Deciphering the Potential of Bioactivated Rock Phosphate and Di-Ammonium Phosphate on Agronomic Performance, Nutritional Quality and Productivity of Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Composted/Bio-Activated Rock Phosphate Preparation and Analysis
2.2. Soil Analysis
2.3. P-sources Analysis
2.4. Climatic Conditions and Growth Period
2.5. Experiment Description
2.6. Plant Sampling and Analysis
2.7. Statistical Data Analysis
3. Results
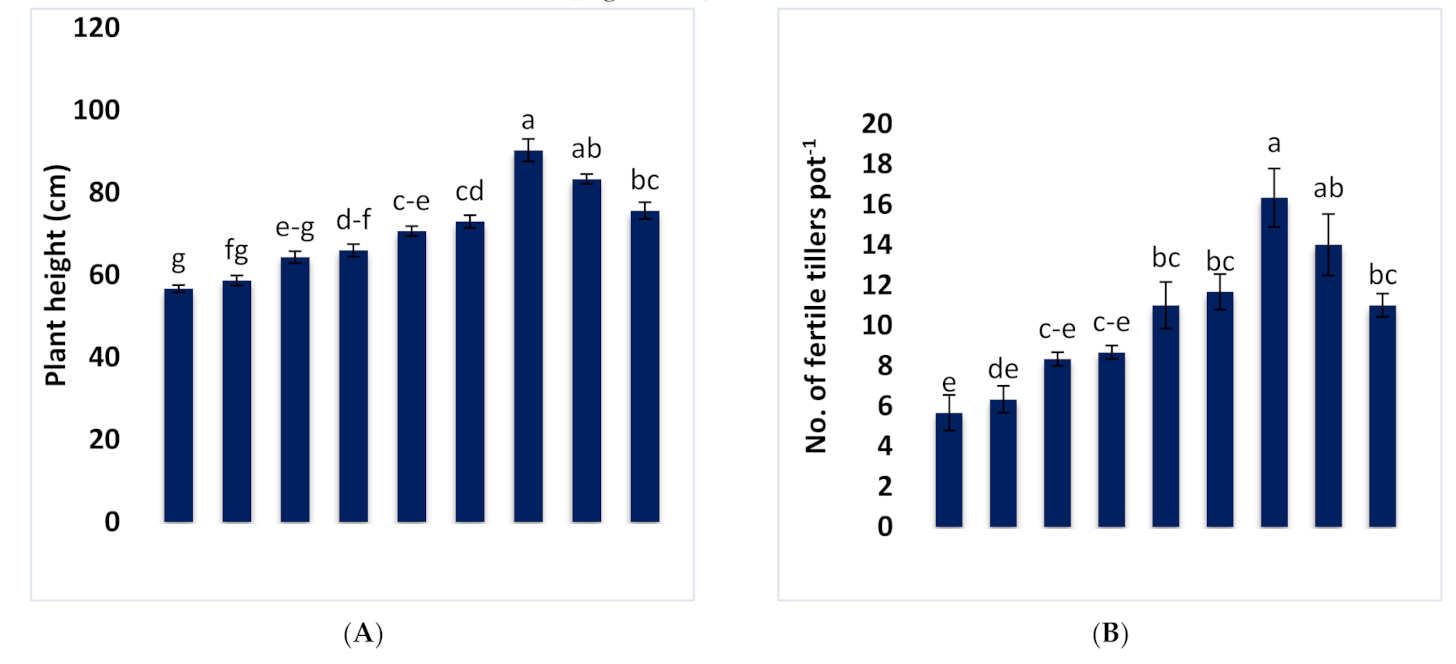
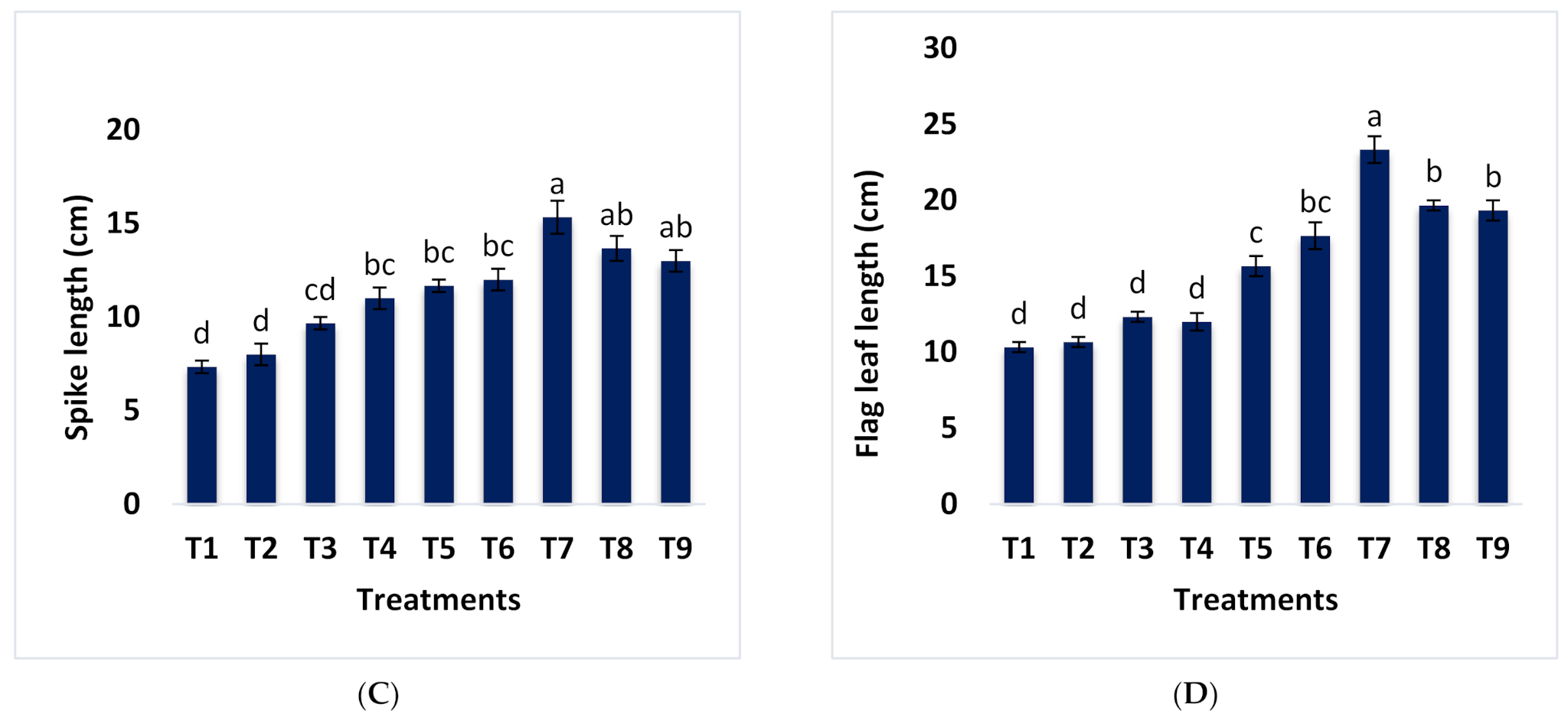
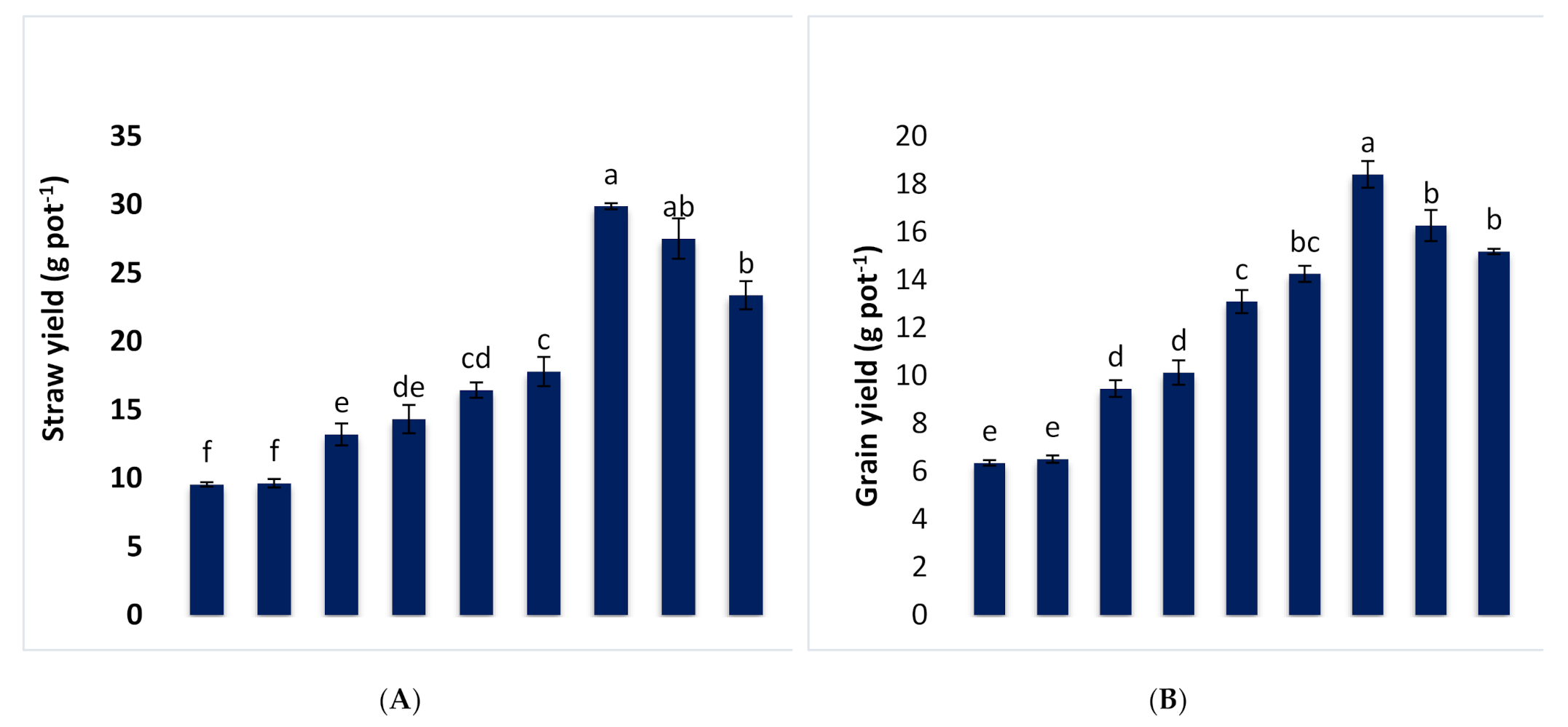
3.1. Effect of Different Combinations of B-RP and DAP on Growth and Yield Parameters of Wheat
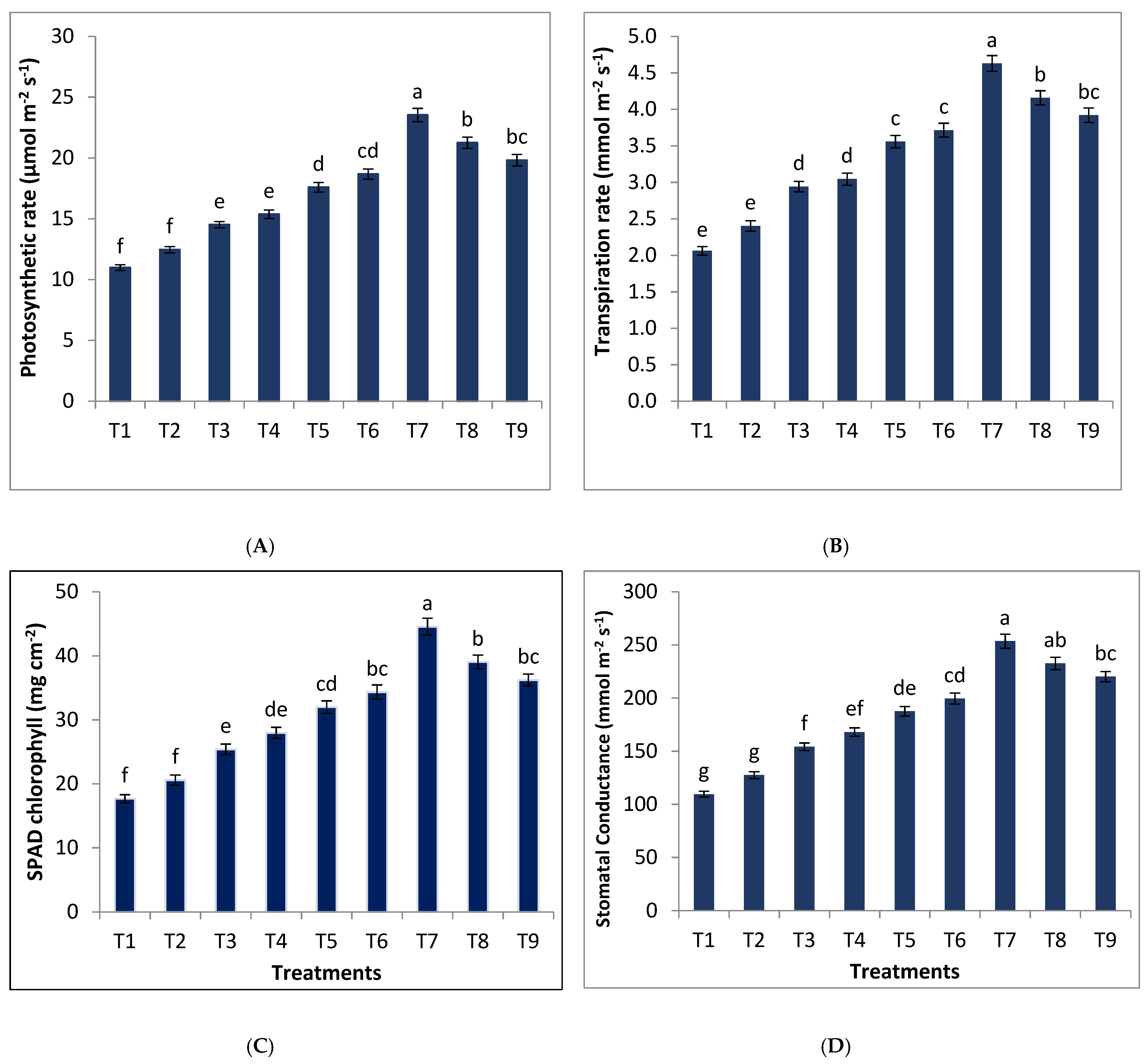
3.2. Effect of Different Combinations of B-RP and DAP on Physiological Parameters of Wheat
3.3. Effect of Different Combinations of B-RP and DAP on Chemical Parameters of Wheat
3.4. Effect of Different Combinations of B-RP and DAP on Wheat Nutritional Quality
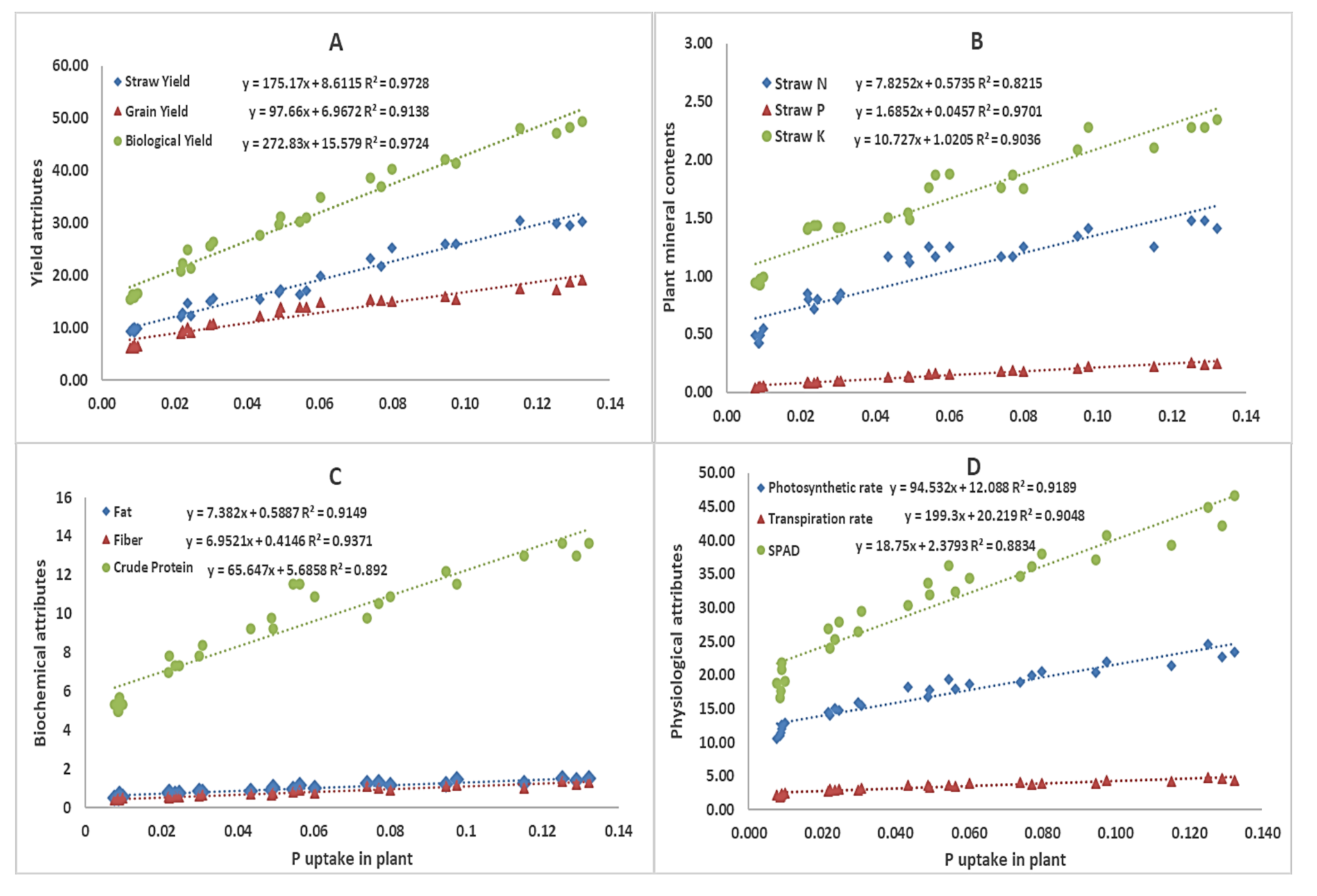
3.5. Correlation and Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiaz, S.; Noor, M.A.; Aldosri, F.O. Achieving food security in the Kingdom of Saudi Arabia through innovation: Potential role of agricultural extension. J. Saudi Soc. Agric. Sci. 2018, 17, 365–375. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Abbas, T.; Saeed, Q.; Hussain, A.; Ashraf, M.N.; Minggang, X. Growth response of wheat and associated weeds to plant antagonistic rhizobacteria and fungi. Ital. J. Agron. 2019, 14, 191–198. [Google Scholar] [CrossRef]
- Naz, T.; Akhtar, J.; Anwar-ul-Haq, V.; Shahid, M. Genetic variability in wheat genotypes for salt tolerance, growth and physiological responses. Soil Environ. 2015, 34, 10–30. [Google Scholar]
- Economic Survey of Pakistan 2017–2018; Agriculture Division, Economic Advisory Wing: Islamabad, Pakistan, 2018.
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of phosphate solubilization and physiological functions of phosphate solubilizing microorganisms. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Springer International Publishing Switzerland: Cham, Switzerland, 2014; pp. 31–62. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Naveed, M.; Wang, X.; Fatima, K.; Saeed, Q.; Mustafa, A. Polymer-Paraburkholderia phytofirmans PsJN coated diammonium phosphate enhanced microbial survival, phosphorous use efficiency, and production of Wheat. Agronomy 2020, 10, 1344. [Google Scholar] [CrossRef]
- Kirkby, E.A.; Johnston, E.A. Soil and fertilizer phosphorus in relation to crop nutrition. In The Eco-Physiology of Plant Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 177–223. [Google Scholar]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Mehta, P.; Walia, A.; Kulshrestha, S.; Chauhan, A.; Shirkot, C.K. Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microb. 2014, 53, 1–12. [Google Scholar]
- Timilsena, Y.P.; Adhikarib, R.; Caseyb, P.; Musterb, T.; Gilla, H.; Adhikaria, B. Enhanced efficiency fertilizers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef]
- Trolove, S.N.; Hedley, M.J.; Kirk, G.J.D.; Bolan, N.S.; Loganathan, P. Progress in selected areas of rhizosphere research on P acquisition. Aust. J. Soil Sci. 2003, 41, 471–499. [Google Scholar] [CrossRef]
- Yaseen, M.; Aziz, M.Z.; Manzoor, A.; Naveed, M.; Hamid, Y.; Noor, S.; Khalid, M.A. Promoting growth, yield, and phosphorus-use efficiency of crops in maize–wheat cropping system by using polymer-coated diammonium phosphate. Commun. Soil Sci. Plant Anal. 2017, 48, 646–655. [Google Scholar] [CrossRef]
- Umar, W.; Ayub, M.A.; ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and Phosphorus Use Efficiency in Agroecosystems. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 213–257. [Google Scholar]
- Akande, M.O.; Makinde, E.A.; Oluwatoyinbo, F.I.; Adetunji, M.T. Effects of phosphate rock application on dry matter yield and phosphorus recovery of maize and cowpea grown in sequence. Afr. J. Environ. Sci. Technol. 2010, 4, 293–303. [Google Scholar]
- Matiullah, K.; Sharif, M. Solubility enhancement of phosphorus from rock phosphate through composting with poultry litter. Sarhad J. Agric. 2012, 28, 415–420. [Google Scholar]
- Van Straaten, P. Rocks for Crops: Agro Minerals of Sub-Saharan Africa; ICRAF-International Center for Research in Agro forestry; University of Guelph: Nairobi, Kenya, 2002; p. 338. [Google Scholar]
- Park, J.; Bolan, N.; Mallavarapu, M.; Naidu, R. Enhancing the solubility of insoluble phosphorus compounds by phosphate solubilizing bacteria. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Autralia, 1–6 August 2010. [Google Scholar]
- Muhammad, D.; Khattak, R.A. Growth and nutrient concentration of maize in pressmud treated saline-sodic soils. Soil Environ. 2009, 28, 145–155. [Google Scholar]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N. Calcium-enriched animal manure alleviates the adverse effects of salt stress on growth, physiology and nutrients homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Deksissa, T.; Wyche-Moore, G.S.; Hare, W.W. Occurrence, fate and transport of 17beta-estradiol and testosterone in the environment. In Proceedings of the 2007 AWRA Summer Specialty Conference, Vail, CO, USA, 25–27 June 2007. [Google Scholar]
- Garg, S.; Bahla, G.S. Phosphorus availability to maize as influenced by organic manures and fertilizer P associated phosphatase activity in soil. Bioresour. Technol. 2008, 99, 5773–5777. [Google Scholar] [CrossRef]
- Ng’etich, O.K.; Aguyoh, J.N.; Ogweno, J.O. Effects of composted farmyard manure on growth and yield of spider plant (Cleome gynandra). Int. J. Sci. Nat. 2012, 3, 514–520. [Google Scholar]
- Singh, H.; Reddy, M.S. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 2011, 47, 30–34. [Google Scholar] [CrossRef]
- Munir, A.; Nawaz, S.A.-H.; Bajwa, M.A. Farm manure improved soil fertility in mungbean-wheat cropping system and rectified the deleterious effects of brackish water. Pak. J. Agric. Sci. 2012, 49, 511–519. [Google Scholar]
- Naveed, M.; Aziz, M.Z.; Yaseen, M. Perspectives of endophytic microbes for legume improvement. In Microbes for Legume Improvement; Zaidi, A., Khan, M.S., Musarrat, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 277–299. [Google Scholar]
- Sharif, M.; Arif, M.; Burni, T.; Khan, F.; Jan, B.; Khan, I. Growth and P uptake of sorghum plants in salt affected soil as affected by organic materials composted with rock phosphate. Pak. J. Bot. 2014, 46, 173–180. [Google Scholar]
- Pérez, E.; Sulbara, N.M.; Ball, M.M.; Yarzábal, L.A. Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol. Biochem. 2007, 39, 2905–2914. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Olivieri, R.; Roberttielle, A.; Degen, L. Mar. Pollut. Bull. 1978, 9, 217–220. [Google Scholar] [CrossRef]
- Buscot, F. What are soils? In Microorganisms in Soils: Roles in Genesis and Functions; Buscot, F., Varma, S., Eds.; Springer: Heidelberg, Germany, 2005; Volume 3, pp. 3–18. [Google Scholar]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate solubilizing fungi. Adv. Agron. 2000, 69, 99–151. [Google Scholar]
- Joseph, S.; Jisha, M.S. Buffering reduces phosphate solubilizing ability of selected strains of bacteria. World J. Agric. Sci. 2009, 5, 135–137. [Google Scholar]
- Khan, A.; Jilani, V.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Aziz, M.Z.; Naveed, M. Enhancing phosphorus use efficiency of wheat via controlled release of microbes by coating alginate loaded bacteria on diammonium phosphate. In Proceedings of the International Conference on Research for Food Security, Natural Resource Management and Rural Development, Vienna, Austria, 18–21 September 2016. [Google Scholar]
- U.S. Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; USDA Handbook No. 60; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. J. Agron. 1962, 53, 464–465. [Google Scholar] [CrossRef]
- Jackson, M.L. Chemical composition of soil. In Chemistry of Soil; Bear, F.E., Ed.; Van Nostrand Reinhold Co: New York, NY, USA, 1962; pp. 71–144. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Aanalysis Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Chemical and Microbiological Properties: Madison, WI, USA, 1982; pp. 152–153. [Google Scholar]
- Gallardo, J.E.; Saavedra, J.; Martin-Patino, T.; Millan, A. Soil organic matter determination. Commun. Soil Sci. Plant Anal. 1987, 18, 699–707. [Google Scholar] [CrossRef]
- Olsen, R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular 939 United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Brake, J.D. A Practical Guide for Composting Poultry Litter; MAFES Bulletin 981; Department of Poultry Science Mississippi State University: Starkville, MS, USA, 1992. [Google Scholar]
- van Schouwenberg, J.C.H.; Walinge, I. Methods of Analysis for Plant Material; Agriculture University: Wageningen, The Netherlands, 1973. [Google Scholar]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Lab Manual, 2nd ed.; International Centre for Agricultural Research in Dry Areas: Aleppo, Syria, 2001. [Google Scholar]
- Anderson, J.M.; Ingram, J.S. Colorimetric determination of ammonium and phosphorus. In Tropical Soil Biology and Fertility—A Hand Book of Methods; C.A.B. International: Wallingfold, UK, 1993; pp. 70–80. [Google Scholar]
- Winkleman, G.E.; Amine, R.; Rice, W.A.; Tahir, M.B. Potassium in plant. In Methods Manual Soils Laboratory. Barani Agricultural Research and Development Project; National Agricultural Research Centre: Islamabad, Pakistan, 1986. [Google Scholar]
- AOAC. Official Method of Analysis of AOAC International, 17th ed.; William Horwitz: Gaithersburg, MD, USA, 2000; Volume 1. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics, 3rd ed.; McGraw Hill. Inc. Book Co: New York, NY, USA, 1997; pp. 352–358. [Google Scholar]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient use Efficiency in Agriculture; International Fertilizer Industry Association (IFA): Paris, France, 2010. [Google Scholar]
- Fayiga, A.O.; Ipinmoroti, M.O.; Chirenje, T. Environmental pollution in Africa Environ. Dev. Sustain. 2018, 20, 41–73. [Google Scholar] [CrossRef]
- Prasanna, A.; Deepa, V.; Murthy, P.B.; Deecaraman, M.; Sridhar, R.; Dhandapani, P. Insoluble phosphate solubilization by bacterial strains isolated from rice rhizosphere soils from Southern India. Int. J. Soil Sci. 2011, 6, 134–141. [Google Scholar] [CrossRef]
- Bashan, Y.; Kamney, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils. 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Musa, N.; Manzoor, M. Phosphorus release capacity of soluble P fertilizers and insoluble rock phosphate in response to phosphate solubilizing bacteria and poultry manure and their effect on plant growth promotion and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosci. Discuss. 2015, 12, 1839–1873. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2006, 26, 1–15. [Google Scholar]
- He, X.S.; Xi, B.D.; Jiang, Y.H.; He, L.S.; Li, D.; Pan, H.W. Structural transformation study of water-extractable organic matter during the industrial composting of cattle manure. Microchem. J. 2013, 106, 160–166. [Google Scholar] [CrossRef]
- Gupta, N.; Sabat, J.; Parida, R.; Kerkatta, D. Solubilization of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Bot. Croat. 2007, 66, 197–204. [Google Scholar]
- Maliha, R.; Samina, K.; Najma, A.; Sadia, A.; Farooq, L. Organic acid production and phosphate solubilization by phosphate solubilizing microorganisms under in vitro conditions. Pak. J. Biol. Sci. 2004, 7, 187–196. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi-current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Rodriguez, H.; Fraga, R.; Gonzelaz, T.; Bashan, Y. Genetics of phosphate solubilization and its potential application for improving plant growth promoting bacteria. Plant Soil. 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Yadav, B.K.; Tarafdar, J.C. Availability of unavailable phosphate compounds as a phosphorus source for cluster bean (Cyamopsis tetragonoloba L.) through the activity of phosphatase and phytase produced by actinomycetes. J. Arid Legume 2007, 4, 110–116. [Google Scholar]
- Maougal, R.T.; Brauman, A.; Plassard, C.; Abadie, J.; Djekoun, A.; Drevon, J.J. Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. Eur. J. Soil Biol. 2014, 62, 8–14. [Google Scholar] [CrossRef]
- Reyes, I.; Bernier, L.; Simard, R.; Antoun, H. Effect of nitrogen source on solubilization of different inorganic phosphates by bacterial strain of Penicillium rugulosum and two UV induced mutants. Microbiol. Ecol. 2001, 28, 281–290. [Google Scholar] [CrossRef]
- Richardson, A.E. Soil microorganisms and phosphorus availability. In Management of the Soil Biota in Sustainable Farming Systems; Pankhurst, C.E., Doube, B.M., Gupta, V.V.S.R., Grace, P.R., Eds.; CSIRO Publishing: Melbourne, Australia, 2001; pp. 50–62. [Google Scholar]
- Lim, L.Y.; Chua, L.S.; Chew, T.L. Effects of microbial additive on the physiochemical and biological properties of oil palm empty fruit bunches compost. J. Eng. Sci. Technol. 2015, 5, 10–18. [Google Scholar]
- Torkashvand, A.M. Improvement of compost quality by addition of some amendments. Aus. J. Crop Sci. 2010, 4, 252–257. [Google Scholar]
- Sanchez, A.; Ysunza, F.; Beltran-Garcia, M.J.; Esqueda, M. Biodegradation of viticulture wastes by Pleurotus: A source of microbial and human food and its potential use in animal feeding. J. Agric. Food Chem. 2002, 50, 2537–2542. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Schuchardt, F.; Sheng, F.L.; Zhang, R.Z.; Cao, Z.Y. Assessment of maturity of vineyard pruning compost by Fourier Transform Infrared Spectroscopy, biological and chemical analyses. Landbauforsch Volkenrode 2005, 54, 163–169. [Google Scholar]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itavaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Schuchardt, F. Effect of C/N ratio on the composting of vineyard pruning residues. Agric. For. Res. 2010, 3, 131–138. [Google Scholar]
- Kumari, K.; Phogat, V.K. Rock phosphate: Its availability and solubilization in the soil. A review. Agric. Rev. 2008, 29, 108–116. [Google Scholar]
- Agyarko, K.; Abunyewa, A.A.; Asiedu, A.K.; Heva, E. Dissolution of rock phosphate in animal manure soil amendment and lettuce growth. Eurasian J. Soil Sci. 2016, 5, 84–88. [Google Scholar] [CrossRef][Green Version]
- Bashir, M.A.; Naveed, M.; Ahmad, Z.; Gao, B.; Mustafa, A.; Nú~nez-Delgado, A. Combined application of biochar and sulfur regulated growth, physiological, antioxidant responses and Cr removal capacity of maize (Zea mays L.) in tannery polluted soils. J. Environ. Manag. 2020, 259, 110051. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.N.-A.; Hussain, A.; Mumtaz, M.Z.; Rafique, M.; Bashir, M.A.; Alamri, S.; Siddiqui, M.H. Impact of facile coating of urea with Bacillus augmented zinc oxide on physiology, antioxidant activity, nutrients efficiency and productivity of wheat grown under salinity stress. Plants 2020, 9, 1375. [Google Scholar]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núnez-Delgado, A.; Saeed, Q.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Z.; Naveed, M.; Imran, M.; Bashir, M.A.; Sattar, A.; Mustafa, A.; Hussain, A.; Xu, M. Combined use of Enterobacter sp. MN17 and zeolite reverts the adverse effects of cadmium on growth, physiology and antioxidant activity of Brassica napus. PLoS ONE 2019, 14, e0213016. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A. Studies on the Possible use of Rock phosphate in Agriculture. Int. J. Chem. Tech. Res. 2015, 8, 53–68. [Google Scholar]
- Agyarko, K.; Frimpong, K.A.; Abunyewa, A.D. Phosphorus release dynamics under phosphate rock and ammonium sulphate in soil amendment. Eurasian J. Soil Sci. 2017, 6, 312–318. [Google Scholar] [CrossRef]
- Yasmeen, H.; Yaseen, M.; Aziz, M.Z.; Naveed, M.; Arfan-ul-Haq, M.; Abbas, T. Wheat residue management improves soil fertility and productivity of maize. Int. J. Agric. Biol. 2018, 20, 2181–2188. [Google Scholar]
- Gyaneshwar, P.; Naresh, K.G.; Parekh, L.J.; Poole, P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Naveed, M.; Shahid, M. Alginate-entrapped Enterobacter spp. MN17 coated diammonium phosphate improves growth, yield and phosphorus use efficiency of wheat. Int. J. Agric. Biol. 2018, 20, 2401–2407. [Google Scholar]
- Nishanth, D.; Biswas, D.R. Kinetics of phosphorus and potassium release from rock phosphate and waste mica enriched compost and their effect on yield and nutrient uptake by wheat (Triticum aestivum L.). Bioresour. Technol. 2008, 99, 3342–3353. [Google Scholar] [CrossRef]
- Shrivastava, M.; Kale, S.P.; D’Souza, S.F. Rock phosphate enriched post-methanation bio-sludge from kitchen waste based biogas plant as P source for mung bean and its effect on rhizosphere phosphatase activity. Eur. J. Soil Biol. 2011, 47, 205–212. [Google Scholar] [CrossRef]
- Billah, M.; Bano, A. Role of plant growth promoting rhizobacteria in modulating the efficiency of poultry litter composting with rock phosphate and its effect on growth and yield of wheat. Waste Manag. Res. 2015, 33, 63–72. [Google Scholar] [CrossRef]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethid, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef]
- Niemiera, A.X.; Leda, C.E. Nitrogen leaching from Osmocote-fertilized pine bark at leaching fractions of 0 to 0.4. J. Environ. Hort. 2007, 11, 75–77. [Google Scholar]
- Warren, R.; Arnell, N.; Nicholls, R.; Levy, P.; Price, J. Understanding the Regional Impacts of Climate Change. 2006. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.737.6215&rep=rep1&type=pdf (accessed on 2 April 2021).
- Oyedeji, S.; Animasaun, D.A.; Bello, A.A.; Agboola, O.O. Effect of NPK and poultry manure on growth, yield and proximate composition of three Amaranths. J. Bot. 2014, 2014, 828750. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Nisa, S.; Hussain, A.; Dawar, K.M.; Munir, A.; Khan, N. Rock phosphate-enriched compost in combination with Rhizobacteria; A cost-effective source for better soil health and wheat (Triticum aestivum) productivity. Agronomy 2020, 10, 1390. [Google Scholar] [CrossRef]
- Deepshika, T.; Kaushal, R.; Shyam, V. Phosphate solubilising microorganisms: Role in phosphorus nutrition of crop plants-A review. Agri. Rev. 2014, 35, 159–171. [Google Scholar]
- Santana, E.B.; Marques, E.L.S.; Dias, J.C.T. Effects of phosphate-solubilizing bacteria, native microorganisms and rock dust on Jatropha curcas L. growth. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. A review on phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar]
- Kumar, A.; Kumar, A.; Patel, H. Role of microbes in phosphorus availability and acquisition by plants. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1344–1347. [Google Scholar] [CrossRef]
- Mustafa, A.; Minggang, X.; Shah, S.A.A.; Abrar, M.M.; Nan, S.; Baoren, W.; Zejiang, C.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef]
- Morales-Corts, M.R.; Pérez-Sánchez, R.; Sánchez, M.A.G. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soil borne pathogens. Scientia Agricola 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Paredes, C.; Pe´rez-Murcia, M.D.; Pe´rez-Espinosa, A.; Bustamante, M.; Moreno-Caselles, J. Recycling of Two-Phase Olive-Mill Cake “Alperujo” by Co-composting with Animal Manures. Commun. Soil Sci. Plant Anal. 2015, 46, 238–247. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Z.; Gao, H.; Yan, X.; Chang, J.; Wang, C.; Hu, J.; Zhang, L. Chemical structures and characteristics of animal manures and composts during composting and assessment of maturity indices. PLoS ONE 2017, 12, e0178110. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Mustafa, A.; Hamid, Y.; Saeed, Q.; Xu, M.G. Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Mpanga, I.A.; Nkebiwe, P.M.; Kuhlmann, K.; Cozzolino, V.; Piccolo, A.; Geistlinger, G.; Berger, N.; Ludewig, U.; Neumann, G. The form of N supply determines plant growth promotion by P-solubilizing microorganisms in maize. Microorganisms 2019, 7, 38. [Google Scholar] [CrossRef] [PubMed]


| Properties | Soil | Animal Manure | Poultry Manure | Bio-Activated Rock Phosphate | LSD |
|---|---|---|---|---|---|
| Sand (%) | 46 ± 2.66 | - | - | - | |
| Silt (%) | 29 ± 2.66 | - | - | - | |
| Clay (%) | 25 ± 1.73 | - | - | - | |
| Texture | Sandy clay loam | - | - | - | |
| Saturation percentage (%) | 31.5 ± 1.68 | - | - | - | |
| Cation exchange capacity (CEC) (Cmolc kg−1) | 13.6 ± 1.22 | - | - | - | |
| pHs | 7.819 ± 0.01 | - | - | - | |
| Electrical conductivity (ECe) (dS m−1) | 1.98 ± 0.11 | - | - | - | |
| Organic matter (%) | 0.68 ± 0.16 | 63.51 ± 3.57 a | 69.93 ± 2.72 a | 37.90 ± 4.11 b | 7.02 |
| Carbon (%) | - | 35.28 ± 1.98 a | 38.85 ± 1.51 a | 21.06 ± 2.29 b | 3.91 |
| Ash (%) | - | 36.49 ± 3.57 b | 30.07 ± 2.72 b | 62.10 ± 4.11 a | 7.02 |
| Olsen phosphorus (mg kg−1) | 6.43 ± 1.45 | - | - | - | |
| Total phosphorus (%) | - | 0.69 ± 0.08 b | 1.25 ± 0.07 b | 4.92 ± 0.55 a | 0.64 |
| Total nitrogen (%) | 0.043 ± 0.02 | 1.19 ± 0.04 c | 1.85 ± 0.12 b | 2.77 ± 0.11 a | 0.19 |
| Extractable potassium (mg kg−1) | 120 ± 18.33 | - | - | - | |
| Total potassium (%) | - | 0.54 ± 0.10 c | 1.10 ± 0.07 b | 2.25 ± 0.07 a | 0.16 |
| Carbon: Nitrogen (C:N) | - | 29.64 ± 0.94 a | 21.00 ± 1.99 b | 7.60 ± 0.68 c | 2.66 |
| Treatments | Grain | Straw | ||||
|---|---|---|---|---|---|---|
| N (%) | P (%) | K (%) | N (%) | P (%) | K (%) | |
| T1: Control (0% P) | 0.82±0.03 f | 0.06 ± 0.01 f | 0.65 ± 0.03 e | 0.44 ± 0.44 e | 0.05 ± 0.01 g | 0.93 ± 0.01 d |
| T2: RP (100% P) | 0.89 ± 0.04 f | 0.07 ± 0.01 f | 0.67 ± 0.02 e | 0.51 ± 0.04 e | 0.05 ± 0.01 g | 0.96 ± 0.03 d |
| T3: AM (100% P) | 1.18 ± 0.07 e | 0.12 ± 0.01 e | 0.82 ± 0.01 d | 0.79 ± 0.07 d | 0.08 ± 0.01 f | 1.42 ± 0.027 c |
| T4: PM (100% P) | 1.25 ± 0.08 e | 0.15 ± 0.01 e | 0.84 ± 0.02 d | 0.82 ± 0.03 d | 0.10 ± 0.01 f | 1.42 ± 0.01 c |
| T5: B-RP (100% P) | 1.51 ± 0.05 d | 0.19 ± 0.01 d | 1.13 ± 0.01 c | 1.15 ± 0.03 c | 0.14 ± 0.01 e | 1.70 ± 0.03 b |
| T6: B-RP (75% P) + DAP (25% P) | 1.81 ± 0.06 bc | 0.20 ± 0.01 cd | 1.18 ± 0.01 c | 1.22 ± 0.05 bc | 0.16 ± 0.01 d | 1.84 ± 0.07 b |
| T7: B-RP (50% P) + DAP (50% P) | 2.15 ± 0.058 a | 0.30 ± 0.02 a | 1.47 ± 0.05 a | 1.46 ± 0.04 a | 0.25 ± 0.01 a | 2.30 ± 0.04 a |
| T8: B-RP (25% P) + DAP (75% P) | 1.96 ± 0.115 ab | 0.26 ± 0.01 b | 1.32 ± 0.01 b | 1.33 ± 0.08 ab | 0.22 ± 0.01 b | 2.16 ± 0.11 a |
| T9: DAP (100% P) | 1.67 ± 0.09 cd | 0.26 ± 0.02 c | 1.16 ± 0.04 c | 1.20 ± 0.05 bc | 0.18 ± 0.01 c | 1.80 ± 0.07 b |
| HSD | 0.201 | 0.024 | 0.069 | 0.139 | 0.016 | 0.148 |
| Treatments | Dry Matter (%) | Crude Protein (%) | Fats/Lipids (%) | Fibre (%) | Mineral Matter (%) |
|---|---|---|---|---|---|
| T1: Control (0% P) | 73.6 ± 1.6 d | 5.10 ± 0.18 f | 0.55 ± 0.04 g | 0.44 ± 0.03 g | 0.36 ± 0.05 g |
| T2: RP (100% P) | 73.9 ± 1.8 d | 5.56 ± 0.22 f | 0.63 ± 0.07 fg | 0.48 ± 0.04 fg | 0.44 ± 0.03 fg |
| T3: AM (100% P) | 75.5 ± 2.2 cd | 7.38 ± 0.41 e | 0.77 ± 0.03 efg | 0.55 ± 0.05 fg | 0.58 ± 0.07ef |
| T4: PM (100% P) | 77.2 ± 0.8 bcd | 7.83 ± 0.53 e | 0.84 ± 0.05 ef | 0.62 ± 0.06 ef | 0.65 ± 0.07 de |
| T5: B-RP (100% P) | 82.2 ± 2.3 abc | 9.44 ± 0.32 d | 0.95 ± 0.09 de | 0.73 ± 0.05 de | 0.81 ± 0.07 cd |
| T6: B-RP (75% P) + DAP (25% P) | 82.5 ± 2.4 ab | 11.33 ± 0.40 bc | 1.07 ± 0.11 cd | 0.85 ± 0.06 cd | 0.96 ± 0.13 bc |
| T7: B-RP (50% P) + DAP (50% P) | 85.8 ± 2.6 a | 13.42 ± 0.36 a | 1.49 ± 0.05 a | 1.29 ± 0.07 a | 1.28 ± 0.05 a |
| T8: B-RP (25% P) + DAP (75% P) | 84.0 ± 3.3 a | 12.25 ± 0.72 ab | 1.30 ± 0.14 ab | 1.11 ± 0.08 b | 1.17 ± 0.09 a |
| T9: DAP (100% P) | 82.7 ± 3.2 ab | 10.42 ± 0.55 cd | 1.25 ± 0.07 bc | 1.02 ± 0.09 bc | 1.15 ± 0.03 ab |
| HSD | 6.742 | 1.261 | 0.224 | 0.174 | 0.201 |
| Variables | DM | SY | SN | SP | SK | Fat | Fib | CP | GY | PU | PR | TR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SY | 0.920 | |||||||||||
| SN | 0.988 | 0.919 | ||||||||||
| SP | 0.966 | 0.988 | 0.964 | |||||||||
| SK | 0.938 | 0.963 | 0.963 | 0.978 | ||||||||
| Fat | 0.955 | 0.984 | 0.957 | 0.994 | 0.974 | |||||||
| Fib | 0.941 | 0.991 | 0.932 | 0.993 | 0.961 | 0.994 | ||||||
| CP | 0.975 | 0.943 | 0.986 | 0.979 | 0.986 | 0.971 | 0.958 | |||||
| GY | 0.986 | 0.959 | 0.990 | 0.988 | 0.977 | 0.986 | 0.971 | 0.989 | ||||
| PU | 0.928 | 0.992 | 0.920 | 0.989 | 0.959 | 0.982 | 0.994 | 0.950 | 0.960 | |||
| PR | 0.974 | 0.970 | 0.981 | 0.992 | 0.980 | 0.993 | 0.980 | 0.987 | 0.994 | 0.971 | ||
| TR | 0.980 | 0.958 | 0.990 | 0.985 | 0.976 | 0.984 | 0.966 | 0.986 | 0.995 | 0.958 | 0.998 | |
| SPAD | 0.971 | 0.966 | 0.977 | 0.988 | 0.977 | 0.990 | 0.977 | 0.984 | 0.991 | 0.970 | 0.998 | 0.995 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfan-ul-Haq, M.; Yaseen, M.; Naveed, M.; Mustafa, A.; Siddique, S.; Alamri, S.; Siddiqui, M.H.; Al-Amri, A.A.; D. Alsubaie, Q.; Ali, H.M. Deciphering the Potential of Bioactivated Rock Phosphate and Di-Ammonium Phosphate on Agronomic Performance, Nutritional Quality and Productivity of Wheat (Triticum aestivum L.). Agronomy 2021, 11, 684. https://doi.org/10.3390/agronomy11040684
Arfan-ul-Haq M, Yaseen M, Naveed M, Mustafa A, Siddique S, Alamri S, Siddiqui MH, Al-Amri AA, D. Alsubaie Q, Ali HM. Deciphering the Potential of Bioactivated Rock Phosphate and Di-Ammonium Phosphate on Agronomic Performance, Nutritional Quality and Productivity of Wheat (Triticum aestivum L.). Agronomy. 2021; 11(4):684. https://doi.org/10.3390/agronomy11040684
Chicago/Turabian StyleArfan-ul-Haq, Muhammad, Muhammad Yaseen, Muhammad Naveed, Adnan Mustafa, Sulman Siddique, Saud Alamri, Manzer H. Siddiqui, Abdullah A. Al-Amri, Qasi D. Alsubaie, and Hayssam M. Ali. 2021. "Deciphering the Potential of Bioactivated Rock Phosphate and Di-Ammonium Phosphate on Agronomic Performance, Nutritional Quality and Productivity of Wheat (Triticum aestivum L.)" Agronomy 11, no. 4: 684. https://doi.org/10.3390/agronomy11040684
APA StyleArfan-ul-Haq, M., Yaseen, M., Naveed, M., Mustafa, A., Siddique, S., Alamri, S., Siddiqui, M. H., Al-Amri, A. A., D. Alsubaie, Q., & Ali, H. M. (2021). Deciphering the Potential of Bioactivated Rock Phosphate and Di-Ammonium Phosphate on Agronomic Performance, Nutritional Quality and Productivity of Wheat (Triticum aestivum L.). Agronomy, 11(4), 684. https://doi.org/10.3390/agronomy11040684









