Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Analysis
2.2. Properties of Chemicals Used in This Study
2.3. Production of Different Biochars
2.4. Adsorption and Desorption Experiments
2.5. Statistical Analyses
3. Results and Discussion
3.1. Physiochemical Properties of Soil and Biochars
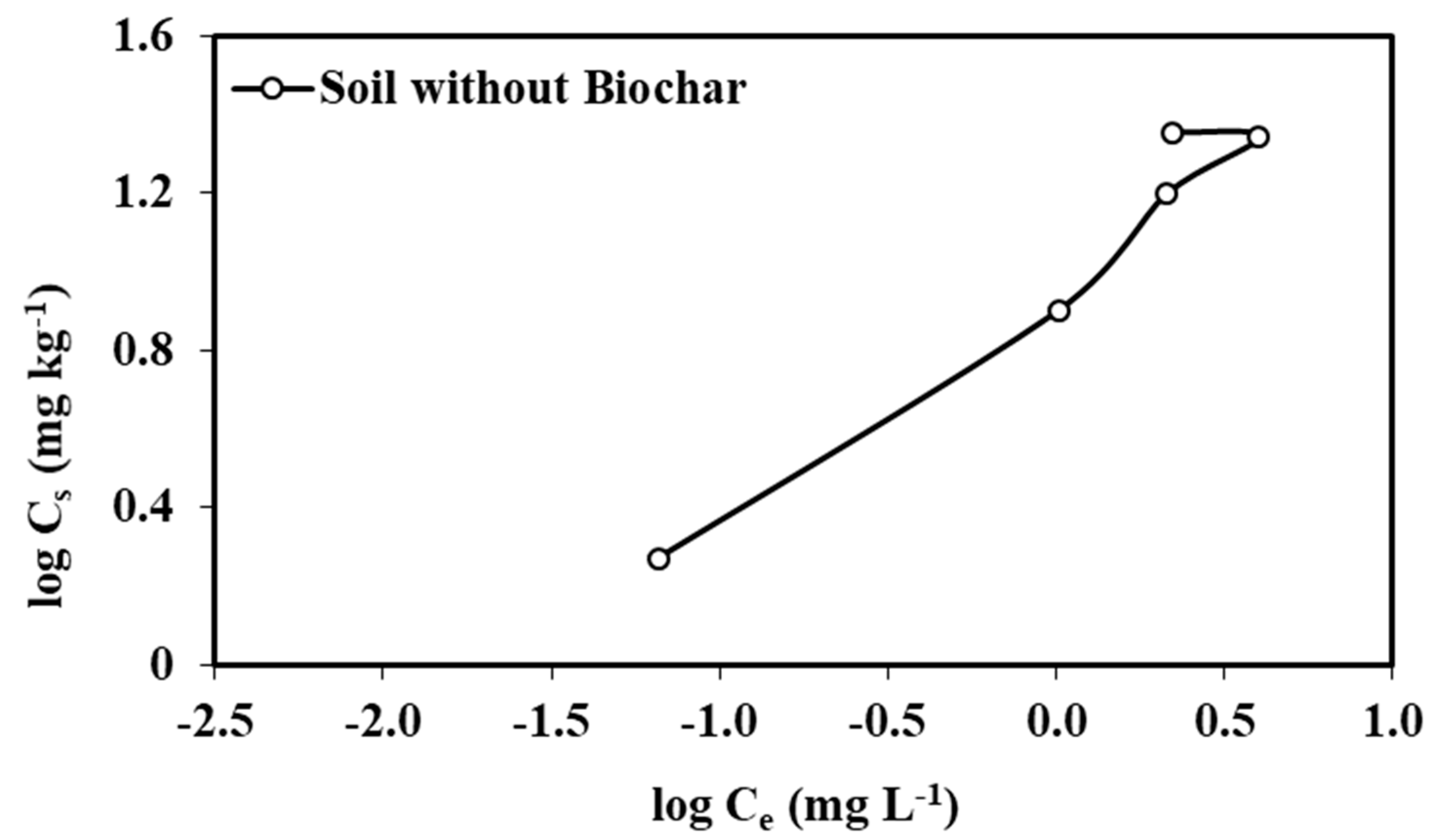
3.2. Adsorption–Desorption Isotherms of Atrazine
3.3. Environmental Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates. US Environmental Protection Agency: Washington, DC, USA, 2017. [Google Scholar]
- Bridges, D.C. The Triazine Herbicides, 1st ed.; Elsevier: Berkeley, CA, USA, 2011; pp. 163–174. [Google Scholar]
- Lima, D.L.; Silva, C.P.; Schneider, R.J.; Esteves, V.I. Development of an ELISA procedure to study sorption of atrazine onto a sewage sludge-amended luvisol soil. Talanta 2011, 85, 1494–1499. [Google Scholar] [CrossRef]
- Lasserre, J.P.; Fack, F.; Revets, D.; Planchon, S.; Renaut, J.; Hoffmann, L.; Bohn, T. Effects of the endocrine disruptor’s atrazine and PCB 153 on the protein expression of MCF-7 human cells. J. Proteome. Res. 2009, 8, 5485–5496. [Google Scholar] [CrossRef]
- Hayes, T.B.; Khoury, V.; Narayan, A.; Nazir, M.; Park, A.; Brown, T.; Gallipeau, S. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc. Natl. Acad. Sci. USA 2010, 107, 4612–4617. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.X.; Arbestain, M.C.; Virgel, S.; Blanco, F.; Arostegui, J.; Maciá-Agulló, J.A.; Macìas, F. Simulated geochemical weathering of a mineral ash-rich biochar in a modified Soxhlet reactor. Chemosphere 2010, 80, 724–732. [Google Scholar] [CrossRef] [PubMed]
- McHenry, M.P. Carbon-based stock feed additives: A research methodology that explores ecologically delivered C biosequestration, alongside live weights, feed use efficiency, soil nutrient retention, and perennial fodder plantations. J. Sci. Food Agric. 2010, 90, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Fidel, R.B.; Laird, D.A.; Parkin, T.B. Effect of biochar on soil greenhouse gas emissions at the laboratory and field scales. Soil Syst. 2019, 3, 8. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Garcia, M.; Förster, B.; Zech, W. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 2008, 51, 359–366. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef]
- Mesa, A.C.; Spokas, K.A. Impacts of biochar (black carbon) additions on the sorption and efficacy of herbicides. Herbic. Environ. 2011, 13, 315–340. [Google Scholar]
- Chen, Z.; Chen, B.; Zhou, D.; Chen, W. Bisolute sorption and thermodynamic behavior of organic pollutants to biomass-derived biochars at two pyrolytic temperatures. Environ. Sci. Technol. 2012, 46, 12476–12483. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Khorram, M.S.; Zhang, Q.; Lin, D.; Zheng, Y.; Fang, H.; Yu, Y. Biochar: A review of its impact on pesticide behavior in soil environments and its potential applications. J. Environ. Sci. 2016, 44, 269–279. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef]
- Mylavarapu, R.; Nair, V.; Morgan, K. An Introduction to Biochars and Their Uses in Agriculture. EDIS 2013, 2013. [Google Scholar] [CrossRef]
- Johnson, R.; Crafton, R.E.; Upton, H.F. Invasive Species: Major Laws and the Role of Selected Federal Agencies; Congressional Research Service: Washington, DC, USA, 2017. [Google Scholar]
- Delwiche, K.B.; Lehmann, J.; Walter, M.T. Atrazine leaching from biochar-amended soils. Chemosphere 2014, 95, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Yu, H.; Chen, M.; Ge, C. Sorption of atrazine in tropical soil by biochar prepared from cassava waste. Bioresources 2014, 9, 6627–6643. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Zhang, P.; Cao, M.; Xu, G.; Wu, H.; Rong, Q. Effects of biochar amendment on the sorption and degradation of atrazine in different soils. Soil Sediment Contam. 2018, 27, 643–657. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N. Optimization of atrazine and imidacloprid removal from water using biochars: Designing single or multi-staged batch adsorption systems. Int. J. Hyg. Environ. Health 2017, 220, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Lieurance, D.; Gettys, L.A. Lost in the Weeds?: A Comprehensive Guide to Florida’s Many Non-Native Plant Lists. EDIS 2019, 5, 6. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Chemical Analysis of Wood Charcoal; ASTME: Conshohocken, PA, USA, 2007. [Google Scholar]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Test No. 106: Adsorption-Desorption Using a Batch Equilibrium Method; OECD Publishing: Paris, France, 2000. [Google Scholar]
- García-Jaramillo, M.; Cox, L.; Cornejo, J.; Hermosín, M.C. Effect of soil organic amendments on the behavior of bentazone and tricyclazole. Sci. Total Environ. 2014, 466, 906–913. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Schomberg, H. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A.; de Melo, I.C.N.; Melo, L.C.; Magriotis, Z.M.; Sanchez-Monedero, M.A. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, e0176884. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 1st ed.; Routledge: Abingdon, UK, 2009; pp. 1–12. [Google Scholar]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. In Biochar for Environmental Management: Science and Technology, 1st ed.; Routledge: Abingdon, UK, 2009; pp. 14–32. [Google Scholar]
- Cabrera, A.; Cox, L.; Spokas, K.A.; Celis, R.; Hermosín, M.C.; Cornejo, J.; Koskinen, W.C. Comparative sorption and leaching study of the herbicides fluometuron and 4-chloro-2-methylphenoxyacetic acid (MCPA) in a soil amended with biochars and other sorbents. J. Agric. Food Chem. 2011, 59, 12550–12560. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Glob. Chang. Biol. Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Lentz, R.D. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Kocsis, T.; Biró, B.; Ulmer, Á.; Szántó, M.; Kotroczó, Z. Time-lapse effect of ancient plant coal biochar on some soil agrochemical parameters and soil characteristics. Environ. Sci. Pollut. Res. 2018, 25, 990–999. [Google Scholar] [CrossRef]
- Kocsis, T.; Kotroczó, Z.; Kardos, L.; Biró, B. Optimization of increasing biochar doses with soil–plant–microbial functioning and nutrient uptake of maize. Environ. Technol. Innov. 2020, 20, 101191. [Google Scholar] [CrossRef]
- Batista, E.M.; Shultz, J.; Matos, T.T.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar's role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Bachmann, R.T.; Rafiq, M.T.; Shang, Z.; Joseph, S.; Long, R. Influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L.) biochar and feasibility for carbon capture and energy balance. PLoS ONE 2016, 11, e0156894. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Giudicianni, P.; Cardone, G.; Ragucci, R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2013, 100, 213–222. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G.; Kaliakatsou, G.; Kritikaki, A. Efficiency of pecan shells and sawdust biochar on Pb and Cu adsorption. Desalin. Water Treat. 2016, 57, 3237–3246. [Google Scholar] [CrossRef]
- Pan, M. Biochar Adsorption of Antibiotics and its Implications to Remediation of Con-taminated Soil. Water Air Soil Pollut. 2020, 231, 1–15. [Google Scholar] [CrossRef]
- Krutz, L.J.; Senseman, S.A.; McInnes, K.J.; Zuberer, D.A.; Tierney, D.P. Adsorption and desorption of atrazine, desethylatrazine, deisopropylatrazine, and hydroxyatrazine in vegetated filter strip and cultivated soil. J. Agric. Food Chem. 2003, 51, 7379–7384. [Google Scholar] [CrossRef]
- Hao, F.; Zhao, X.; Ouyang, W.; Lin, C.; Chen, S.; Shan, Y.; Lai, X. Molecular structure of corncob-derived Biochars and the mechanism of Atrazine sorption. Agron. J. 2013, 105, 773–782. [Google Scholar] [CrossRef]
- Ghosh, R.K.; Singh, N. Adsorption–desorption of metolachlor and atrazine in Indian soils: Effect of fly ash amendment. Environ. Monit. Assess. 2013, 185, 1833–1845. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, K.; Wang, H.; Gan, J. Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ Pollut. 2010, 158, 2821–2825. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Mu, C.L.; Gu, C.; Liu, C.; Liu, X.J. Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils. Chemosphere 2011, 85, 1284–1289. [Google Scholar] [CrossRef]
- Grube, A.H.; Donaldson, D.; Kiely, T. Pesticides Industry Sales and Usage: 2000 and 2001 Market Estimates; Biological and Economic Analysis Division, US Environmental Protection Agency: Washington, DC, USA, 2004; p. 114. [Google Scholar]
- Barco-Bonilla, N.; Romero-González, R.; Plaza-Bolaños, P.; Vidal, J.L.M.; Frenich, A.G. Systematic study of the contamination of wastewater treatment plant effluents by organic priority compounds in Almeria province (SE Spain). Sci. Total Environ. 2013, 447, 381–389. [Google Scholar] [CrossRef]
- Moreno-González, R.; Campillo, J.A.; García, V.; León, V.M. Seasonal input of regulated and emerging organic pollutants through surface watercourses to a Mediterranean coastal lagoon. Chemosphere 2013, 92, 247–257. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Mean Values |
|---|---|
| pH † | 7.52 ± 0.04 |
| C (%) ‡ | 9.90 ± 0.55 |
| N (%) ‡ | 0.55 ± 0.03 |
| OM (%) § | 15.49 ± 0.34 |
| OC (%) § | 8.98 ± 0.20 |
| Sand (%) ¶ | 76.44 |
| Silt (%) ¶ | 21.65 |
| Clay (%) ¶ | 1.91 |
| Proximate Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample † | Feedstock | Production Temperature (°C) | Biochar Yield (%) | Volatile Matter Content ‡ (%) | Ash Content § (%) | Moisture Content ¶ (%) | Fixed C # (%) | pH †† | CEC ‡‡ (cmol kg−1) | SSA §§ (m2 g−1) | TPV ¶¶ (cm3 g−1) | Average Pore Size (nm) |
| AP350 | Australian pine (Casaurina equisetifolia) | 350 | 41.00 | 79.24 ± 2.39 AB | 5.32 ± 1.11 B | 5.85 ± 0.09 ABCD | 15.44 ± 3.50 B | 8.58 ± 0.03 C | 16.31 ± 5.50 A | 0.98 ± 0.07 | 0.003 | 12.46 |
| AP500 | 500 | 33.10 | 61.35 ± 5.67 ABC | 10.19 ± 0.91 A | 2.81 ± 0.19 DE | 28.46 ± 6.59 AB | 9.37 ± 0.03 B | 8.19 ± 1.43 A | 2.59 ± 0.29 | 0.006 | 9.40 | |
| BP350 | Brazilian pepper (Schinus terebinthifolius) | 350 | 41.60 | 66.47 ± 9.32 ABC | 2.06 ± 1.00 CD | 4.40 ± 0.47 BCDE | 31.48 ± 8.32 AB | 7.72 ± 0.08 DE | 8.47 ± 2.51 A | 0.57 ± 0.08 | 0.002 | 12.26 |
| BP500 | 500 | 33.00 | 55.80 ± 3.79 BC | 4.02 ± 0.02 BC | 1.96 ± 0.98 E | 40.18 ± 3.82 AB | 9.65 ± 0.02 AB | 7.92 ± 2.30 A | 2.29 ± 0.26 | 0.008 | 14.60 | |
| CH350 | Coconut husk (Cocos nusifera) | 350 | 47.20 | 85.05 ± 2.45 A | 3.74 ± 0.48 BC | 8.81 ± 0.81 A | 11.21 ± 1.97 B | 9.40 ± 0.09 B | 16.32 ± 3.46 A | 0.89 ± 0.15 | 0.003 | 13.31 |
| CH500 | 500 | 40.30 | 79.37 ± 1.48 AB | 8.88 ± 0.38 A | 4.96 ± 0.92 BCDE | 11.75 ± 1.85 B | 9.89 ± 0.10 A | 12.04 ± 1.07 A | 1.94 ± 0.22 | 0.004 | 7.99 | |
| Cy350 | Cypress (Taxodium distichum) | 350 | 37.70 | 72.75 ± 1.17 ABC | 0.55 ± 0.05 D | 6.51 ± 0.57 ABC | 26.71 ± 1.22 AB | 7.11 ± 0.01 G | 10.55 ± 0.20 A | 0.41 ± 0.07 | 0.001 | 10.01 |
| Cy500 | 500 | 30.00 | 62.66 ± 7.56 ABC | 1.59 ± 0.11 CD | 2.45 ± 0.52 E | 36.76 ± 7.67 AB | 7.67 ± 0.01 DE | 9.18 ± 2.46 A | 4.18 ± 0.47 | 0.002 | 2.39 | |
| L350 | Loblolly pine (Pinus taeda) | 350 | 39.60 | 71.31 ± 6.00 ABC | 1.70 ± 0.10 CD | 3.46 ± 0.46 CDE | 26.98 ± 6.11 AB | 7.63 ± 0.05 EF | 8.51 ± 1.84 A | 0.30 ± 0.06 | 0.001 | 12.81 |
| L500 | 500 | 32.20 | 48.59 ± 7.53 C | 3.20 ± 0.19 BCD | 2.36 ± 0.56 E | 48.21 ± 7.73 A | 7.84 ± 0.01 DE | 7.93 ± 4.34 A | 5.21 ± 0.56 | 0.004 | 3.13 | |
| P350 | Pecan shell (Carya illinoinensis) | 350 | 46.80 | 68.04 ± 4.12 ABC | 2.18 ± 0.12 CD | 6.83 ± 0.09 AB | 29.78 ± 4.24 AB | 7.36 ± 0.02 FG | 6.14 ± 1.18 A | 0.36 ± 0.05 | 0.001 | 14.56 |
| P500 | 500 | 39.20 | 56.33 ± 1.12 ABC | 3.82 ± 0.29 BC | 3.50 ± 0.50 CDE | 39.85 ± 1.42 AB | 7.94 ± 0.03 D | 4.66 ± 1.41 A | 2.14 ± 0.34 | 0.002 | 4.41 | |
| Atomic Ratio of the Elements in Biochar | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample † | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Oxygen (%) | Sulfur (%) | H/C ‡ | O/C § | (N+O)/C ¶ |
| AP350 | 64.93 | 4.00 | 0.94 | 21.80 | 0.07 | 0.06 | 0.34 | 0.35 |
| AP500 | 66.65 | 3.07 | 1.10 | 15.66 | 0.04 | 0.05 | 0.23 | 0.25 |
| BP350 | 67.54 | 3.97 | 0.5 | 20.89 | 0.11 | 0.06 | 0.31 | 0.32 |
| BP500 | 77.37 | 3.04 | 0.51 | 11.76 | 0.13 | 0.04 | 0.15 | 0.16 |
| CH350 | 66.69 | 4.02 | 0.51 | 22.81 | 0.03 | 0.06 | 0.34 | 0.35 |
| CH500 | 67.00 | 3.01 | 0.69 | 18.29 | 0.03 | 0.04 | 0.27 | 0.28 |
| Cy350 | 76.10 | 4.39 | 0.5 | 17.31 | 0.01 | 0.06 | 0.23 | 0.23 |
| Cy500 | 83.59 | 3.39 | 0.5 | 11.11 | 0.04 | 0.04 | 0.13 | 0.14 |
| L350 | 67.71 | 4.25 | 0.5 | 17.13 | 0.09 | 0.06 | 0.25 | 0.26 |
| L500 | 79.47 | 3.52 | 0.5 | 12.97 | 0.08 | 0.04 | 0.16 | 0.17 |
| P350 | 68.45 | 3.59 | 0.5 | 22.85 | 0.04 | 0.05 | 0.33 | 0.34 |
| P500 | 78.96 | 3.21 | 0.5 | 12.10 | 0.03 | 0.04 | 0.15 | 0.16 |
| Treatment † | Kf ads ‡ | 1/nads § | Freundlich r2 | Kd ads ¶ | KOC # | %Adsorption |
|---|---|---|---|---|---|---|
| Soil | 9.12 ± 1.07 | 0.59 ± 0.04 | 0.99 | 9.12 BCDE | 101.53 BCDE | 73.24–93.44 (81.25) †† |
| Soil + AP350 | 10.47 ± 1.15 | 0.47 ± 0.06 | 0.96 | 10.47 ABCDE | 116.56 ABCDE | 75.77–97.63 (83.09) |
| Soil + AP500 | 9.17 ± 1.48 | 0.43 ± 0.14 | 0.77 | 9.17 E | 102.09 E | 58.72–98.96 (79.20) |
| Soil + BP350 | 8.32 ± 1.45 | 0.42 ± 0.19 | 0.72 | 8.32 CDE | 92.63 CDE | 52.26–98.28 (76.73) |
| Soil + BP500 | 9.33 ± 1.45 | 0.36 ± 0.15 | 0.73 | 9.33 CDE | 103.87 CDE | 54.51–98.73 (77.72) |
| Soil + CH350 | 13.80 ± 1.02 | 0.52 ± 0.02 | 0.99 | 13.80 A | 153.63 A | 80.96–100.0 (90.31) |
| Soil + CH500 | 10.96 ± 1.02 | 0.54 ± 0.02 | 0.99 | 10.96 ABC | 122.02 ABC | 75.03–100.0 (87.05) |
| Soil + Cy350 | 7.94 ± 1.05 | 0.60 ± 0.03 | 0.99 | 7.94 DE | 88.40 DE | 71.35–93.99 (78.52) |
| Soil + Cy500 | 11.40 ± 1.07 | 0.40 ± 0.03 | 0.99 | 11.40 ABCDE | 126.92 ABCDE | 71.32–98.90 (83.97) |
| Soil + L350 | 11.39 ± 1.12 | 0.60 ± 0.07 | 0.98 | 11.39 BCDE | 126.80 BCDE | 80.10–95.48 (85.42) |
| Soil + L500 | 12.30 ± 1.15 | 0.33 ± 0.05 | 0.96 | 12.30 ABCD | 136.93 ABCD | 76.84–99.65 (85.12) |
| Soil + P350 | 9.12 ± 1.02 | 0.60 ± 0.02 | 0.99 | 9.12 CDE | 101.53 CDE | 72.24–93.01 (80.82) |
| Soil + P500 | 13.49 ± 1.48 | 0.52 ± 0.21 | 0.75 | 13.49 AB | 150.18 AB | 78.45–97.18 (87.53) |
| Treatment † | Kf des ‡ | 1/ndes § | Freundlich r2 | Kd des ¶ | H # | %Desorption |
|---|---|---|---|---|---|---|
| Soil | 10.00 ± 1.22 | 0.62 ± 0.07 | 0.96 | 10.00 | 1.07 A | 3.52 |
| Soil + AP350 | 11.48 ± 1.15 | 0.49 ± 0.07 | 0.94 | 11.48 | 1.04 AB | 2.69 |
| Soil + AP500 | 10.72 ± 1.35 | 0.45 ± 0.17 | 0.70 | 10.72 | 1.05 B | 2.30 |
| Soil + BP350 | 10.00 ± 1.41 | 0.43 ± 0.19 | 0.62 | 10.00 | 1.02 AB | 3.19 |
| Soil + BP500 | 10.96 ± 1.38 | 0.38 ± 0.16 | 0.66 | 10.96 | 1.06 A | 3.12 |
| Soil + CH350 | 16.98 ± 1.12 | 0.48 ± 0.18 | 0.70 | 16.98 | 0.92 AB | 2.11 |
| Soil + CH500 | 15.85 ± 1.09 | 0.54 ± 0.11 | 0.79 | 15.85 | 1.00 AB | 6.03 |
| Soil + Cy350 | 8.71 ± 1.12 | 0.64 ± 0.08 | 0.95 | 8.71 | 1.07 AB | 2.82 |
| Soil + Cy500 | 12.30 ± 1.09 | 0.42 ± 0.04 | 0.97 | 12.30 | 1.05 AB | 2.66 |
| Soil + L350 | 12.02 ± 1.09 | 0.61 ± 0.06 | 0.97 | 12.02 | 1.02 AB | 4.94 |
| Soil + L500 | 13.49 ± 1.12 | 0.35 ± 0.05 | 0.95 | 13.49 | 1.06 AB | 4.45 |
| Soil + P350 | 9.77 ± 1.12 | 0.63 ± 0.08 | 0.96 | 9.77 | 1.05 AB | 2.26 |
| Soil + P500 | 15.85 ± 1.41 | 0.53 ± 0.21 | 0.68 | 15.85 | 1.02 AB | 1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffar, S.; Dattamudi, S.; Baboukani, A.R.; Chanda, S.; Novak, J.M.; Watts, D.W.; Wang, C.; Jayachandran, K. Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil. Agronomy 2021, 11, 716. https://doi.org/10.3390/agronomy11040716
Gaffar S, Dattamudi S, Baboukani AR, Chanda S, Novak JM, Watts DW, Wang C, Jayachandran K. Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil. Agronomy. 2021; 11(4):716. https://doi.org/10.3390/agronomy11040716
Chicago/Turabian StyleGaffar, Shagufta, Sanku Dattamudi, Amin Rabiei Baboukani, Saoli Chanda, Jeffrey M. Novak, Donald W. Watts, Chunlei Wang, and Krishnaswamy Jayachandran. 2021. "Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil" Agronomy 11, no. 4: 716. https://doi.org/10.3390/agronomy11040716
APA StyleGaffar, S., Dattamudi, S., Baboukani, A. R., Chanda, S., Novak, J. M., Watts, D. W., Wang, C., & Jayachandran, K. (2021). Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil. Agronomy, 11(4), 716. https://doi.org/10.3390/agronomy11040716









