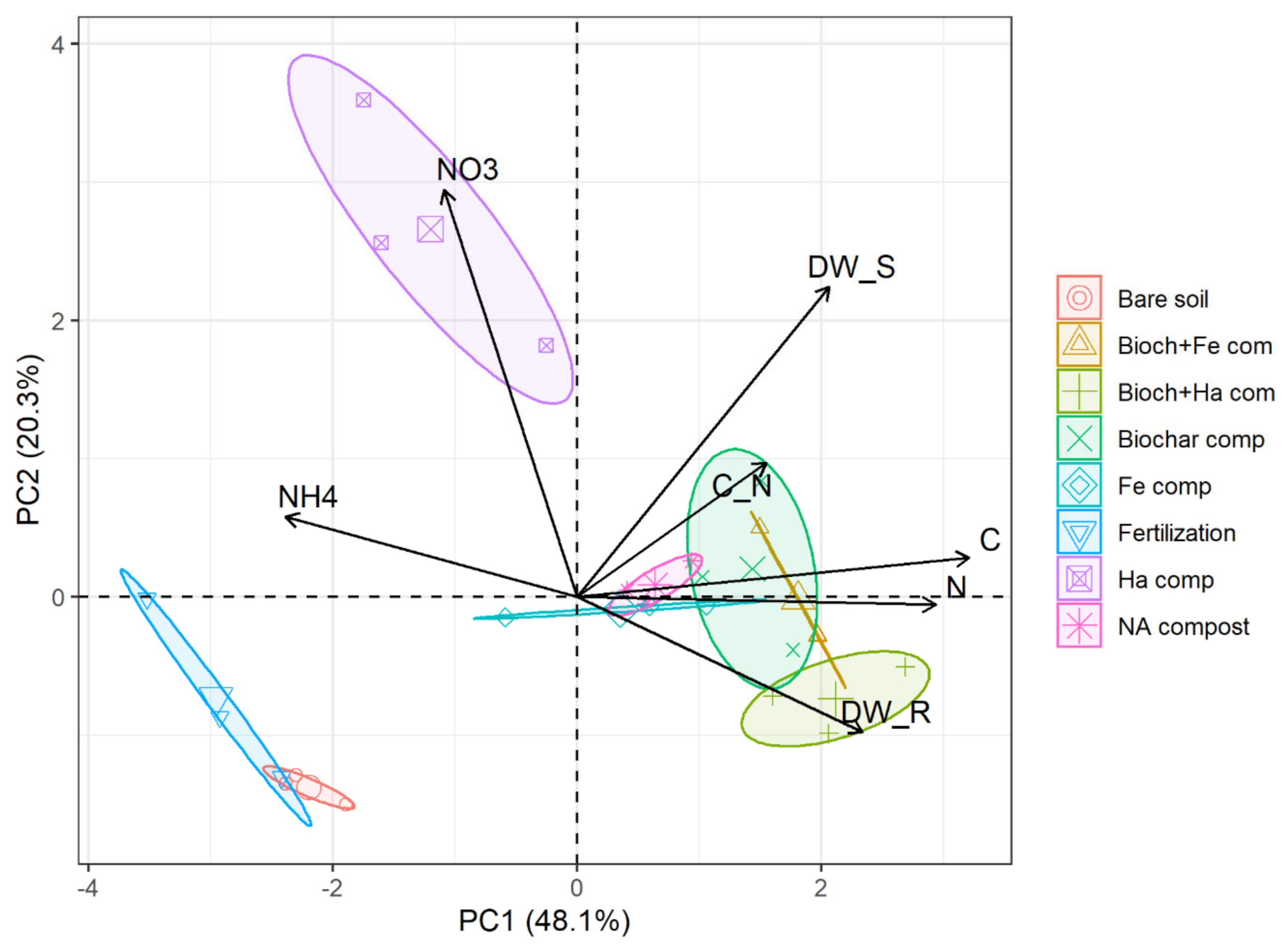
3.4. Multivariate Relationships
4. Discussion
4.1. Effect of Compost on Elemental Composition and Nutrient Status on Soil
4.2. Effect of Compost on Plant Biomass and Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; de la Luz Mora, M. Smart Fertilizers as a Strategy for Sustainable Agriculture, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 147. [Google Scholar]
- Veeken, A.; Adani, F.; Nierop, K.; Jager, P.; Hamelers, H.V.M. Degradation of Biomacromolecules during High-Rate Composting of Wheat Straw–Amended Feces. J. Environ. Qual. 2001, 30, 1675–1684. [Google Scholar] [CrossRef]
- Dunford, N.T.; Edwards, J. Nutritional bioactive components of wheat straw as affected by genotype and environment. Bioresour. Technol. 2010, 101, 422–425. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Sun, J.; Peng, H.; Chen, J.; Wang, X.; Wei, M.; Li, W.; Yang, L.; Zhang, Q.; Wang, W.; Mellouki, A. An estimation of CO2 emission via agricultural crop residue open field burning in China from 1996 to 2013. J. Clean. Prod. 2016, 112, 2625–2631. [Google Scholar] [CrossRef]
- Udeigwe, T.K.; Teboh, J.M.; Eze, P.N.; Hashem Stietiya, M.; Kumar, V.; Hendrix, J.; Mascagni, H.J.; Ying, T.; Kandakji, T. Implications of leading crop production practices on environmental quality and human health. J. Environ. Manag. 2015, 151, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Borie, F.; Rubio, R.; Rouanet, J.L.; Morales, A.; Borie, G.; Rojas, C. Effects of tillage systems on soil characteristics, glomalin and mycorrhizal propagules in a Chilean Ultisol. Soil Tillage Res. 2006, 88, 253–261. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R.; Morales, A.; Curaqueo, G.; Cornejo, P. Arbuscular mycorrhizae in agricultural and forest ecosystems in Chile. J. Soil Sci. Plant Nutr. 2010, 10, 185–206. [Google Scholar] [CrossRef][Green Version]
- Calabi-Floody, M.; Medina, J.; Suazo, J.; Ordiqueo, M.; Aponte, H.; de la Luz Mora, M.; Rumpel, C. Optimization of wheat straw co-composting for carrier material development. Waste Manag. 2019, 98, 37–49. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Chen, L.; Shen, M.; Zhang, X.; Zhang, M.; Bian, X.; Zhang, J.; Zhang, W. Integrative effects of soil tillage and straw management on crop yields and greenhouse gas emissions in a rice-wheat cropping system. Eur. J. Agron. 2015, 63, 47–54. [Google Scholar] [CrossRef]
- De Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.; Barea, J.M.; Arriagada, C.; Borie, F.; Cornejo, P. Crop residue stabilization and application to agricultural and degraded soils: A review. Waste Manag. 2015, 42, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Tapia, Y.; Antilén, M.; Casanova, M.; Vidal, C.; Santander, C.; Aponte, H.; Cornejo, P. Interactive effect of compost application and inoculation with the fungus Claroideoglomus claroideum in Oenothera picensis plants growing in mine tailings. Ecotoxicol. Environ. Saf. 2021, 208, 111495. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tan, W.; Zhao, Y.; Wu, J.; Sun, Q.; Qi, H.; Xie, X.; Wei, Z. Diversity in the Mechanisms of Humin Formation during Composting with Different Materials. Environ. Sci. Technol. 2019, 53, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Kunhikrishnan, A.; Choppala, G.K.; Thangarajan, R.; Chung, J.W. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Godbout, S.; Verma, M.; Larouche, J.P.; Potvin, L.; Chapman, A.M.; Lemay, S.P.; Pelletier, F.; Brar, S.K. Methane production potential (B0) of swine and cattle manures—A Canadian perspective. Environ. Technol. 2010, 31, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Paetsch, L.; Mueller, C.W.; Rumpel, C.; Houot, S.; Kögel-Knabner, I. Urban waste composts enhance OC and N stocks after long-term amendment but do not alter organic matter composition. Agric. Ecosyst. Environ. 2016, 223, 211–222. [Google Scholar] [CrossRef]
- Mikutta, R.; Kaiser, K. Organic matter bound to mineral surfaces: Resistance to chemical and biological oxidation. Soil Biol. Biochem. 2011, 43, 1738–1741. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Awasthi, M.K.; Zhao, J.; Wang, J.; Liu, T.; Li, R.; Zhang, Z. Improvement of cleaner composting production by adding Diatomite: From the nitrogen conservation and greenhouse gas emission. Bioresour. Technol. 2019, 286, 121377. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bolan, N.S.; Seshadri, B.; Kunhikrishnan, A.; Wijesekara, H.; Xu, Y.; Yang, J.; Kim, G.H.; Sparks, D.; Rumpel, C. Co-composting solid biowastes with alkaline materials to enhance carbon stabilization and revegetation potential. Environ. Sci. Pollut. Res. 2016, 23, 7099–7110. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Fu, Q.; Zhong, M.; Li, R.; Zhai, B.; Wang, Z.; Zhou, L. Effects of four additives in pig manure composting on greenhouse gas emission reduction and bacterial community change. Bioresour. Technol. 2019, 292, 121896. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Wilk, R.; Nowicki, M.; Nowosielski, R.; Cebula, J. Application of micro- and nanostructural multifunctional halloysite-based sorbents from DUNINO deposit in selected biotechnological processes. J. Achiev. Mater. Manuf. Eng. 2015, 69, 69–78. [Google Scholar]
- Medina, J.; Monreal, C.M.; Orellana, L.; Calabi-Floody, M.; González, M.E.; Meier, S.; Borie, F.; Cornejo, P. Influence of saprophytic fungi and inorganic additives on enzyme activities and chemical properties of the biodegradation process of wheat straw for the production of organo-mineral amendments. J. Environ. Manag. 2020, 255, 109922. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Monreal, C.M.; Antilén, M.; Calabi-Floody, M.; Velasco-Molina, M.; Meier, S.; Borie, F.; Cornejo, P.; Knicker, H. Influence of inorganic additives on wheat straw composting: Characterization and structural composition of organic matter derived from the process. J. Environ. Manag. 2020, 260. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Monreal, C.; Chabot, D.; Meier, S.; González, M.E.; Morales, E.; Parillo, R.; Borie, F.; Cornejo, P. Microscopic and spectroscopic characterization of humic substances from a compost amended copper contaminated soil: Main features and their potential effects on Cu immobilization. Environ. Sci. Pollut. Res. 2017, 24, 14104–14116. [Google Scholar] [CrossRef] [PubMed]
- Calabi-Floody, M.; Bendall, J.S.; Jara, A.A.; Welland, M.E.; Theng, B.K.G.; Rumpel, C.; de la Luz Mora, M. Nanoclays from an Andisol: Extraction, properties and carbon stabilization. Geoderma 2011, 161, 159–167. [Google Scholar] [CrossRef]
- Calabi-Floody, M.; Rumpel, C.; Velásquez, G.; Violante, A.; Bol, R.; Condron, L.M.; Mora, M.L. Role of nanoclays in carbon stabilization in andisols and cambisols. J. Soil Sci. Plant Nutr. 2015, 15, 587–604. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Q.; Dong, D.; Strong, P.J.; Wang, H.; Sun, B.; Wu, W. Spectroscopic evidence for biochar amendment promoting humic acid synthesis and intensifying humification during composting. J. Hazard. Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef]
- Curaqueo, G.; Sánchez-Monedero, M.Á.; Meier, S.; Medina, J.; Panichini, M.; Borie, F.; Navia, R. Characterization of a soil amendment derived from co-composting of agricultural wastes and biochar. Geophys. Res. Abstr. 2016, 18, EGU2016–18184–1. [Google Scholar]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Rilling, J.; Vidal, C.; Fernández, N.; Acuña, J.; González, M.E.; Cornejo, P.; et al. Effects of biochar on copper immobilization and soil microbial communities in a metal-contaminated soil. J. Soils Sediments 2017, 17, 1237–1250. [Google Scholar] [CrossRef]
- Prost, K.; Borchard, N.; Siemens, J.; Kautz, T.; Séquaris, J.-M.; Möller, A.; Amelung, W. Biochar Affected by Composting with Farmyard Manure. J. Environ. Qual. 2013, 42, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, B.; Sinicco, T.; D’Hose, T.; Vanden Nest, T.; Mondini, C. Biochar amendment before or after composting affects compost quality and N losses, but not P plant uptake. J. Environ. Manag. 2016, 168, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Subdiaga, E.; Orsetti, S.; de la Rosa, J.M.; Knicker, H.; Schmidt, H.P.; Kappler, A.; Behrens, S. Effect of biochar amendment on compost organic matter composition following aerobic compositing of manure. Sci. Total Environ. 2018, 613–614, 20–29. [Google Scholar] [CrossRef]
- Ngo, P.T.; Rumpel, C.; Janeau, J.L.; Dang, D.K.; Doan, T.T.; Jouquet, P. Mixing of biochar with organic amendments reduces carbon removal after field exposure under tropical conditions. Ecol. Eng. 2016, 91, 378–380. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Paradelo, R.; Dignac, M.F. The effects of worms, clay and biochar on CO2 emissions during production and soil application of co-composts. Soil 2016, 2, 673–683. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Calabi-Floody, M.; Mora, M.L.; Bolan, N.S.; Dignac, M.F. Adding worms during composting of organic waste with red mud and fly ash reduces CO2 emissions and increases plant available nutrient contents. J. Environ. Manag. 2018, 222, 207–215. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Melero, S.; Porras, J.C.R.; Herencia, J.F.; Madejon, E. Chemical and biochemical properties in a silty loam soil under conventional and organic management. Soil Tillage Res. 2006, 90, 162–170. [Google Scholar] [CrossRef]
- Liu, M.; Hu, F.; Chen, X.; Huang, Q.; Jiao, J.; Zhang, B.; Li, H. Organic amendments with reduced chemical fertilizer promote soil microbial development and nutrient availability in a subtropical paddy field: The influence of quantity, type and application time of organic amendments. Appl. Soil Ecol. 2009, 42, 166–175. [Google Scholar] [CrossRef]
- Weerasinghe, T.K.; De Silva, I.H.W.K. Effect of applying different ratios of compost made of municipal solid waste on the growth of Zea mays L. (Corn). J. Soil Sci. Environ. Manag. 2017, 8, 52–60. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong, J.W.C. Reducing nitrogen loss and salinity during “struvite” food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130. [Google Scholar]
- Meena, M.D.; Biswas, D.R. Phosphorus and Potassium Transformations in Soil Amended with Enriched Compost and Chemical Fertilizers in a Wheat-Soybean Cropping System. Commun. Soil Sci. Plant Anal. 2014, 45, 624–652. [Google Scholar] [CrossRef]
- Mihreteab, H.T.; Ceglie, F.G.; Aly, A.; Tittarelli, F. Rock phosphate enriched compost as a growth media component for organic tomato (Solanum lycopersicum L.) seedlings production. Biol. Agric. Hortic. 2016, 32, 7–20. [Google Scholar] [CrossRef]
- Nishanth, D.; Biswas, D.R. Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat (Triticum aestivum). Bioresour. Technol. 2008, 99, 3342–3353. [Google Scholar] [CrossRef] [PubMed]
- López-Martín, M.; Velasco-Molina, M.; Knicker, H. Variability of the quality and quantity of organic matter in soil affected by multiple wildfires. J. Soils Sediments 2016, 16, 360–370. [Google Scholar] [CrossRef]
- Blume, H.-P. Page, A. L., R. H. Miller and D. R. Keeney (Ed., 1982): Methods of soil analysis; 2. Chemical and microbiological properties, 2. Aufl. 1184 S., American Soc. of Agronomy (Publ.), Madison, Wisconsin, USA, gebunden 36 Dollar. J. Plant Nutr. Soil Sci. 1985, 148, 363–364. [Google Scholar] [CrossRef]
- Jolanun, B.; Towprayoon, S. Novel bulking agent from clay residue for food waste composting. Bioresour. Technol. 2010, 101, 4484–4490. [Google Scholar] [CrossRef]
- Mahimairaja, S.; Bolan, N.S.; Hedley, M.J.; Macgregor, A.N. Losses and transformation of nitrogen during composting of poultry manure with different amendments: An incubation experiment. Bioresour. Technol. 1994, 47, 265–273. [Google Scholar] [CrossRef]
- Merino, C.; Nannipieri, P.; Matus, F. Soil carbon controlled by plant, microorganism and mineralogy interactions. J. Soil Sci. Plant Nutr. 2015, 15, 321–332. [Google Scholar] [CrossRef][Green Version]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Jouquet, E.; Bloquel, E.; Doan, T.T.; Ricoy, M.; Orange, D.; Duc, T.T.; Jouquet, E.; Bloquel, E.; Ricoy, M.; Orange, D.; et al. Do Compost and Vermicompost Improve Macronutrient Retention and Plant Growth in Degraded Tropical Soils? Compos. Sci. Util. 2011, 19, 15–24. [Google Scholar] [CrossRef]
- Lazcano, C.; Arnold, J.; Tato, A.; Zaller, J.G.; Domínguez, J. Compost and vermicompost as nursery pot components: Effects on tomato plant growth and morphology. Span. J. Agric. Res. 2009, 7, 944. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y.; Kumari, U.R. An experimental study of vermi-biowaste composting for agricultural soil improvement. Bioresour. Technol. 2008, 99, 1672–1681. [Google Scholar] [CrossRef]
- Vidal, A.; Lenhart, T.; Dignac, M.F.; Biron, P.; Höschen, C.; Barthod, J.; Vedere, C.; Vaury, V.; Bariac, T.; Rumpel, C. Promoting plant growth and carbon transfer to soil with organic amendments produced with mineral additives. Geoderma 2020, 374, 114454. [Google Scholar] [CrossRef]
- Soudejani, H.T.; Kazemian, H.; Inglezakis, V.J.; Zorpas, A.A. Application of zeolites in organic waste composting: A review. Biocatal. Agric. Biotechnol. 2019, 22, 101396. [Google Scholar] [CrossRef]
- Guaya, D.; Mendoza, A.; Valderrama, C.; Farran, A.; Sauras-Yera, T.; Cortina, J.L. Use of nutrient-enriched zeolite (NEZ) from urban wastewaters in amended soils: Evaluation of plant availability of mineral elements. Sci. Total Environ. 2020, 727, 138646. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wong, J.W.C. Effects of lime amendment on availability of heavy metals and maturation in sewage sludge composting. Environ. Pollut. 1999, 106, 83–89. [Google Scholar] [CrossRef]
- Samaras, P.; Papadimitriou, C.A.; Haritou, I.; Zouboulis, A.I. Investigation of sewage sludge stabilization potential by the addition of fly ash and lime. J. Hazard. Mater. 2008, 154, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Genesio, L.; Miglietta, F.; Lugato, E.; Baronti, S.; Pieri, M.; Vaccari, F.P. Surface albedo following biochar application in durum wheat. Environ. Res. Lett. 2012, 7. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ju, X.-T.; Chen, X.-P.; Zhang, F.-S.; Romheld, V. Nitrogen recommendations for summer maize in northern China using the Nmin test and rapid plant tests. Pedosphere 2005, 15, 246–254. [Google Scholar]
- Ågren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [PubMed]
| Variable | Factor | F-Value |
|---|---|---|
| C (%) | Time | 19.79 *** |
| Amendments | 61.20 *** | |
| Time × Amendments | 9.99 *** | |
| N (%) | Time | 102.58 *** |
| Amendments | 55.74 *** | |
| Time × Amendments | 11.39 *** | |
| C/N | Time | 37.56 *** |
| Amendments | 17.61 *** | |
| Time × Amendments | 3.67 ** | |
| NH4 | Time | 1608.92 *** |
| Amendments | 15.56 *** | |
| Time × Amendments | 11.97 *** | |
| NO3 | Time | 4735.10 *** |
| Amendments | 336.20 *** | |
| Time × Amendments | 271.10 *** | |
| Dry shoot weight (g/pot) | Amendments | 40.42 *** |
| Dry root weight (g/pot) | Amendments | 12.36 *** |
| Treatment | B | Ca | Cu | Fe | K | Mg | P | S |
|---|---|---|---|---|---|---|---|---|
| Bare soil | 3.8 ± 0.6 ab | 1755.4 ± 22 d | 37.0 ± 0.4 abc | 35,518.0 ± 33.1 c | 2225.1 ± 293.7 c | 2579.4 ± 12.9 d | 382.6 ± 1.2 e | 216.5 ± 2.8 e |
| Fertilization | 3.7 ± 0.4 ab | 1778.1 ± 13.6 d | 36.9 ± 0.1 abcd | 35,621.9 ± 13.7 c | 3138.3 ± 146.0 b | 2541.5 ± 18.8 d | 382.5 ± 1.7 e | 224.6 ± 1.2 e |
| NA compost | 4.7 ± 0.2 a | 2909.3 ± 14.7 b | 37.5 ± 0.1 ab | 35,148.6 ± 347.5 cd | 4694.1 ± 161.0 a | 3082.9 ± 42.2 a | 861.8 ± 0.1 b | 543.7 ± 1.5 bc |
| Biochar comp | 3.9 ± 0.0 ab | 2702.4 ± 6.8 c | 37.1 ± 0.1 ab | 33,869.3 ± 34.9 de | 4206.3 ± 95.4 a | 2778.4 ± 0.9 c | 831.7 ± 0.3 c | 498.2 ± 3.9 d |
| Fe comp | 4.0 ± 0.0 ab | 3091.1 ± 25.4 a | 38.0 ± 0.0 a | 40,019.8 ± 142.6 a | 4667.5 ± 17.5 a | 2927.3 ± 12.4 b | 864.6 ± 5.7 ab | 634.4 ± 1.8 a |
| Ha comp | 2.9 ± 0.3 b | 2762.8 ± 13.3 c | 35.8 ± 0.1 cd | 33,137.2 ± 135.0 e | 4053.5 ± 173.7 a | 2795.0 ± 35.4 bc | 825.5 ± 0.8 c | 531.7 ± 4.5 c |
| Bioch+Fe com | 2.8 ± 0.0 b | 2684.6 ± 25.8 c | 35.7 ± 0.1 d | 38,301.0 ± 103.4 b | 4252.3 ± 55.1 a | 2811.2 ± 1.4 bc | 800.3 ± 2.9 d | 488.7 ± 4.6 d |
| Bioch+Ha com | 3.3 ± 0.4 ab | 2870.7 ± 10.8 b | 36.6 ± 0.6 bcd | 33,263.0 ± 794.5 e | 4686.2 ± 269.2 a | 2771.1 ± 47.0 c | 878.5 ± 5.9 a | 547.9 ± 1.9 b |
| Treatment | Al | As | Cd | Co | Cr | Mn | Na | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Bare soil | 19,567.9 ± 1285 b | 23.2 ± 0.6 ab | 0.19 ± 0.00 a | 13.5 ± 0.1 bc | 32.1 ± 1.1 a | 1134.9 ± 4.8 ab | 117.8 ± 32.3 c | 31.7 ± 0.2 ab | 43.9 ± 0.3 a | 70.5 ± 0.2 cd |
| Fertilization | 20,732.9 ± 582 ab | 23.5 ± 1.0 a | 0.11 ± 0.01 cd | 13.5 ± 0.1 bc | 33.1 ± 0.5 a | 1158.8 ± 2.3 ab | 160.7 ± 15.2 c | 32.8 ± 0.2 a | 44.5 ± 0.1 a | 69.8 ± 0.4 d |
| NA compost | 23,382.1 ± 547.6 ab | 21.7 ± 0.2 ab | 0.13 ± 0.00 bc | 13.7 ± 0.1 b | 32.9 ± 0.3 a | 1120.1 ± 3.1 ab | 664.3 ± 25.6 b | 32.2 ± 0.8 ab | 40.0 ± 0.0 c | 77.5 ± 1.2 a |
| Biochar comp | 21,444.8 ± 391.1 ab | 21.6 ± 0.1 ab | 0.13 ± 0.0 b | 12.8 ± 0.0 c | 33.2 ± 1.4 a | 1065.7 ± 0.9 c | 599.4 ± 15.0 b | 30.9 ± 0.3 ab | 40.2 ± 0.2 bc | 72.3 ± 0.1 cd |
| Fe comp | 21,362.0 ± 113.5 ab | 21.3 ± 0.3 ab | 0.14 ± 0.00 b | 14.8 ± 0.0 a | 32.4 ± 0.2 a | 1144.6 ± 5.3 ab | 852.6 ± 4.6 a | 30.9 ± 0.1 ab | 41.2 ± 0.1 b | 76.6 ± 0.8 ab |
| Ha comp | 21,630.7 ± 803.4 ab | 20.9 ± 0.1 b | 0.09 ± 0.00 de | 12.7 ± 0.0 c | 31.3 ± 0.2 a | 1061.5 ± 2.2 c | 620.3 ± 21.1 b | 31.7 ± 0.2 ab | 38.2 ± 0.2 d | 72.6 ± 0.5 cd |
| Bioch+Fe com | 20,706.3 ± 1428 ab | 21.2 ± 0.1 ab | 0.09 ± 0.01 e | 13.5 ± 0.1 bc | 32.3 ± 0.7 a | 1112.4 ± 9.6 bc | 655.5 ± 4.4 b | 30.2 ± 0.1 b | 40.9 ± 0.2 bc | 73.8 ± 0.0 bc |
| Bioch+Ha com | 24,108.6 ± 111.9 a | 21.9 ± 0.5 ab | 0.10 ± 0.00 de | 13.5 ± 0.5 bc | 33.5 ± 1.5 a | 1184.4 ± 32.9 a | 687.0 ± 40.7 b | 31.5 ± 0.8 ab | 40.9 ± 0.4 bc | 72.0 ± 1.2 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, J.; Calabi-Floody, M.; Aponte, H.; Santander, C.; Paneque, M.; Meier, S.; Panettieri, M.; Cornejo, P.; Borie, F.; Knicker, H. Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy 2021, 11, 767. https://doi.org/10.3390/agronomy11040767
Medina J, Calabi-Floody M, Aponte H, Santander C, Paneque M, Meier S, Panettieri M, Cornejo P, Borie F, Knicker H. Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy. 2021; 11(4):767. https://doi.org/10.3390/agronomy11040767
Chicago/Turabian StyleMedina, Jorge, Marcela Calabi-Floody, Humberto Aponte, Christian Santander, Marina Paneque, Sebastian Meier, Marco Panettieri, Pablo Cornejo, Fernando Borie, and Heike Knicker. 2021. "Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region" Agronomy 11, no. 4: 767. https://doi.org/10.3390/agronomy11040767
APA StyleMedina, J., Calabi-Floody, M., Aponte, H., Santander, C., Paneque, M., Meier, S., Panettieri, M., Cornejo, P., Borie, F., & Knicker, H. (2021). Utilization of Inorganic Nanoparticles and Biochar as Additives of Agricultural Waste Composting: Effects of End-Products on Plant Growth, C and Nutrient Stock in Soils from a Mediterranean Region. Agronomy, 11(4), 767. https://doi.org/10.3390/agronomy11040767













