Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects on Growth Rate and Biomass Productivity by Variation of Soil Moisture
2.1.1. Growth Rate over Time
2.1.2. Biomass Productivity
2.2. Effects on Photosynthetic Response and Biomass Productivity by Drought Conditions and Elevated CO2 Levels
2.2.1. Physiological Responses
2.2.2. Partitioned Biomass
2.3. Effect on Seed Germination from Exposure to Varying Temperatures
2.4. Statistical Analysis
3. Results
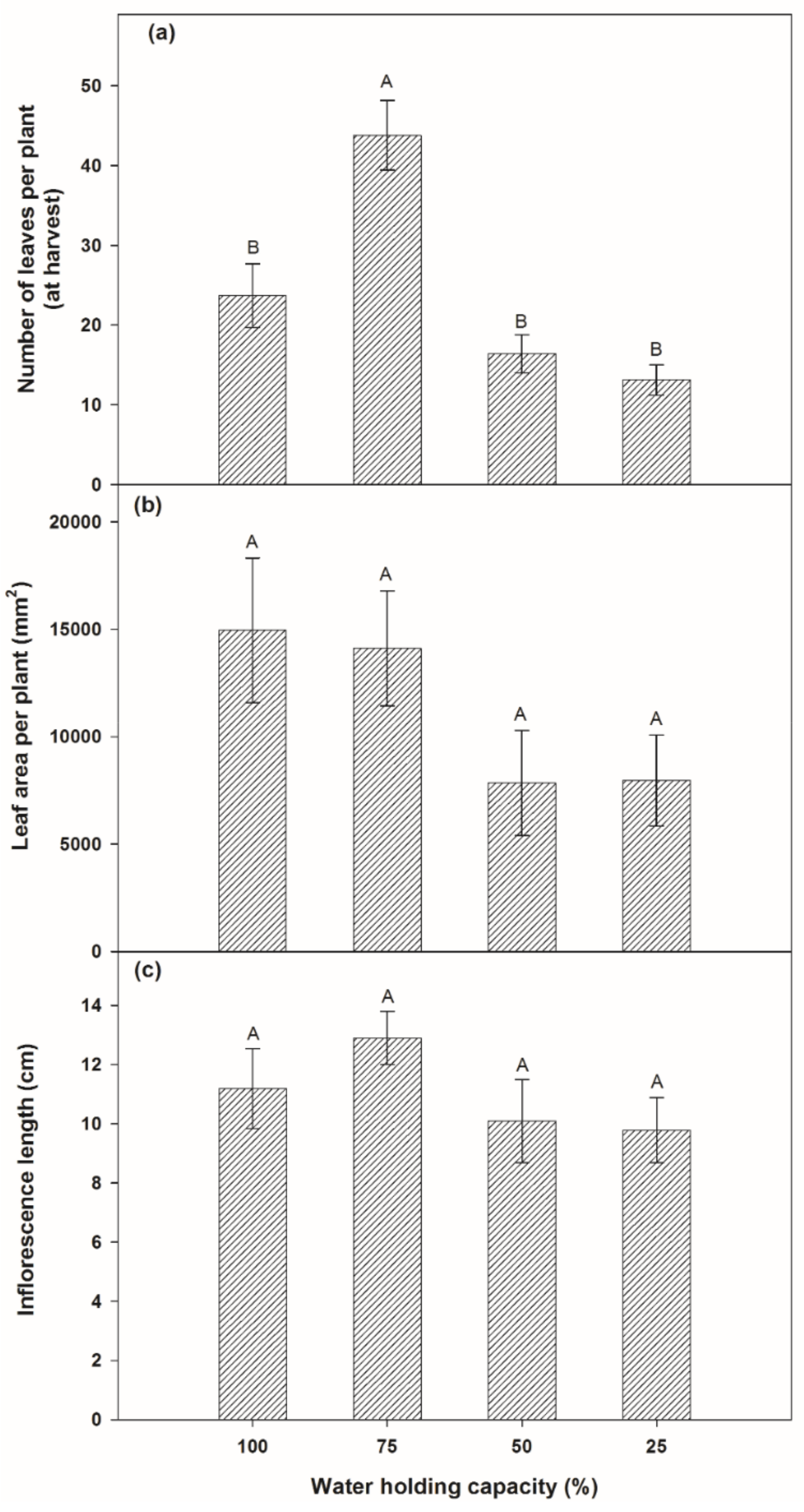
3.1. Effects on Growth Rate and Biomass Productivity by Variation of Soil Moisture
3.1.1. Growth Rate of Plants
3.1.2. Partitioned Biomass of Plants
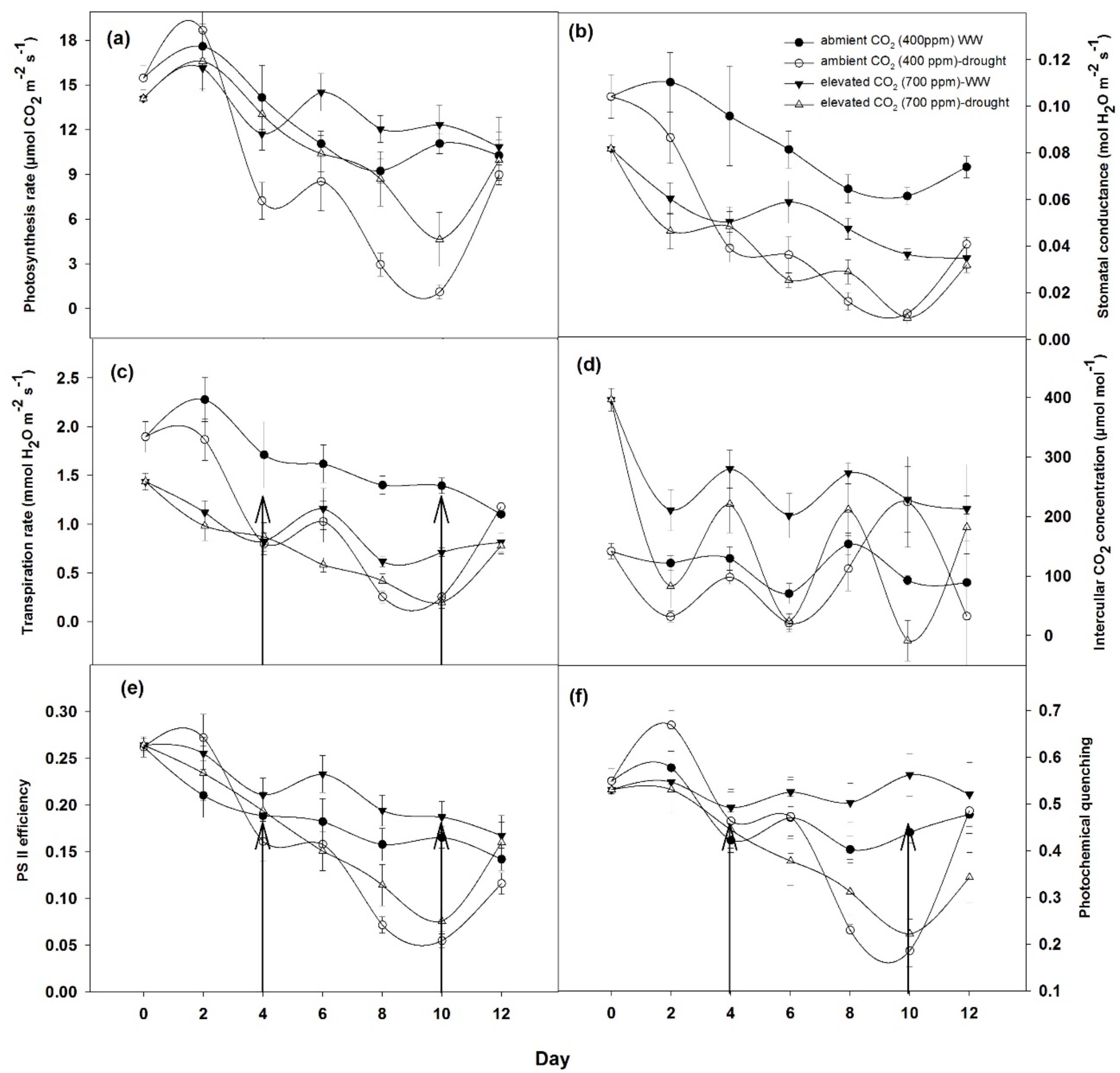
3.2. Effects on Photosynthetic Response and Biomass Productivity by Drought Conditions and Elevated CO2 Levels
3.2.1. Photosynthetic Response of Plants
3.2.2. Partitioned Biomass of Plants
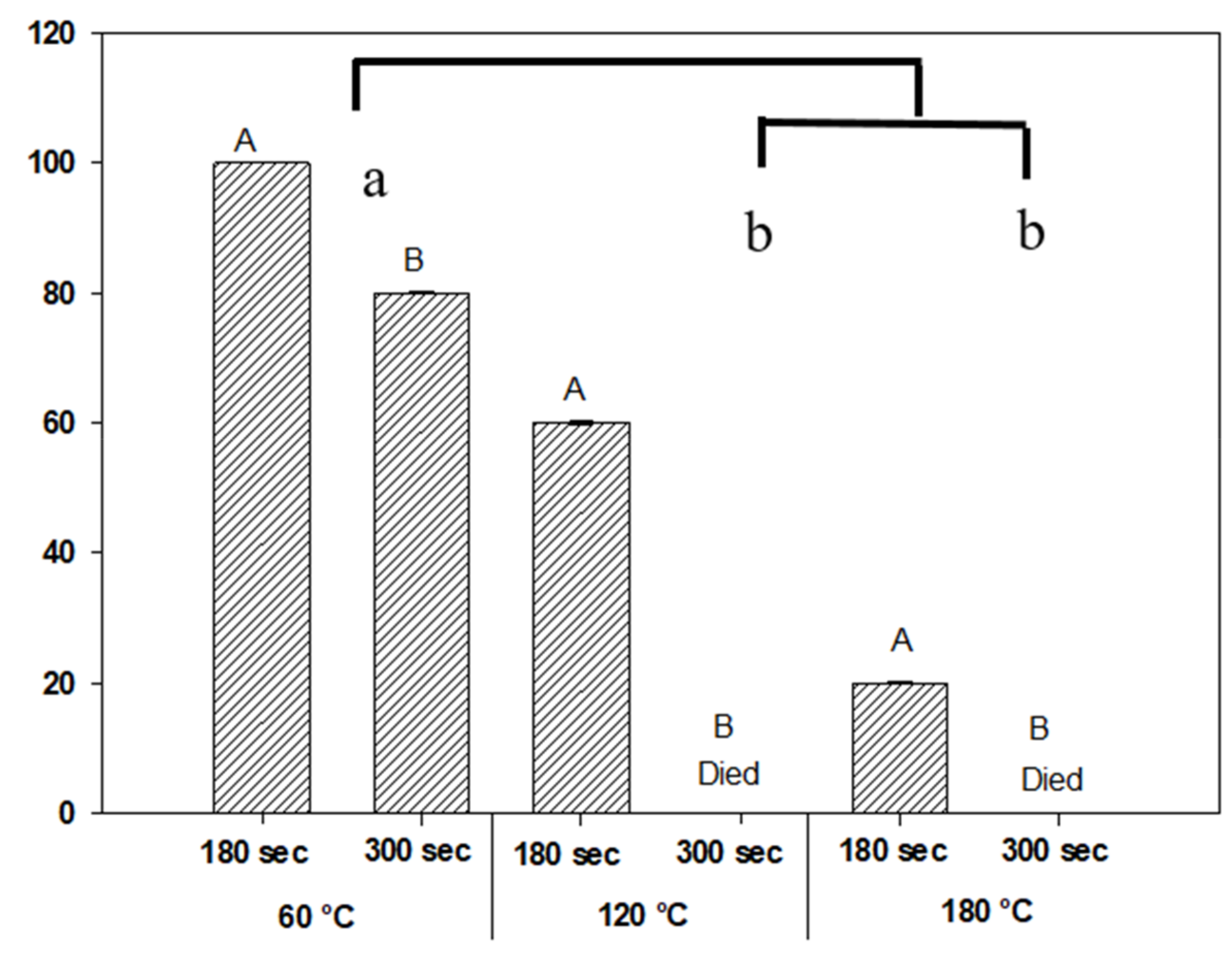
3.3. Effect on Seed Germination from Exposure to Varying Temperatures
4. Discussion
4.1. Effects on Growth Rate and Biomass Productivity by Variation of Soil Moisture
4.2. Effects on Photosynthetic Response and Biomass Productivity by Drought Conditions and Elevated CO2 Levels
4.3. Effect on Seed Germination from Exposure to Varying Temperatures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.E.; McWilliams, E.L. The biology of Canadian weeds. 44. Amaranthus retroflexus L., A. powellii S. Wats A. hybridus L. Can. J. Plant Sci. 1980, 60, 1215–1234. [Google Scholar] [CrossRef]
- AVH. Occurrence Records: Amaranthus Retroflexus. The Australasian Virtual Herbarium. Available online: https://avh.ala.org.au/occurrences/search?taxa=Amaranthus+retroflexus#tab_mapView (accessed on 28 July 2020).
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Can. J. Plant Sci. 2004, 84, 631–668. [Google Scholar] [CrossRef]
- Sauer, J.D. The grain amaranths: A survey of their history and classification. Ann. Mo. Bot. 1950, 37, 561–632. [Google Scholar] [CrossRef]
- Stevens, O. Weights of seeds and numbers per plant. Weeds 1957, 5, 46–55. [Google Scholar] [CrossRef]
- Egley, G.H.; Chandler, J.M. Germination and viability of weed seeds after 2.5 years in a 50-year buried seed study. Weed Sci. 1978, 26, 230–239. [Google Scholar] [CrossRef]
- Siriwardana, T.; Zimdahl, R. Competition between barnyard grass (Echinochloa crus-galli) and red-root pigweed (Amaranthus retroflexus). Weed Sci. 1984, 32, 218–222. [Google Scholar] [CrossRef]
- Mohammadi, V.; Moghaddam, S.R. Influence of weed densities and different nitrogen levels on growth indices of corn, red root pigweed (Amaranthus retroflexus L.) and millet (Panicum miliaceum L.). Agroecology 2018, 9, 1084–1098. [Google Scholar]
- CABI. Datasheet: Amaranthus retroflexus. (Redroot Pigweed) Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/4652 (accessed on 7 March 2020).
- Athanassova, D.P. Allelopathic effect of Amaranthus retroflexus L. on weeds and crops. In Proceedings of the Seizième Conférence du COLUMA, Journées Internationales sur la Lutte Contre les Mauvaises Herbes, Sixteenth COLUMA Conference, International Days on Weed Control, Reims, France, 6–8 December 1995; Association Nationale Pour la Protection des Plantes (ANPP): Paris, France, 1996; Volume 1, pp. 437–442. [Google Scholar]
- Nuss, R.; Loewus, F.A. Further studies on oxalic acid biosynthesis in oxalate-accumulating plants. Plant Physiol. 1978, 61, 590–592. [Google Scholar] [CrossRef]
- Torres, M.B.; Kommers, G.D.; Dantas, A.F.; de Barros, C.L. Redroot pigweed (Amaranthus retroflexus) poisoning of cattle in southern Brazil. Vet. Hum. Toxicol. 1997, 39, 94–96. [Google Scholar]
- Casteel, S.W.; Johnson, G.C.; Miller, M.A.; Chudomelka, H.J.; Cupps, D.E.; Haskins, H.E.; Gosser, H.S. Amaranthus retroflexus (redroot pigweed) poisoning in cattle. J. Vet. Am. 1994, 204, 1068–1070. [Google Scholar]
- Stuart, B.P.; Nicholson, S.S.; Smith, J.B. Perirenal edema and toxic nephrosis in cattle, associated with ingestion of pigweed. J. Vet. Am. 1975, 167, 949–950. [Google Scholar]
- Heap, I.M. International Survey of Herbicide-Resistant Weeds; Annual Report; Weed Science Society of America: Westminster, CO, USA, 1997. [Google Scholar]
- McNaughton, K.E.; Letarte, J.; Lee, E.A.; Tardif, F.J. Mutations in ALS confer herbicide resistance in redroot pigweed (Amaranthus retroflexus) and Powell amaranth (Amaranthus powellii). Weed Sci. 2005, 53, 17–22. [Google Scholar] [CrossRef]
- Davis, G.; Letarte, J.; Grainger, C.M.; Rajcan, I.; Tardif, F.J. Widespread herbicide resistance in pigweed species in Ontario carrot production is due to multiple Photosystem II mutations. Can. J. Plant Sci. 2019, 100, 56–67. [Google Scholar] [CrossRef]
- Khan, A.; Hereward, J.; Werth, J.; Walter, G.; Chauhan, B. Amaranthus: Emerging weeds of cotton systems in Australia? In Proceedings of the Cotton Research Conference, Armidale, Australia, 5–7 September 2017; CRCD: Narrabri, Australia, 2017. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Norsworthy, J.K.; Green, J.K.; Barber, T.; Roberts, T.L.; Walsh, M.J. Seed destruction of weeds in southern US crops using heat and narrow-windrow burning. Weed. Technol. 2020, 34, 589–596. [Google Scholar] [CrossRef]
- IPCC. Carbon Dioxide—Data Distribution Centre, IPCC. Available online: https://www.ipcc-data.org/observ/ddc_co2.html (accessed on 13 March 2020).
- Ferrari, F.N.; Parera, C.A. Germination of six native perennial grasses that can be used as potential soil cover crops in drip-irrigated vineyards in semiarid environs of Argentina. J. Arid Environ. 2015, 113, 1–5. [Google Scholar] [CrossRef]
- Jomo, M.O.; Netondo, G.W.; Musyim, D.M. Growth changes of seven Amaranthus (spp.) during the vegetative and reproductive stages of development as influenced by variations in soil water deficit. Int. J. Res. Innov. Earth Sci. 2015, 2, 157–167. [Google Scholar]
- Mlakar, S.G.; Bavec, M.; Jakop, M.; Bavec, F. The Effect of Drought Occurring at Different Growth Stages on Productivity of Grain Amaranth Amaranthus cruentus G6. J. Life Sci. 2012, 6, 283–286. [Google Scholar]
- Pace, P.F.; Cralle, H.T.; El-Halawany, S.H.; Cothren, J.T.; Senseman, S.A. Drought-induced changes in shoot and root growth of young cotton plants. J. Cotton Sci. 1999, 3, 183–187. [Google Scholar]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic-Amst. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Lynch, A.L.; Godin, V.J.; Reid, D.M. Single and interactive effects of temperature, carbon dioxide, and watering regime on the invasive weed black knapweed (Centaurea nigra). Ecoscience 2013, 20, 328–338. [Google Scholar] [CrossRef]
- Ward, J.K.; Tissue, D.T.; Thomas, R.B.; Strain, B.R. Comparative responses of model C3 and C4 plants to drought in low and elevated CO2. Glob. Chang. Biol. 1999, 5, 857–867. [Google Scholar] [CrossRef]
- Hamim, H. Photosynthesis of C3 and C4 species in response to increased CO2 concentration and drought stress. Hayati J. Biosci. 2005, 12, 131–138. [Google Scholar] [CrossRef]
- Wang, A.; Lam, S.K.; Hao, X.; Li, F.Y.; Zong, Y.; Wang, H.; Li, P. Elevated CO2 reduces the adverse effects of drought stress on a high-yielding soybean (Glycine max (L.) Merr.) cultivar by increasing water use efficiency. Plant Physiol. Bioch. 2018, 132, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Mathobo, R.; Marais, D.; Steyn, J.M. The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric. Water Manag. 2017, 180, 118–125. [Google Scholar] [CrossRef]
- Li, Q.M.; Liu, B.B.; Wu, Y.; Zou, Z.R. Interactive effects of drought stresses and elevated CO2 concentration on photochemistry efficiency of cucumber seedlings. J. Integr. Plant Biol. 2008, 50, 1307–1317. [Google Scholar] [CrossRef]
- Choi, E.Y.; Seo, T.C.; Lee, S.G.; Cho, I.H.; Stangoulis, J. Growth and physiological responses of Chinese cabbage and radish to long-term exposure to elevated carbon dioxide and temperature. Hortic. Environ. Biotechnol. 2011, 52, 376. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Garbutt, K.; Williams, W.E.; Bazzaz, F.A. Analysis of the differential response of five annuals to elevated CO2 during growth. Ecology 1990, 71, 1185–1194. [Google Scholar] [CrossRef]
- Poorter, H. Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. In CO2 and Biosphere; Rozema, J., Lambers, H., Van de Geijn, S.C., Cambridge, M.L., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 77–98. [Google Scholar]
- Tremmel, D.C.; Patterson, D.T. Responses of soybean and five weeds to CO2 enrichment under two temperature regimes. Can. J. Plant Sci. 1993, 73, 1249–1260. [Google Scholar] [CrossRef][Green Version]
- Rogers, H.H.; Runion, G.B.; Krupa, S.V. Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollut 1994, 83, 155–189. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A. Influence of increasing carbon dioxide concentration on the photosynthetic and growth stimulation of selected C4 crops and weeds. Photosynth. Res. 1997, 54, 199–208. [Google Scholar] [CrossRef]
- Ziska, L.H. The impact of elevated CO2 on yield loss from a C3 and C4 weed in field-grown soybean. Glob. Chang. Biol. 2000, 6, 899–905. [Google Scholar] [CrossRef]
- Lovelli, S.; Perniola, M.; Ferrara, A.; Amato, M.; Di Tommaso, T. Photosynthetic response to water stress of pigweed (Amaranthus retroflexus) in a Southern-Mediterranean area. Weed Sci. 2010, 58, 126–131. [Google Scholar] [CrossRef]
- Valerio, M.; Tomecek, M.B.; Lovelli, S.; Ziska, L.H. Quantifying the effect of drought on carbon dioxide-induced changes in competition between a C3 crop (tomato) and a C4 weed (Amaranthus retroflexus). Weed Res. 2011, 51, 591–600. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, H.; Kaushik, M.; Nautiyal, R.; Singh, O. Adaptive physiological response, carbon partitioning, and biomass production of Withania somnifera (L.) Dunal grown under elevated CO2 regimes. 3. Biotechnology 2018, 8, 267. [Google Scholar] [CrossRef]
- Wong, S.C. Elevated atmospheric partial pressure of CO2 and plant growth. Oecologia 1979, 44, 68–74. [Google Scholar] [CrossRef]
- Florentine, S.K.; Weller, S.; Graz, P.F.; Westbrooke, M.; Florentine, A.; Javaid, M.; Fernando, N.; Chauhan, B.S.; Dowling, K. Influence of selected environmental factors on seed germination and seeding survival of the arid zone invasive species tobacco bush (Nicotiana glauca R. Graham). Rangel. J. 2016, 38, 417–425. [Google Scholar] [CrossRef]
- Weller, S.L.; Florentine, S.K.; Chauhan, B.S. Influence of selected environmental factors on seed germination and seedling emergence of Dinebra panicea var. brachiata (Steud.). Crop. Prot. 2019, 117, 121–127. [Google Scholar] [CrossRef]





| Parameter | Moisture | Time | Time ∗ Moisture | |||
|---|---|---|---|---|---|---|
| p-Values | HSD at 0.05 | p-Values | HSD at 0.05 | p-Values | HSD at 0.05 | |
| Plant height (cm) | 0.000 | 5.458 | 0.000 | 9.106 | 0.072 | 22.713 |
| Stem diameter (mm) | 0.000 | 0.662 | 0.000 | 1.105 | 0.002 | 2.756 |
| Number of leaves | 0.000 | 4.212 | 0.000 | 7.027 | 0.002 | 17.529 |
| Growth Parameter | p-Value | HSD at 0.05 |
|---|---|---|
| Shoot height (cm) | 0.000 | 13.167 |
| Shoot fresh weight (g) | 0.723 | 10.511 |
| Shoot dry weight (g) | 0.854 | 3.283 |
| Root length (cm) | 0.487 | 7.069 |
| Root fresh weight (g) | 0.757 | 2.479 |
| Root dry weight (g) | 0.794 | 0.768 |
| Number of leaves | 0.000 | 12.703 |
| Leaf area (cm2) | 0.126 | 10250 |
| Inflorescence length (cm) | 0.267 | 4.588 |
| Post-Harvest Parameter | CO2 | Water | CO2 ∗ Water | |||
|---|---|---|---|---|---|---|
| p-Values | HSD at 0.05 | p-Values | HSD at 0.05 | p-Values | HSD at 0.05 | |
| Significant difference according to treatment, including interaction | ||||||
| Plant height (cm) | 0.000 | 3.086 | 0.000 | 3.08 | 0.000 | 5.889 |
| Root fresh weight per plant (g) | 0.000 | 0.136 | 0.000 | 0.136 | 0.000 | 0.261 |
| Shoot dry weight per plant (g) | 0.000 | 0.157 | 0.000 | 0.157 | 0.000 | 0.300 |
| Shoot fresh weight per plant (g) | 0.001 | 1.594 | 0.000 | 1.594 | 0.028 | 3.041 |
| Significant difference according to treatment, but interaction is not significant | ||||||
| Number of leaves per plant | 0.000 | 3.670 | 0.000 | 3.673 | 0.534 | 7.009 |
| Leaf Area Per Plant (cm2) | 0.000 | 24.025 | 0.000 | 24.025 | 0.068 | 45.839 |
| Leaves fresh weight per plant (g) | 0.000 | 0.678 | 0.000 | 0.679 | 0.134 | 1.295 |
| Number of branches per plant | 0.003 | 1.966 | 0.000 | 1.967 | 0.070 | 3.753 |
| Leaf Thickness(mm) | 0.010 | 0.098 | 0.000 | 0.098 | 0.975 | 0.187 |
| Root dry weight per plant (g) | 0.006 | 0.111 | 0.004 | 0.111 | 0.355 | 0.212 |
| Leaves dry weight per plant (g) | 0.004 | 0.180 | 0.000 | 0.180 | 0.219 | 0.345 |
| One parameter not significant, other is significant, interaction is not | ||||||
| Stem diameter (mm) | 0.060 | 1.056 | 0.012 | 1.056 | 0.497 | 2.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weller, S.; Florentine, S.; Javaid, M.M.; Welgama, A.; Chadha, A.; Chauhan, B.S.; Turville, C. Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination. Agronomy 2021, 11, 728. https://doi.org/10.3390/agronomy11040728
Weller S, Florentine S, Javaid MM, Welgama A, Chadha A, Chauhan BS, Turville C. Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination. Agronomy. 2021; 11(4):728. https://doi.org/10.3390/agronomy11040728
Chicago/Turabian StyleWeller, Sandra, Singarayer Florentine, Muhammad Mansoor Javaid, Amali Welgama, Aakansha Chadha, Bhagirath Singh Chauhan, and Christopher Turville. 2021. "Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination" Agronomy 11, no. 4: 728. https://doi.org/10.3390/agronomy11040728
APA StyleWeller, S., Florentine, S., Javaid, M. M., Welgama, A., Chadha, A., Chauhan, B. S., & Turville, C. (2021). Amaranthus retroflexus L. (Redroot Pigweed): Effects of Elevated CO2 and Soil Moisture on Growth and Biomass and the Effect of Radiant Heat on Seed Germination. Agronomy, 11(4), 728. https://doi.org/10.3390/agronomy11040728







