Adsorptive and Surface Characterization of Mediterranean Agrifood Processing Wastes: Prospection for Pesticide Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Conditioning of Agrifood Wastes
2.2. Agrifood Waste Biomass Characterization
2.3. Imazalil and Thiabendazole Batch Sorption Experiments
3. Results and Discussion
3.1. Elemental Analysis of the Agrifood Wastes
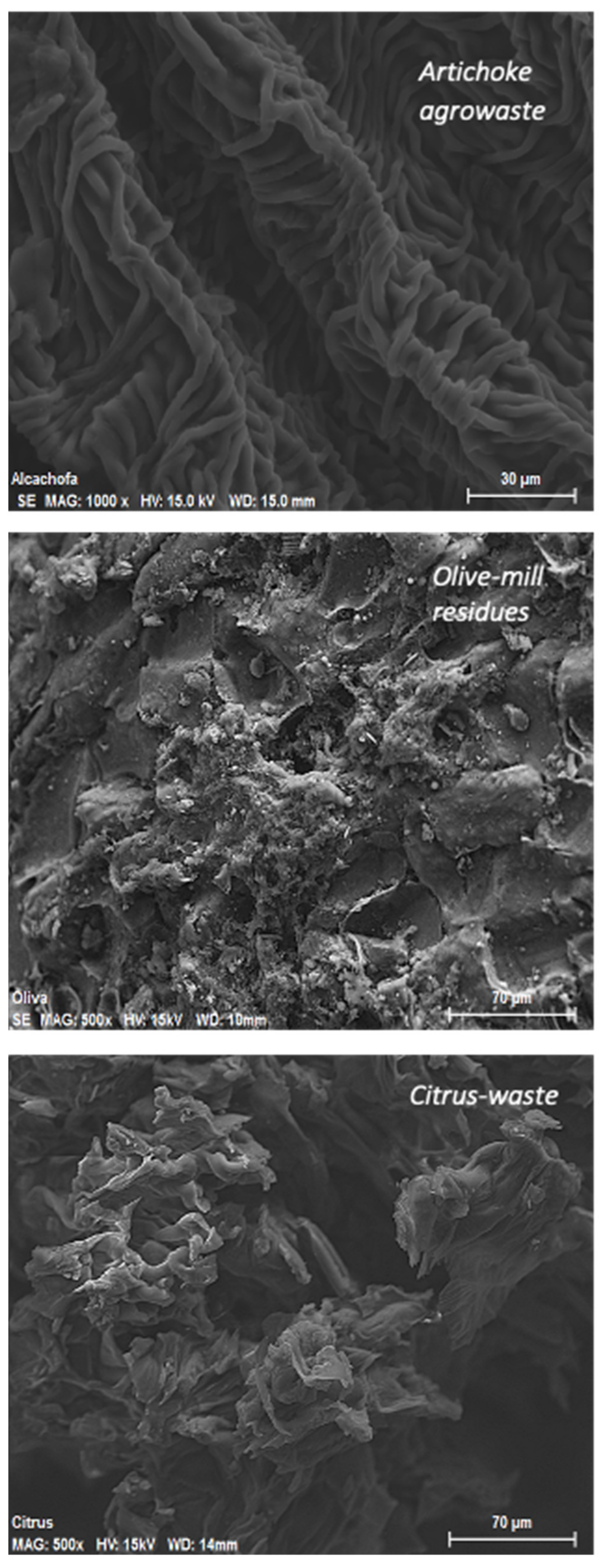
3.2. Scanning Electron Microscopy
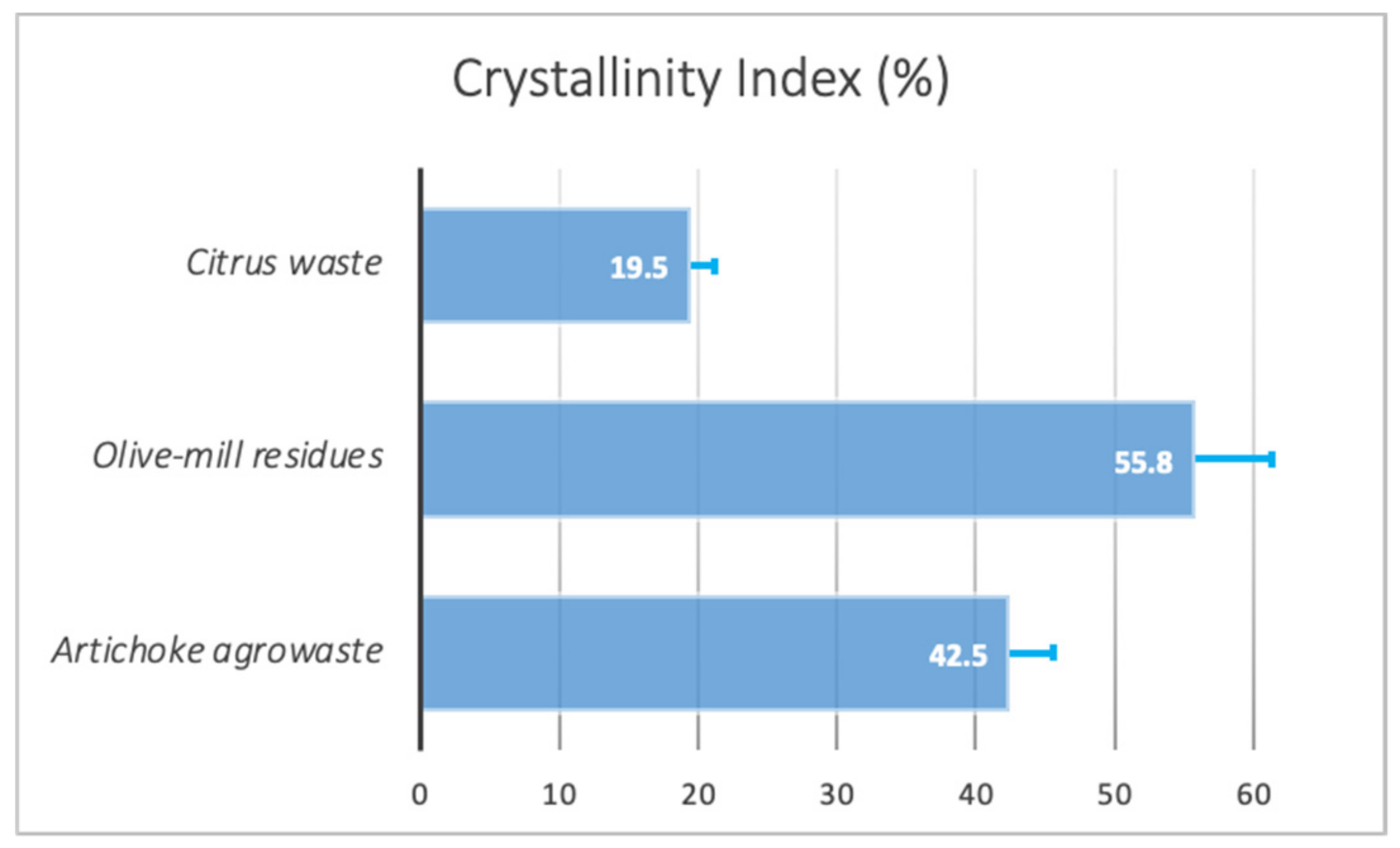
3.3. Crystallinity
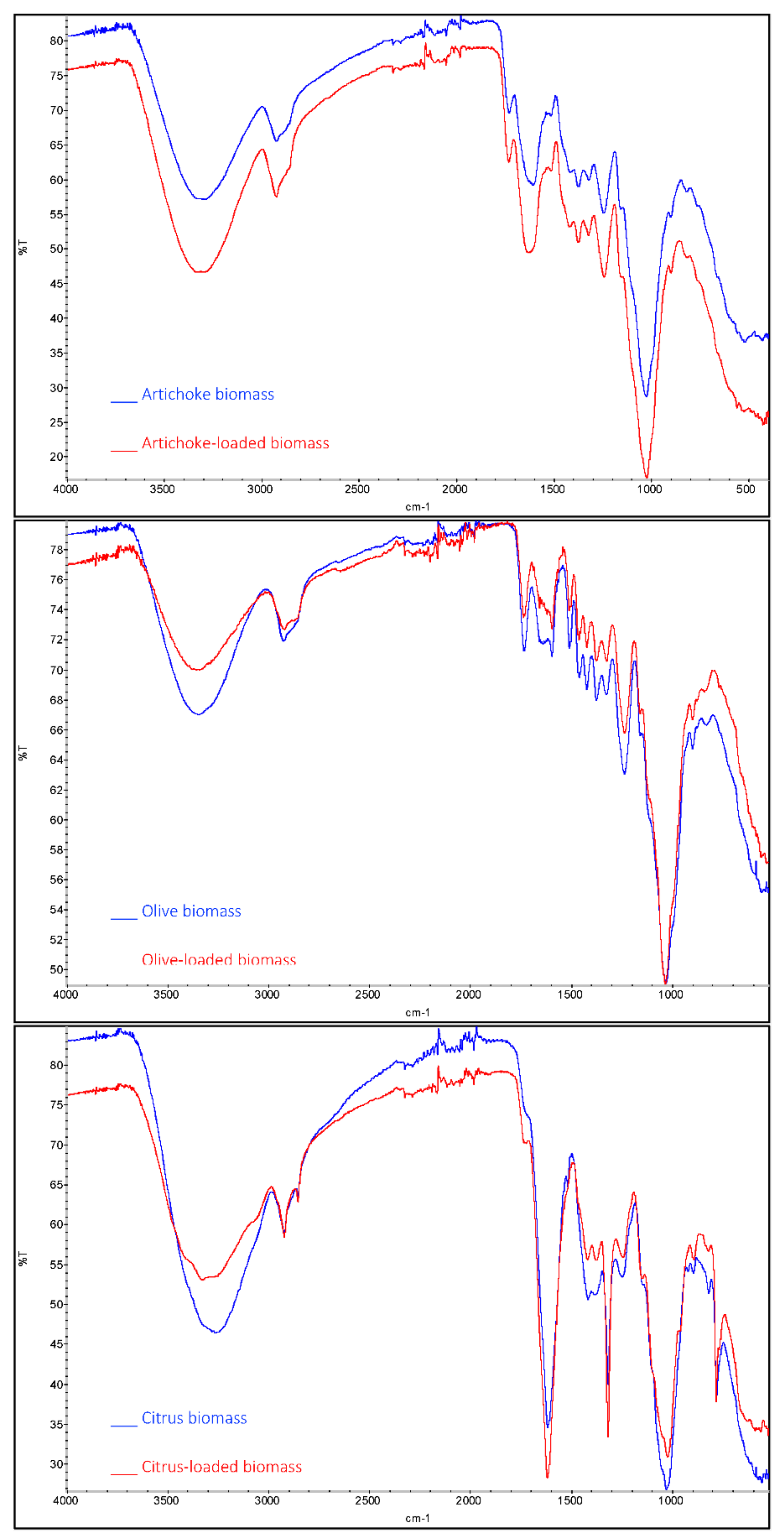
3.4. Fourier-Transform Infrared Spectroscopy
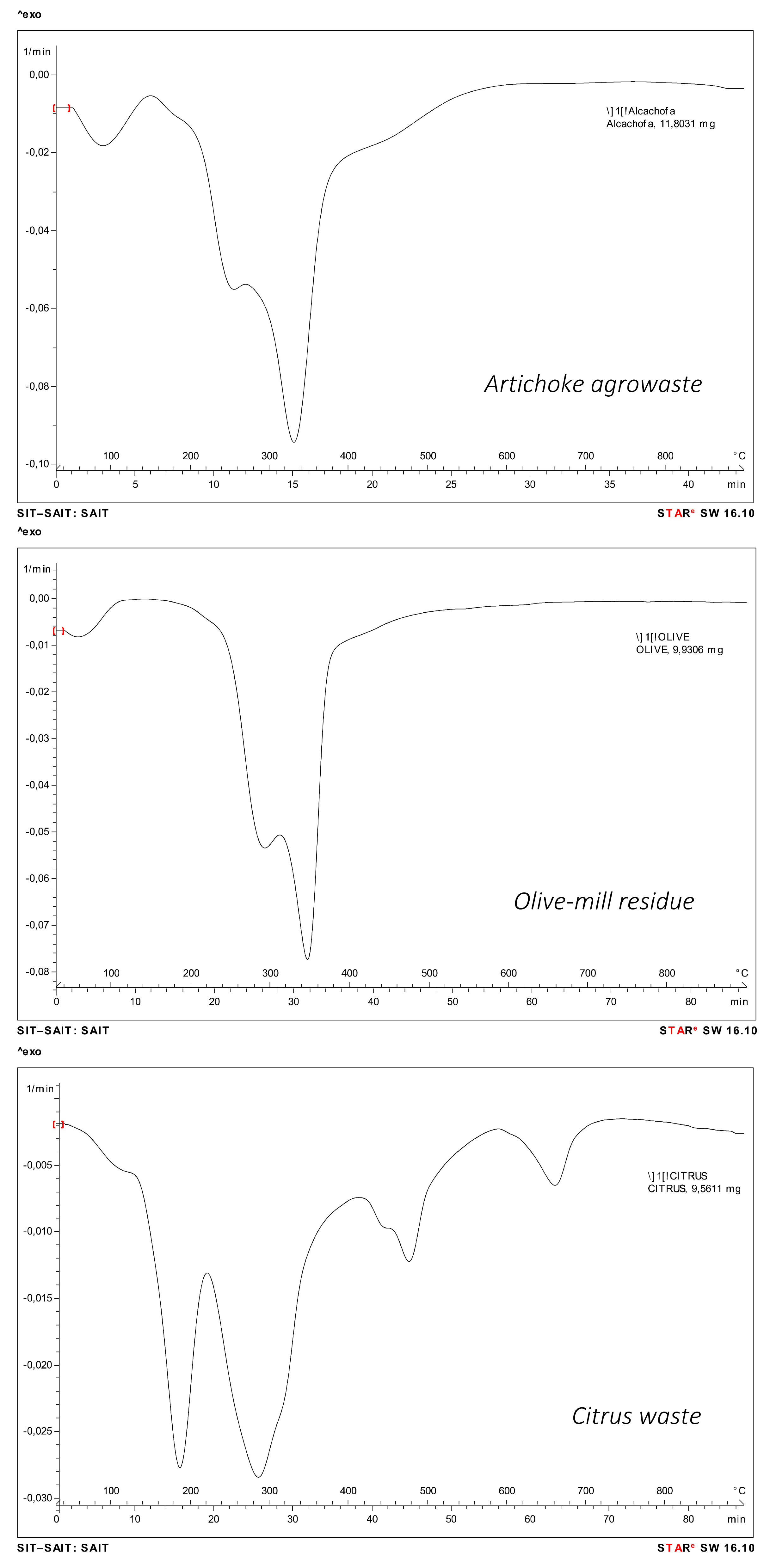
3.5. Thermal Analysis
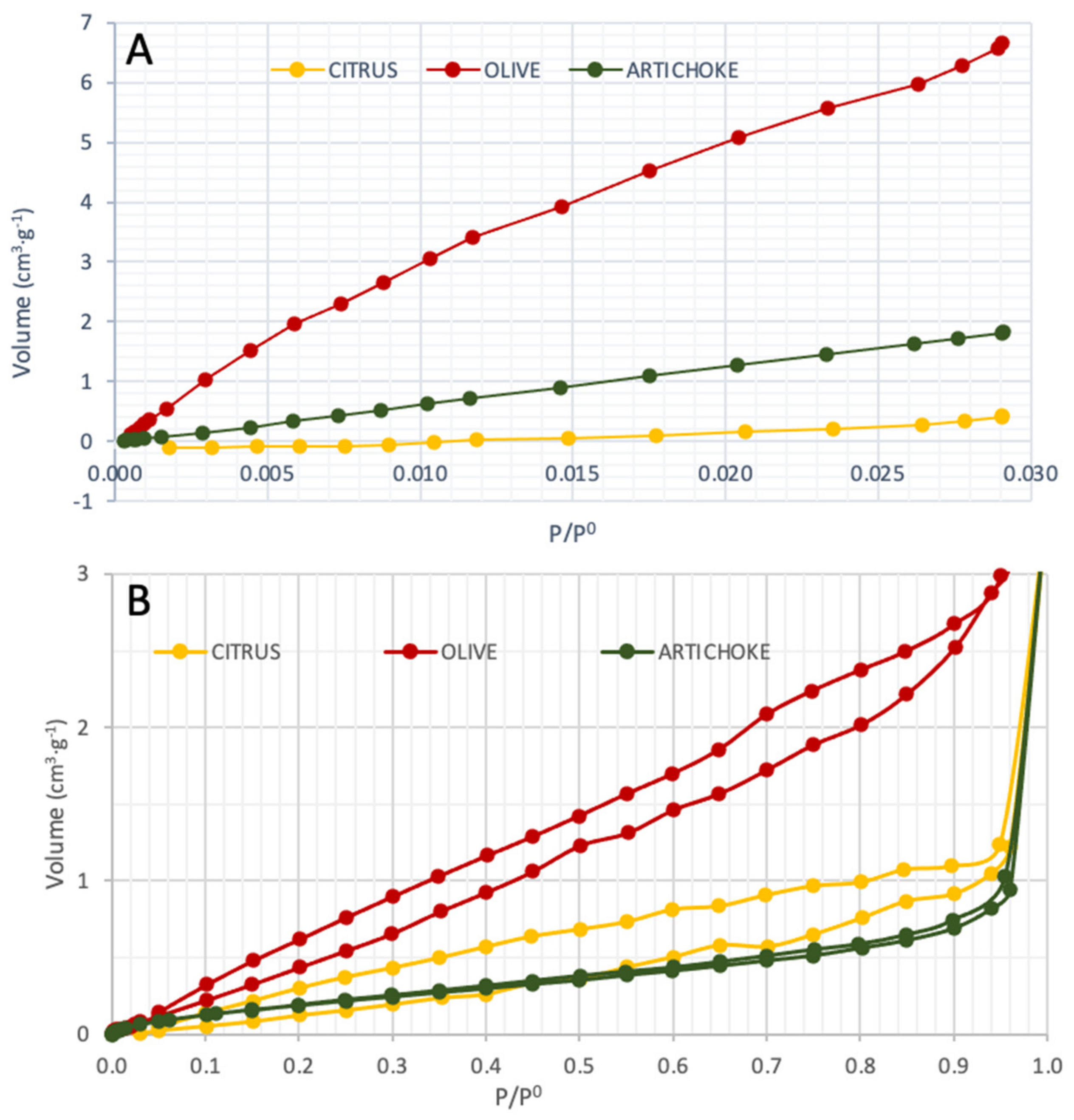
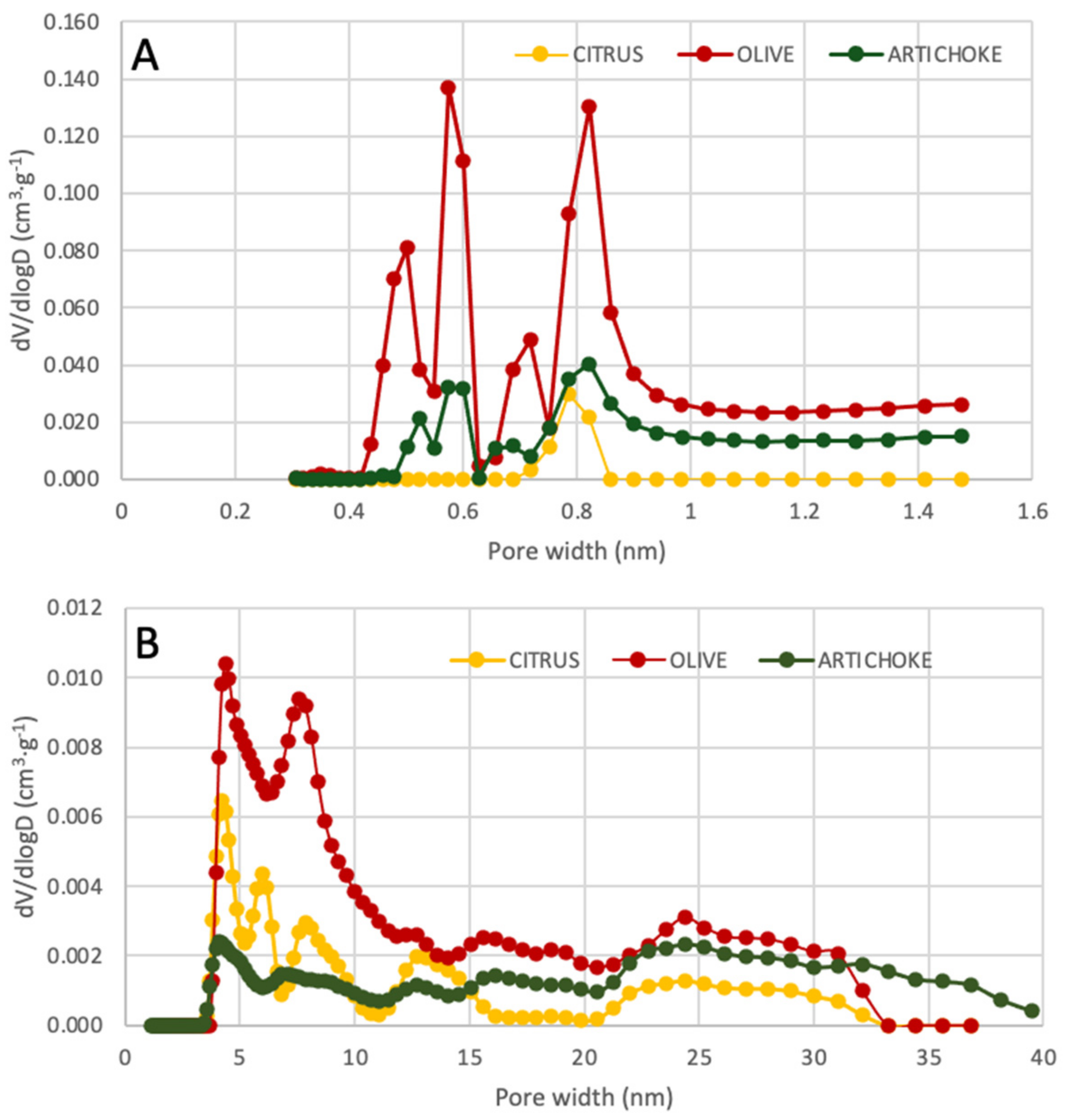
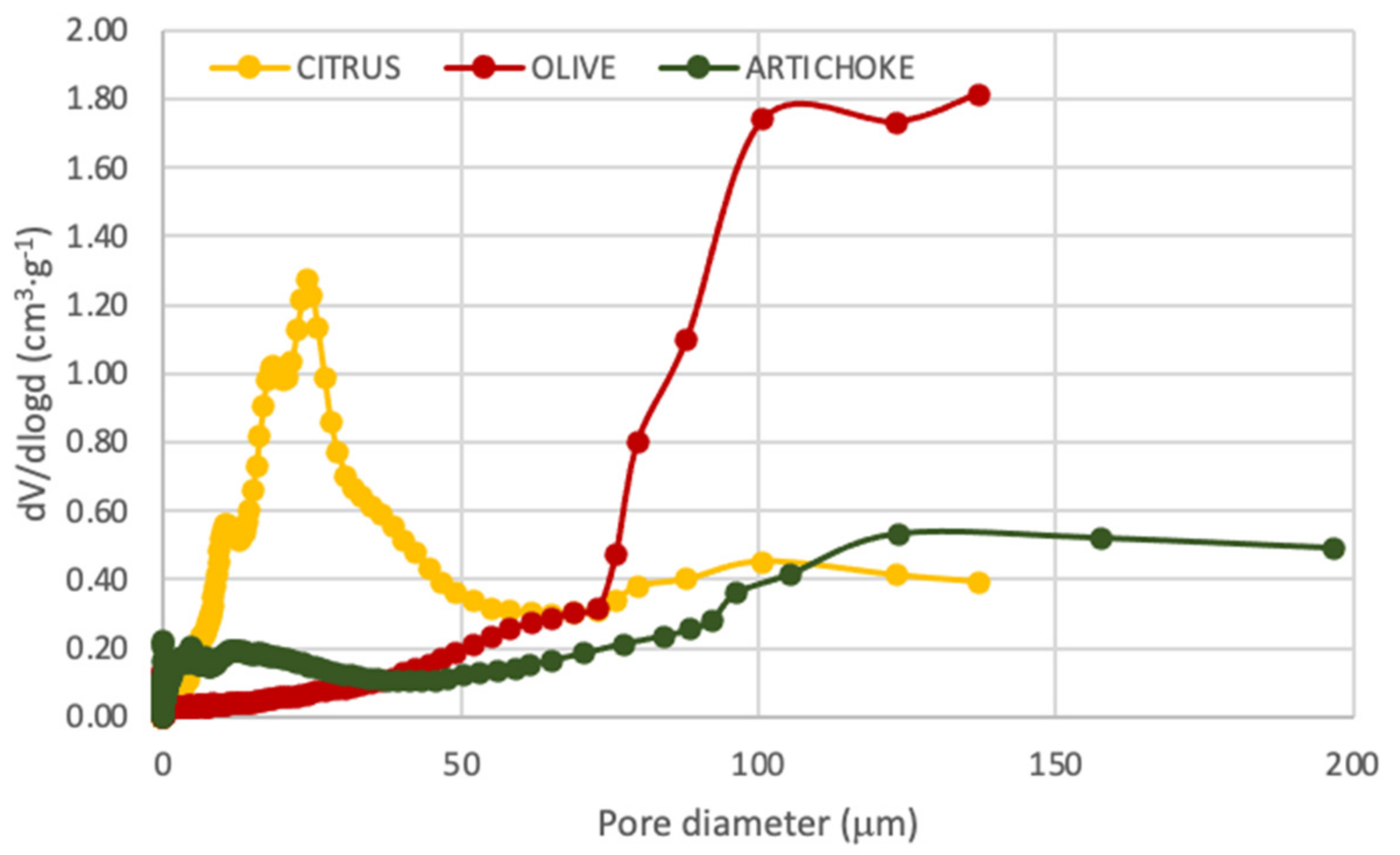
3.6. BET Surface Analysis and Porosimetry
3.7. pH of Zero Point of Charge
3.8. Imazalil and Thiabendazole Sorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boto-Álvarez, A.; García-Fernández, R. Implementation of the 2030 agenda sustainable development goals in Spain. Sustainability 2020, 12, 2546. [Google Scholar] [CrossRef]
- Díaz-Sarachaga, J.M.; Jato-Espino, D.; Castro-Fresno, D. Is the sustainable development goals (SDG) index an adequate framework to measure the progress of the 2030 Agenda? Sustain. Dev. 2018, 26, 663–671. [Google Scholar] [CrossRef]
- Lu, Y.; Nakicenovic, N.; Visbeck, M.; Stevance, A.S. Five priorities for the UN sustainable development goals. Nature 2015, 520, 432–433. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global water pollution and human health. Ann. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Rosique, M.; Angosto, J.M.; Guibal, E.; Roca, M.J.; Fernández-López, J.A. Factorial design methodological approach for enhanced cadmium ions bioremoval by Opuntia biomass. CLEAN Soil Air Water 2016, 44, 959–966. [Google Scholar] [CrossRef]
- Yusuf, M.; Elfghi, F.M.; Zaidi, S.A.; Abdullah, E.C.; Khan, M.A. Applications of graphene and its derivatives as an adsorbent for heavy metal and dye removal: A systematic and comprehensive overview. RSC Adv. 2015, 5, 50392–50420. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Avilés, M.D. Biosorption of hexavalent chromium from aqueous medium with Opuntia biomass. Sci. World J. 2014, 670249. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S.; Rachna. Water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Pol. J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Mahjoub, B.; Mahjoub, O.; Sillanpää, M. Remediation of emerging pollutants in contaminated wastewater and aquatic environments: Biomass-based technologies. CLEAN Soil Air Water 2017, 45, 1700101. [Google Scholar] [CrossRef]
- Saavedra, M.I.; Doval Miñarro, M.; Angosto, J.M.; Fernández-López, J.A. Reuse potential of residues of artichoke (Cynara scolymus L.) from industrial canning processing as sorbent of heavy metals in multimetallic effluents. Ind. Crops Prod. 2019, 141, 111751. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constanti, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Salem, M.B.; Affes, H.; Ksouda, K.; Dhouibi, R.; Sahnoun, Z.; Hammami, S.; Zeghal, Z.M. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 2015, 70, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Olivo, G.; Gutiérrez-Grijalva, E.P.; Heredia, J.B. Prebiotic compounds from agro-industrial by-products. J. Food Biochem. 2019, 4, e12711. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.; Megías, M.D.; Madrid, J.; Martínez-Teruel, A.; Hernández, F.; Oliva, J. Evaluation of the phytosanitary, fermentative and nutritive characteristics of the silage made from crude artichoke (Cynara scolymus L.) by-product feeding for ruminants. Small Rumin. Res. 2007, 70, 292–296. [Google Scholar] [CrossRef]
- Christoforou, E.; Fokaides, P.A. A review of olive mill solid wastes to energy utilization techniques. Waste Manag. 2016, 49, 346–363. [Google Scholar] [CrossRef]
- Nowicki, P.; Kazmierczak-Razna, J.; Pietrzak, R. Physico-chemical and adsorption properties of carbonaceous sorbents prepared by activation of tropical fruit skins with potassium carbonate. Mater. Des. 2016, 90, 579–585. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals and biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Manna, S.; Toy, D.; Adhikari, B.; Thomas, S.; Das, P. Biomass for water defluoridation and current understanding on biosorption mechanisms: A review. Environ. Prog. Sustain. Energy 2018, 37, 1560–1572. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Guidi, P.; Bernardeschi, M.; Palumbo, M.; Genovese, M.; Scarcelli, V.; Fiorati, A.; Riva, L.; Punta, C.; Corsi, I.; Frenzilli, G. Suitability of a cellulose-based nanomaterial for the remediation of heavy metal contaminated freshwaters: A case-study showing the recovery of cadmium induced DNA integrity loss, cell proliferation increase, nuclear morphology and chromosomal alterations on Dreissena polymorpha. Nanomaterials 2020, 10, 1837. [Google Scholar] [CrossRef]
- Möller, M.; Schröder, U. Hydrothermal production of furfural from xylose and xylan as model compounds for hemicelluloses. RSC Adv. 2013, 3, 22253–22260. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Escudero-Oñate, C.; Fiol, N.; Poch, J.; Villaescusa, I. Valorisation of lignocellulosic biomass wastes for the removal of metal ions from aqueous streams: A review. In Biomass Volume Estimation and Valorization for Energy; Tumuluru, J.S., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 381–407. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Romero, I.; Ruiz, E.; Cara, C.; Romero-García, J.M.; Castro, E. Design and optimization of sulfuric acid pretreatment of extracted olive tree biomass using response surface methodology. BioResources 2017, 12, 1779–1797. [Google Scholar] [CrossRef]
- Machado, M.T.; Eça, K.S.; Vieira, G.S.; Menegalli, F.C.; Martínez, J.; Hubinger, M.D. Prebiotic oligosaccharides from artichoke industrial waste: Evaluation of different extraction methods. Ind. Crops Prod. 2015, 76, 141–148. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Tang, C.; Eriksson, E.; Jönsson, K.; Vollertsen, J.; Bester, K. Biocides in urban wastewater treatment plant influent at dry and wet weather: Concentrations, mass flows and possible sources. Water Res. 2014, 60, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Muller, M.D.; Poiger, T. Azole fungicides: Occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Sinha, P.; Datar, A.; Jeong, C.; Deng, X.; Chung, Y.G.; Lin, L.C. Surface area determination of porous materials using the Brunauer–Emmett–Teller (BET) method: Limitations and improvements. J. Phys. Chem. C 2019, 123, 20195–20209. [Google Scholar] [CrossRef]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Castellar, R.; Obón, J.M.; Almela, L. Screening and mass-spectral confirmation of betalains in cactus pears. Chromatographia 2002, 56, 591–595. [Google Scholar] [CrossRef]
- Parikh, J.; Channiwala, S.A.; Ghosal, G.K. A correlation for calculating elemental composition from proximate analysis of biomass materials. Fuel 2007, 86, 1710–1719. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Biores. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, M.; Pashalidis, I. Copper (II) removal from aqueous solutions by adsorption on non-treated and chemically modified cactus fibres. Water Sci. Technol. 2013, 68, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Rambo, M.K.D.; Ferrerira, M.M.C. Determination of cellulose crystallinity of banana residues using near infrared spectroscopy and multivariate analysis. J. Braz. Chem. Soc. 2015, 26, 1491–1499. [Google Scholar] [CrossRef]
- Mariño, M.; Lopes da Silva, L.; Durán, N.; Tasic, L. Enhanced materials from nature: Nanocellulose from citrus waste. Molecules 2015, 20, 5908–5923. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Uchimiya, M. Comparison of biochar formation from various agricultural by-products using FTIR spectroscopy. Mod. Appl. Sci. 2015, 9, 246–253. [Google Scholar] [CrossRef]
- Wang, F.L.; Li, S.; Sun, Y.X.; Han, H.Y.; Zhang, B.X.; Hu, B.Z.; Gao, Y.F.; Hu, X.M. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef]
- Manara, P.; Vamvuka, D.; Sfakiotakis, S.; Vanderghem, C.; Richel, A.; Zabaniotou, A. Mediterranean agri-food processing wastes pyrolysis after pre-treatment and recovery of precursor materials: A TGA-based kinetic modeling study. Food Res. Int. 2015, 73, 44–51. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Branton, P.; Bradley, R.H. Effects of active carbon pore size distributions on adsorption of toxic organic compounds. Adsorption 2011, 17, 293–301. [Google Scholar] [CrossRef]
- Blacher, S.; Maquet, V.; Pirard, R.; Pirard, J.-P.; Jérôme, R. Image analysis, impedance spectroscopy and mercury porosimetry characterisation of freeze-drying porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 375–383. [Google Scholar] [CrossRef]
- Safinia, L.; Mantalaris, A.; Bismarck, A. Nondestructive technique for the characterization of the pore size distribution of soft porous constructs for tissue engineering. Langmuir 2006, 22, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Kosmulski, M. The pH dependent surface changing and points of zero charge. VII. Update. Adv. Colloid Interfaces Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Gamba, M.; Lázaro-Martínez, J.M.; Olivelli, M.S.; Yarza, F.; Vega, D.; Curutchet, G.; Torres Sánchez, R.M. Kinetic and equilibrium adsorption of two post-harvest fungicides onto copper-exchanged montmorillonite: Synergic and antagonistic effects of both fungicides’ presence. Environ. Sci. Pollut. Res. 2019, 26, 2421–2434. [Google Scholar] [CrossRef]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of diclofenac in wastewater using biosorption and advanced oxidation techniques: Comparative results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Martín-González, M.A.; González-Díaz, O.; Susial, P.; Araña, J.; Herrera-Melián, J.A.; Doña-Rodríguez, J.M.; Pérez-Peña, J. Reuse of Phoenix canariensis palm frond mulch as biosorbent and as precursor of activated carbons for the adsorption of imazalil in aqueous phase. Chem. Eng. J. 2014, 245, 348–358. [Google Scholar] [CrossRef]
- Chatterjee, A.; Abraham, J. Desorption of heavy metals from metal loaded sorbents and e-wastes: A review. Biotechnol. Lett. 2019, 41, 319–333. [Google Scholar] [CrossRef]
- Hashim, M.A.; Tan, H.N.; Chu, K.H. Immobilized marine algal biomass for multiple cycles of copper adsorption and desorption. Sep. Purif. Technol. 2000, 19, 39–42. [Google Scholar] [CrossRef]

| Olive Mill Residue | Artichoke Agrowaste | Citrus Waste | |
|---|---|---|---|
| C | 47.0 ± 1.0 * | 42.3 ± 0.9 | 41.3 ± 0.7 |
| O | 46.2 ± 1.9 | 48.1 ± 1.7 | 49.9 ± 2.2 |
| H | 5.2 ± 0.8 | 6.2 ± 0.5 | 5.8 ± 0.5 |
| N | 1.1 ± 0.5 | 3.0 ± 0.3 | 2.0 ± 0.4 |
| S | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.9 ± 0.2 |
| Ash | 0.7± 0.2 | 5.3 ± 0.8 | 8.9 ± 1.2 |
| O/C | 0.98 | 1.14 | 1.21 |
| HHV † (MJ/kg) | 17.77 | 16.97 | 15.96 |
Imazalil (mg·g−1) | Thiabendazole (mg·g−1)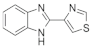 | |
|---|---|---|
| Artichoke agrowaste | 1.9 ± 0.4 * | 6.6 ± 1.0 |
| Olive mill residue | 9.0 ± 0.8 | 8.6 ± 0.9 |
| Citrus waste | 6.6 ± 0.6 | 6.1 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-López, J.A.; Doval Miñarro, M.; Angosto, J.M.; Fernández-Lledó, J.; Obón, J.M. Adsorptive and Surface Characterization of Mediterranean Agrifood Processing Wastes: Prospection for Pesticide Removal. Agronomy 2021, 11, 561. https://doi.org/10.3390/agronomy11030561
Fernández-López JA, Doval Miñarro M, Angosto JM, Fernández-Lledó J, Obón JM. Adsorptive and Surface Characterization of Mediterranean Agrifood Processing Wastes: Prospection for Pesticide Removal. Agronomy. 2021; 11(3):561. https://doi.org/10.3390/agronomy11030561
Chicago/Turabian StyleFernández-López, José A., Marta Doval Miñarro, José M. Angosto, Javier Fernández-Lledó, and José M. Obón. 2021. "Adsorptive and Surface Characterization of Mediterranean Agrifood Processing Wastes: Prospection for Pesticide Removal" Agronomy 11, no. 3: 561. https://doi.org/10.3390/agronomy11030561
APA StyleFernández-López, J. A., Doval Miñarro, M., Angosto, J. M., Fernández-Lledó, J., & Obón, J. M. (2021). Adsorptive and Surface Characterization of Mediterranean Agrifood Processing Wastes: Prospection for Pesticide Removal. Agronomy, 11(3), 561. https://doi.org/10.3390/agronomy11030561