Recycled Wastewater and Reverse Osmosis Brine Use for Halophytes Irrigation: Differences in Physiological, Nutritional and Hormonal Responses of Crithmum maritimum and Atriplex halimus Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experiment Conditions
2.2. Irrigation Water Treatments and Experimental Design
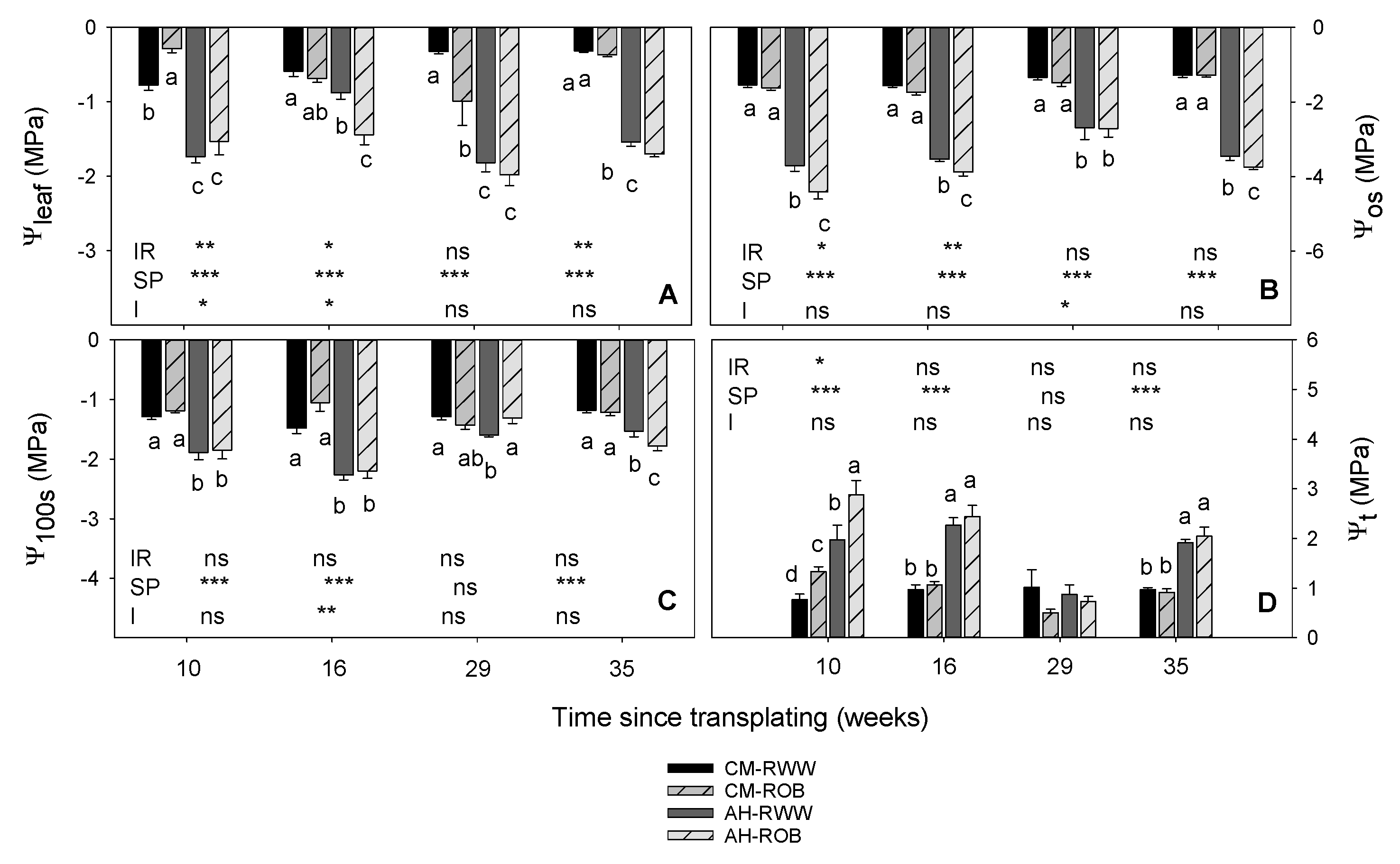
2.3. Plant Water Relations
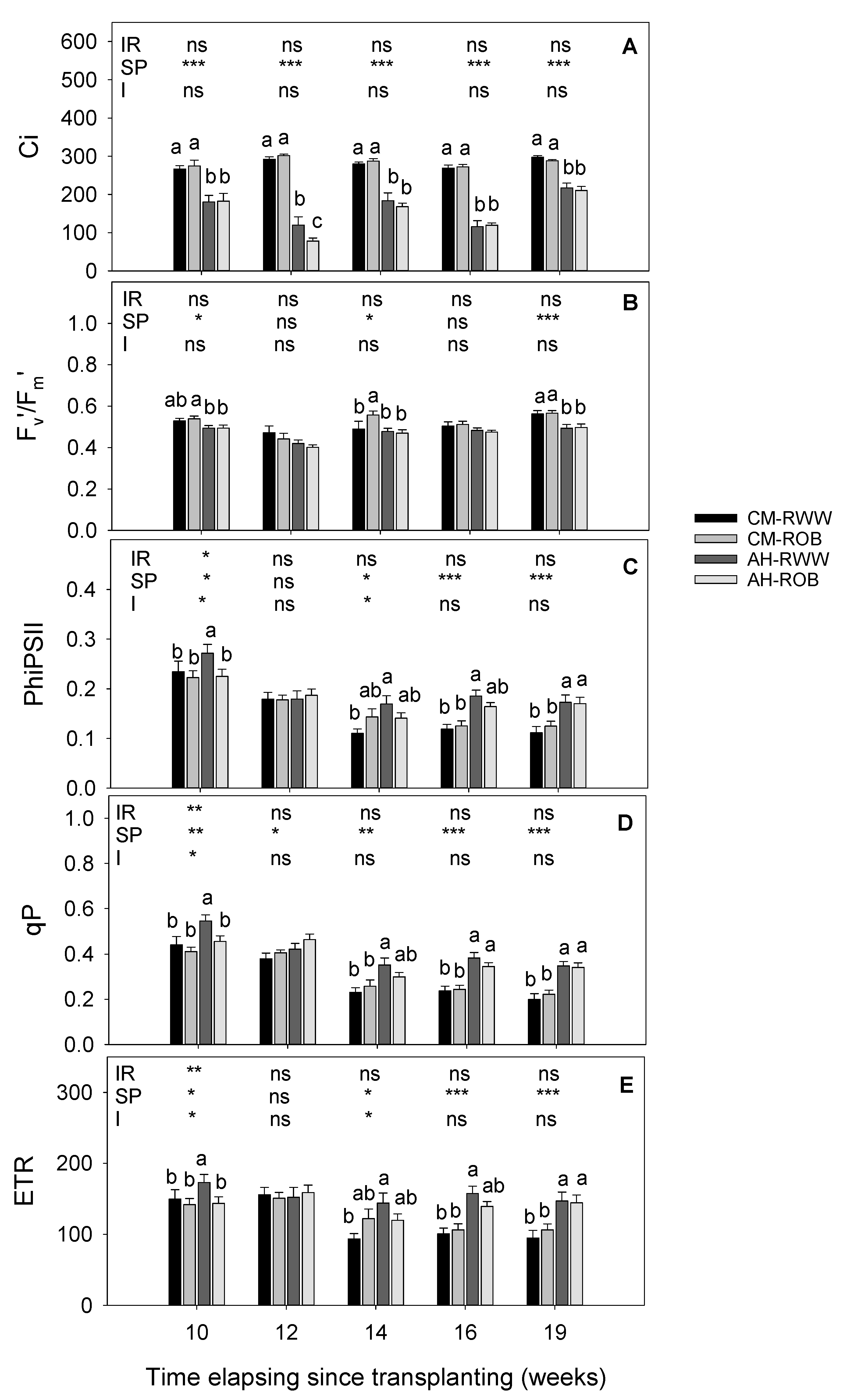
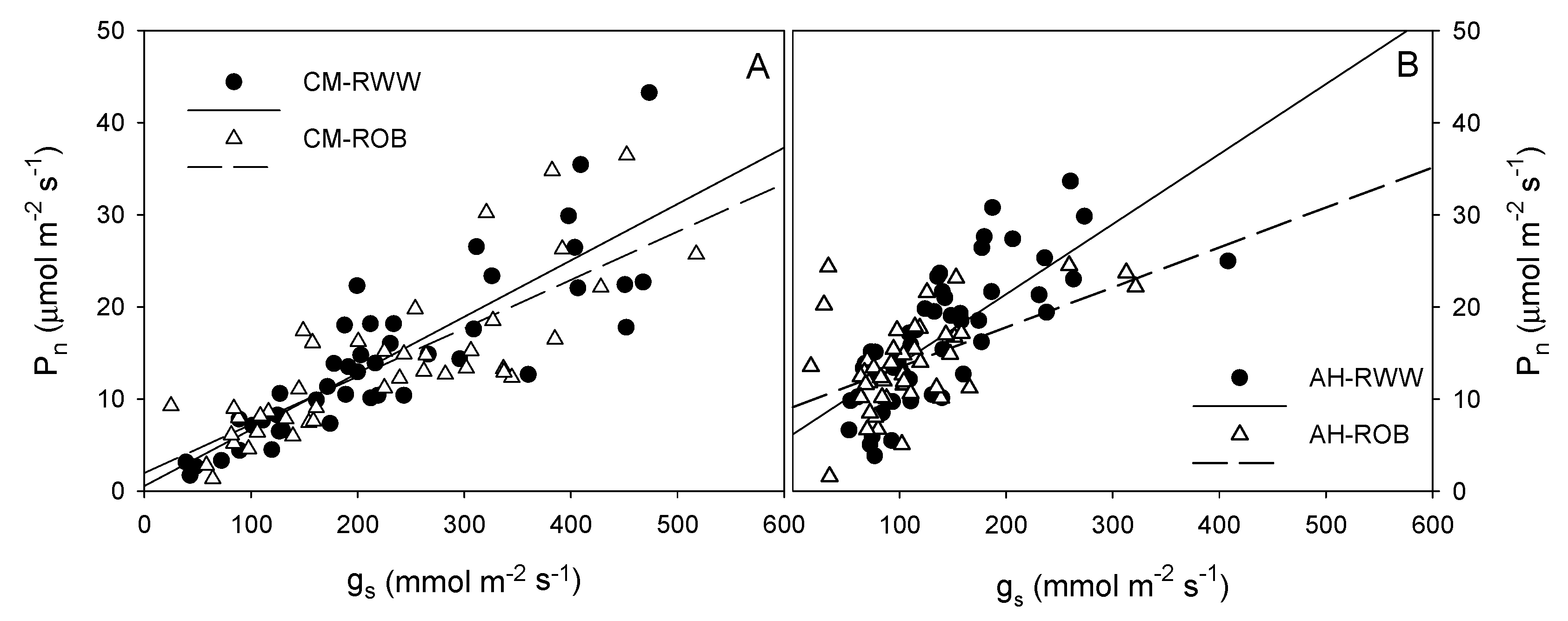
2.4. Gas Exchange and Chlorophyll Fluorescence Parameters
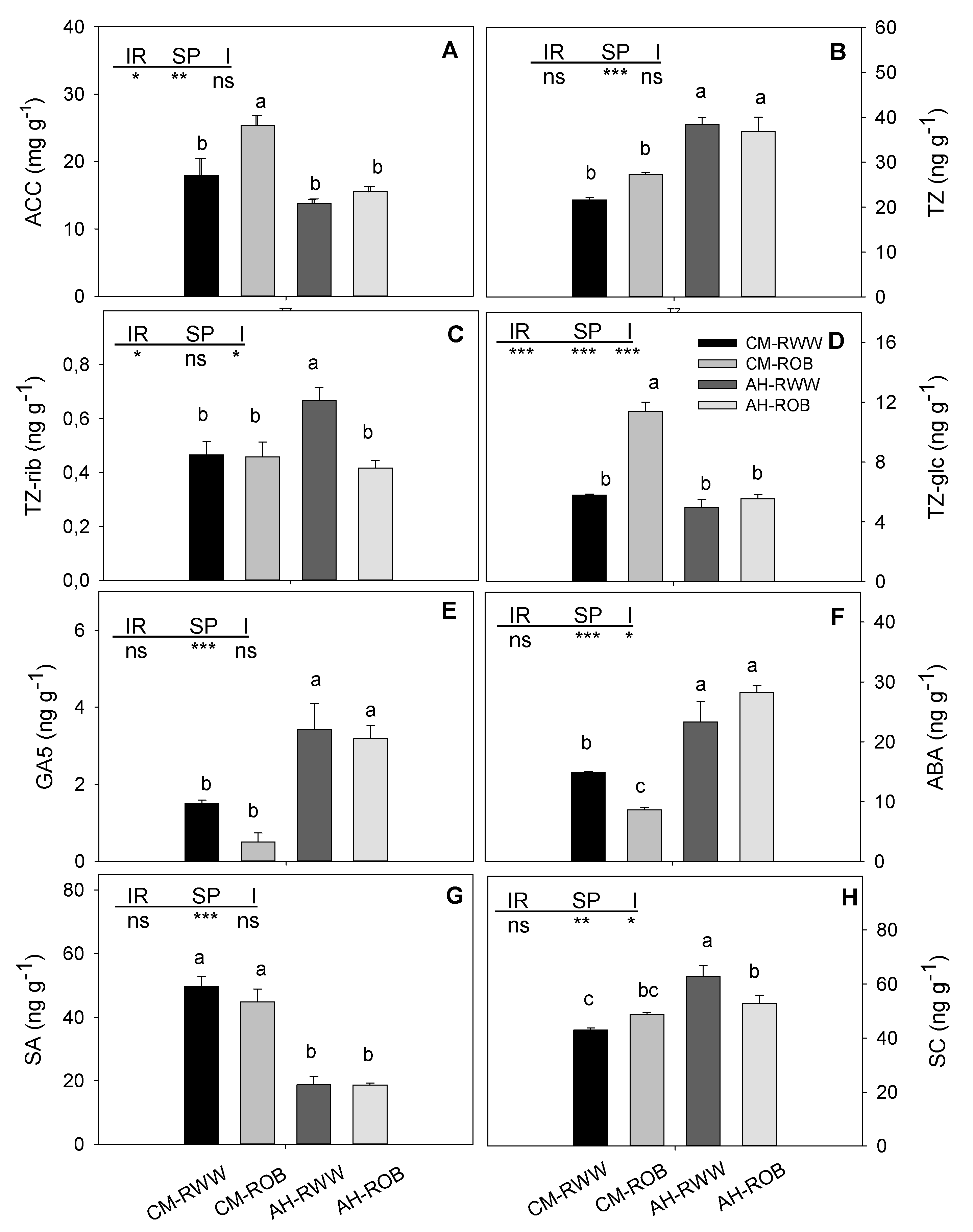
2.5. Qualitative and Quantitative Analysis of Phytohormones and Chlorophyll Content in Leaves
2.6. Determination of Mineral Content in Leaves and Plant Canopy
2.7. Statistics
3. Results
3.1. Plant Water Relations
3.2. Gas Exchange and Chlorophyll Fluorescence Parameters
3.3. Phytohormones and Chlorophyll Content in Leaves
3.4. Leaf Mineral Concentration and Plant Canopy Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benet, A.S.; Castilla, Y.C. Mejora de suelos salinos y control de la erosión en zonas áridas. In Libro de Ponencias y Comunicaciones, XXXII Congreso de la Asociación Española de Parques y Jardines Públicos, Almería, Spain; Asociación Española de Parques y Jardines Públicos: Madrid, Spain, 2005; p. 61. [Google Scholar]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Dogan, E.D.; Yasar, A.; Sen, U.; Aydiner, C. Water recovery from treated urban wastewater by ultrafiltration and reverse osmosis for landscape irrigation. Urban Water J. 2016, 13, 553–568. [Google Scholar] [CrossRef]
- Singh, A. Poor quality water utilization for agricultural production: An environmental perspective. Land Use Policy 2015, 43, 259–262. [Google Scholar] [CrossRef]
- Mizyed, N.R. Challenges to treated wastewater reuse in arid and semi-arid areas. Environ. Sci. Policy 2013, 25, 186–195. [Google Scholar] [CrossRef]
- Pedrero, F.; Kalavrouziotis, I.; Alarcón, J.J.; Koukoulakis, P.; Asano, T. Use of treated municipal wastewater in irrigated agriculture—Review of some practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Bozdoğan, E. Possible use of treated wastewater as irrigation water at urban green area. Turk. J. Agric. Food Sci. Technol. 2015, 3, 35–39. [Google Scholar] [CrossRef][Green Version]
- Saleem, M.; Jabbar, U. Feasibility studies of using domestic wastewater for landscape irrigation purposes near a nuclear power plant. Int. J. Environ. Eng. 2018, 9, 115–129. [Google Scholar] [CrossRef]
- EPA: Guidelines for Water Reuse; US Agency for International Development: Washington, DC, USA, 2012.
- Eslamian, S.; Okhravi, D.; Reyhani, M.N. Urban Water Reuse: Future Policies and Outlooks. In Urban Water Reuse Handbook; Eslamian, S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1097–1104. [Google Scholar]
- Vivaldi, G.A.; Camposeo, S.; Mastro, M.A.; Lacolla, L.; Lonigro, A.; Rubino, P. Effect of irrigation with different municipal wastewaters on ripening indexes and chemical components of nectarine fruits. Acta Hortic. 2015, 1084, 401–407. [Google Scholar] [CrossRef]
- Silber, A.; Israeli, Y.; Elingold, I.; Levi, M.; Levkovitch, I.; Russo, D.; Assouline, S. Irrigation with desalinated water: A step toward increasing water saving and crop yields. Water Resour. Res. 2015, 51, 450–464. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Kalavrouziotis, I.K.; Koukoulakis, P.H.; Vasquez, M.I. The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci. Total Environ. 2011, 409, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Mara, D.; Sleigh, A. Estimation of norovirus infection risks to consumers of wastewater-irrigated food crops eaten raw. J. Water Health 2010, 8, 39–43. [Google Scholar] [CrossRef]
- Intriago, J.C.; López-Gálvez, F.; Allende, A.; Vivaldi, G.A.; Camposeo, S.; Nicolás, E.N.; Salcedo, F.P. Agricultural reuse of municipal wastewater through an integral water reclamation management. J. Environ. Manag. 2018, 213, 135–141. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Ortuño, M.F.; Nortes, P.A.; Vicente-Sánchez, J.; Fernández, F.; Bañón, S.; Sánchez-Blanco, M.J. Protective effects of Glomus iranicum var. tenuihypharum on soil and Viburnum tinus plants irrigated with treated wastewater under field conditions. Mycorrhiza 2015, 25, 399–409. [Google Scholar] [CrossRef]
- Afrasiabi, N.; Shahbazali, E. RO brine treatment and disposal methods. Desalin. Water Treat. 2011, 35, 39–53. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of water: A review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Hamilton, S.J. Review of selenium toxicity in the aquatic food chain. Sci. Total 2004, 326, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Nazer, A.; Guzmán, A.; Bolados, L.; González, L.; Pavez, O. Uso de agua de rechazo de plantas depuradoras en la fabricación de hormigones. Obras Proy. 2018, 24, 21–27. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Ayars, J.E.; Soppe, R.W.O. Integrated Water Management for Saline Drainage Water Disposal. In Proceedings of the Engineering Salinity Solutions, 1st National Salinity Engineering Conference, Perth, Australia, 1 January 2004; Engineers Australia: Perth, Australia, 2004; p. 9. [Google Scholar]
- Miyamoto, S. Appraising Salinity Hazard to Landscape Plants and Soils Irrigated with Moderately Saline Water. 2006. Available online: http://opensiuc.lib.siu.edu/ucowrconfs_2006/102 (accessed on 18 July 2006).
- Romero-Trigueros, C.; Alarcón-Cabañero, J.J.; Tortosa, P.A.; Gambín, J.M.; Maestre-Valero, J.F.; Nicolás, E.N. Medium-long term effects of saline reclaimed water and regulated deficit irrigation on fruit quality of citrus. J. Sci. Food Agric. 2020, 100, 1350–1357. [Google Scholar] [CrossRef]
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2019, 47, 2083–2091. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Riadh, K.; Wided, M.; Hans-Werner, K.; Chedly, A. Responses of halophytes to environmental stresses with special emphasis to salinity. Adv. Bot. Res. 2010, 53, 117–145. [Google Scholar]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Ashraf, M.P.J.C.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Koyro, H.W.; Geissler, N.; Hussin, S. Survival at extreme locations: Life strategies of halophytes. In Salinity and Water Stress; Ashraf, M., Ozturk, M., Athar, H.R., Eds.; Springer: Berlin, Germany, 2009; Volume 17, pp. 167–177. [Google Scholar]
- Moles, T.M.; Pompeiano, A.; Reyes, T.H.; Scartazza, A.; Guglielminetti, L. The efficient physiological strategy of a tomato landrace in response to short-term salinity stress. Plant Physiol. Biochem. 2016, 109, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Polic, D.; Lukovic, J.; Zorić, L.; Boza, P.; Merkulov, L.; Knezević, A. Morpho-anatomical differentiation of Suaeda maritima (L.) Dumort. (Chenopodiaceae) populations from inland and maritime saline area. Cent. Eur. J. Biol. 2009, 4, 117–129. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Polash, M.A.S.; Sakil, A.; Hossain, A. Plants responses and their physiological and biochemical defense mechanisms against salinity: A review. Trop. Plant Res. 2019, 6, 250–274. [Google Scholar] [CrossRef]
- Llanes, A.; Masciarelli, O.; Luna, V. Growth responses to sulfate and chloride are related to different phytohormone profiles in the halophyte Prosopis strombulifera. Emir. J. Food Agric. 2014, 26, 1097–1113. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Maricle, B.R.; Lee, R.W.; Hellquist, C.E.; Kiirats, O.; Edwards, G.E. Effects of salinity on chlorophyll fluorescence and CO2 fixation in C4 estuarine grasses. Photosynthetica 2007, 45, 433–440. [Google Scholar] [CrossRef]
- Moinuddin, M.; Gulzar, S.; Hameed, A.; Gul, B.; Khan, M.A.; Edwards, G.E. Differences in photosynthetic syndromes of four halophytic marsh grasses in Pakistan. Photosynth. Res. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Mackay, A. Ion transport in halophytes. Adv. Bot. Res. 2011, 57, 151–199. [Google Scholar]
- Głoniak, P.; Łoś, R.; Skalicka-Woźniak, K.; Widelski, J.; Burczyk, J.; Malm, A. Activity of Crithmum maritimum L. (Apiaceae) against Gram-positive bacteria. Ann. Univ. Mariae Curie-Sklodowska Lub. Pol. 2006, 19, 123–127. [Google Scholar]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef]
- Abbad, A.; El Hadrami, A.; Benchaabane, A. Germination responses of the Mediterranean saltbush (Atriplex halimus L.) to NaCl treatment. J. Agric. 2004, 3, 111–114. [Google Scholar]
- Soualem, S.; Kouadria, R.; Labdelli, A.; Adda, A. Effect of GA3, ABA and kinetin on the response of the halophyte Atriplex halimus to salinity during germination. Plant Arch. 2018, 18, 609–615. [Google Scholar]
- Hassine, A.B.; Ghanem, M.E.; Bouzid, S.; Lutts, S. An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. J. Exp. Bot. 2008, 59, 1315–1326. [Google Scholar] [CrossRef]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Measurements of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Gucci, R.; Xiloyannis, C.; Flore, J.A. Gas exchange parameters, water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol. Plant. 1991, 83, 497–505. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Photosynthetic response of bottle gourd [Lagenaria siceraria (Molina) Standl.] to drought stress: Relationship between cucurbitacins accumulation and drought tolerance. Sci. Hortic. 2018, 231, 133–143. [Google Scholar] [CrossRef]
- Rivier, L.; Crozier, A. Principles and Practice of Plant Hormone Analysis; Academic Press: London, UK, 1987; pp. 1–401. [Google Scholar]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Jalali, G.A.; Akbarian, H.; Rhoades, C.; Yousefzadeh, H. The effect of the halophytic shrub Lycium ruthenium (Mutt) on selected soil properties of a desert ecosystem in central Iran. Pol. J. Ecol. 2012, 60, 845–850. [Google Scholar]
- Lefèvre, I.; Marchal, G.; Meerts, P.; Corréal, E.; Lutts, S. Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ. Exp. Bot. 2009, 65, 142–152. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E. Viability of nanofiltration and ultra-low pressure reverse osmosis membranes for multi-beneficial use of methane produced water. Sep. Purif. Technol. 2006, 52, 67–76. [Google Scholar] [CrossRef]
- Sucre, B.; Suárez, N. Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation, and leaf growth in Ipomoea pes-caprae. Environ. Exp. Bot. 2011, 70, 192–203. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Labidi, N.; Ammari, M.; Snoussi, S.; Messelini, N.; Gharbi, F.; Abdelly, C. Stimulated growth rate by restriction of P availability at moderate salinity but insensitive to P availability at high salinity in Crithmum maritimum. Acta Biol. Hung. 2011, 62, 302–315. [Google Scholar] [CrossRef]
- Benzarti, M.; Rejeb, K.B.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol. Plant. 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Sikder, S.; Foulkes, J.; West, H.; De Silva, J.; Gaju, O.; Greenland, A.; Howell, P. Evaluation of photosynthetic potential of wheat genotypes under drought condition. Photosynthetica 2015, 53, 47–54. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar]
- Fipps, G. Irrigation Water Quality Standards and Salinity Management Strategies; Texas Agricultural Extension Service; A&M University System: College Station, TX, USA, 2003; pp. 1–19. [Google Scholar]
- Bauder, T.A.; Waskom, R.M.; Sutherland, P.L.; Davis, J.G. Irrigation Water Quality Criteria. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2011. [Google Scholar]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation water quality—A contemporary perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar] [PubMed]
- Cao, W.H.; Liu, J.; He, X.J.; Mu, R.L.; Zhou, H.L.; Chen, S.Y.; Zhang, J.S. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007, 143, 707–719. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Xia, X.; Zhang, W.H. Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul. 2011, 65, 407–413. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Variation in salt tolerance of wheat cultivars: Role of glycinebetaine and ethylene. Pedosphere 2012, 22, 746–754. [Google Scholar] [CrossRef]
- Albacete, A.; Martinez-Andujar, C.; Ghanem, M.E.; Acosta, M.; Sanchez-Bravo, J.; Asins, M. J. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2009, 32, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.E.; Albacete, A.; Martinez-Andujar, C.; Acosta, M.; Romero-Aranda, R.; Dodd, I.C. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 2008, 59, 3039–3050. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Kudoyarova, G.R. Role of endogenous hormonal system in the realization of the antistress action of plant growth regulators on plants. Plant Stress 2010, 4, 32–38. [Google Scholar]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Gnonlonfin, G.B.; Sanni, A.; Brimer, L. Review scopoletin—A coumarin phytoalexin with medicinal properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Tanaka, Y.; Data, E.S.; Hirose, S.; Taniguchi, T.; Uritani, I. Biochemical changes in secondary metabolites in wounded and deteriorated cassava roots. Agric. Biol. Chem. 1983, 47, 693–700. [Google Scholar]
- Hamdani, F.; Derridj, A.; Roger, H.J. Diverse salinity responses in Crithmum maritimum tissues at different salinities over time. J. Soil Sci. Plant Nutr. 2017, 17, 716–734. [Google Scholar] [CrossRef][Green Version]
- Jiménez-Becker, S.; Ramírez, M.; Plaza, B.M. The influence of salinity on the vegetative growth, osmolytes and chloride concentration of four halophytic species. J. Plant Nutr. 2019, 42, 1838–1849. [Google Scholar] [CrossRef]
- Hamed, K.B.; Debez, A.; Chibani, F.; Abdelly, C. Salt response of Crithmum maritimum, an oleagineous halophyte. Trop. Ecol. 2004, 45, 151–159. [Google Scholar]
- Nedjimi, B.; Daoud, Y. Effect of Na2SO4 on the growth, water relations, proline, total soluble sugars and ion content of Atriplex halimus subsp. schweinfurthii through in vitro culture. An. Biol. 2006, 28, 35–43. [Google Scholar]
- Boughalleb, F.; Denden, M. Physiological and biochemical changes of two halophytes, Nitraria retusa (Forssk.) and Atriplex halimus (L.) under increasing salinity. Agric. J. 2011, 6, 327–339. [Google Scholar] [CrossRef]
| RWW | ROB | |
|---|---|---|
| EC (dS m−1) | 0.994 | 5.403 |
| pH | 7.199 | 7.124 |
| SS (mg L−1) | 1.276 | 4.269 |
| Turbidity (NTU) | 0.570 | 0.686 |
| E. coli (UFC 100 mL−1) | 0.00 | 0.00 |
| Fe (ppm) | 0.04 | 0.07 |
| K (ppm) | 17.54 | 98.14 |
| Mg (ppm) | 8.45 | 56.12 |
| Mn (ppm) | 0.05 | 0.27 |
| Na (ppm) | 160.16 | 1003.29 |
| Cl (ppm) | 210.01 | 1208.92 |
| P (ppm) | 2.68 | 10.04 |
| S (ppm) | 1.88 | 9.11 |
| B (ppm) | 0.821 | 0.877 |
| Ni (ppm) | 0.008 | 0.008 |
| Cu (ppm) | 0.009 | 0.009 |
| Zn (ppm) | 0.054 | 0.044 |
| F (ppm) | 0.07 | 0.24 |
| NO2 (ppm) | 0.10 | 0.10 |
| NO3 (ppm) | 5.64 | 15.70 |
| PO4 (ppm) | 8.21 | 30.72 |
| SO4 (ppm) | 129.6 | 877.58 |
| Hormone Z | Retention Time (min) | Ionization Mode | Parent Ion (m/z) | Ion Fragments (m/z) | Fragmentor (V) | Collision Energy (V) |
|---|---|---|---|---|---|---|
| ACC | 1.312 | Positive | 102.1 | 56.0 Y | 80 | 15 |
| 28.0 X | 80 | 15 | ||||
| tZ | 1.724 | Positive | 220.2 | 202.0 | 80 | 15 |
| 136.0 | 80 | 15 | ||||
| tZdeuter | 1.744 | Positive | 225.2 | 136.3 | 80 | 15 |
| tZ-GLC | 1.742 | Positive | 382.4 | 220.0 | 80 | 15 |
| 202.0 | 80 | 15 | ||||
| tZ-Rib | 1.743 | Positive | 352.4 | 219.7 | 80 | 15 |
| 136.0 | 80 | 15 | ||||
| SC | 2.802 | Positive | 193.2 | 132.5 | 80 | 20 |
| 149.1 | 80 | 20 | ||||
| GA5 | 3.095 | Negative | 329.4 | 145.0 | 80 | 39 |
| 285.0 | 80 | 18 | ||||
| ABA | 3.130 | Negative | 263.3 | 152.9 | 80 | 14 |
| 204.1 | 80 | 18 | ||||
| SA | 3.219 | Negative | 137.1 | 93.2 | 80 | 15 |
| 65.4 | 80 | 15 |
| gs (mmol m−2 s−1) | |||||
| Week | 10 | 12 | 14 | 16 | 19 |
| CM-RWW | 268.4 a | 210.9 a | 199.2 b | 110.5 | 333.3 a |
| CM-ROB | 211.9 ab | 177.3 ab | 299.1 a | 122.8 | 285.4 ab |
| AH-RWW | 152.8 bc | 123.6 bc | 120.4 bc | 94.1 | 204.4 b |
| AH-ROB | 105.0 c | 58.4 c | 95.5 c | 92.4 | 196.7 b |
| Sig. | ** | ** | *** | ns | * |
| IR | * | * | ns | ns | ns |
| SP | ** | ** | *** | ns | ** |
| I | ns | ns | * | ns | ns |
| Pn (µmol m−2 s−1) | |||||
| Week | 10 | 12 | 14 | 16 | 19 |
| CM-RWW | 16.93 a | 10.47 b | 10.76 | 6.587 b | 26.69 a |
| CM-ROB | 12.90 ab | 9.56 b | 14.10 | 7.104 b | 24.18 ab |
| AH-RWW | 18.82 a | 16.89 a | 14.52 | 14.68 a | 18.21 b |
| AH-ROB | 10.17 b | 15.60 a | 11.64 | 14.42 a | 17.57 b |
| Sig. | * | ** | ns | *** | * |
| IR | *** | ns | ns | ns | ns |
| SP | ns | *** | ns | *** | ** |
| I | * | ns | ns | ns | ns |
| WUEi (Pn/gs) | |||||
| Week | 10 | 12 | 14 | 16 | 19 |
| CM-RWW | 69.0 b | 51.4 b | 54.8 b | 62.2 b | 80.9 |
| CM-ROB | 65.3 b | 105.8 b | 50.2 b | 60.2 b | 87.6 |
| AH-RWW | 119.1 a | 157.1 b | 113.6 a | 157.6 a | 92.2 |
| AH-ROB | 91.0 b | 369.6 a | 120.0 a | 155.9 a | 93.5 |
| Sig. | *** | ** | *** | *** | ns |
| IR | * | * | ns | ns | ns |
| SP | *** | ** | *** | *** | ns |
| I | * | ns | ns | ns | ns |
| Leaf Mineral Content (mg g−1) | |||
|---|---|---|---|
| Chl A | Chl B | Chl T | |
| CM-RWW | 0.539 b | 0.171 b | 0.710 b |
| CM-ROB | 0.719 b | 0.242 b | 0.961 b |
| AH-RWW | 2.048 a | 0.549 a | 2.597 a |
| AH-ROB | 2.107 a | 0.556 a | 2.663 a |
| Sig. | *** | ** | *** |
| IR | ns | ns | ns |
| SP | ** | * | * |
| I | ns | ns | ns |
| ppm | B | Ca | Fe | K | Mg | Na | P | Zn |
|---|---|---|---|---|---|---|---|---|
| CM-RWW | 329.0 a | 45,143.3 a | 126.5 | 34,673.3 b | 3497.0 b | 24,931.7 b | 3313.2 | 31.1 b |
| CM-ROB | 263.5 b | 44,260.0 a | 112.9 | 19,582.8 b | 3343.0 b | 17,809.2 b | 3324.8 | 40.4 ab |
| AH-RWW | 248.9 b | 11,013.3 b | 92.6 | 76,266.7 a | 9628.3 a | 56,661.7 a | 3218.5 | 52.2 a |
| AH-ROB | 248.0 b | 9726.7 b | 94.5 | 65,616.7 a | 9315.0 a | 71816.7 a | 2879.2 | 50.1 a |
| Sig. | * | * | ns | ** | *** | ** | ns | ns |
| IR | ns | ns | ns | ns | ns | ns | ns | ns |
| SP | * | ** | ns | *** | *** | *** | ns | * |
| I | ns | ns | ns | ns | ns | ns | ns | ns |
| CANOPY (cm) | |||
|---|---|---|---|
| Height (H) | Width (W) | H × W | |
| CM-RWW | 24.44 b | 28.94 b | 770.50 c |
| CM-ROB | 28.72 b | 33.94 b | 1008.17 c |
| AH-RWW | 65.94 a | 104.56 a | 6880.00 a |
| AH-ROB | 57.72 a | 97.72 a | 5723.67 ab |
| *** | *** | *** | |
| IR | ns | ns | ns |
| SP | *** | *** | *** |
| I | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Bellot, M.J.; Lorente, B.; Ortuño, M.F.; Medina, S.; Gil-Izquierdo, Á.; Bañón, S.; Sánchez-Blanco, M.J. Recycled Wastewater and Reverse Osmosis Brine Use for Halophytes Irrigation: Differences in Physiological, Nutritional and Hormonal Responses of Crithmum maritimum and Atriplex halimus Plants. Agronomy 2021, 11, 627. https://doi.org/10.3390/agronomy11040627
Gómez-Bellot MJ, Lorente B, Ortuño MF, Medina S, Gil-Izquierdo Á, Bañón S, Sánchez-Blanco MJ. Recycled Wastewater and Reverse Osmosis Brine Use for Halophytes Irrigation: Differences in Physiological, Nutritional and Hormonal Responses of Crithmum maritimum and Atriplex halimus Plants. Agronomy. 2021; 11(4):627. https://doi.org/10.3390/agronomy11040627
Chicago/Turabian StyleGómez-Bellot, María José, Beatriz Lorente, María Fernanda Ortuño, Sonia Medina, Ángel Gil-Izquierdo, Sebastián Bañón, and María Jesús Sánchez-Blanco. 2021. "Recycled Wastewater and Reverse Osmosis Brine Use for Halophytes Irrigation: Differences in Physiological, Nutritional and Hormonal Responses of Crithmum maritimum and Atriplex halimus Plants" Agronomy 11, no. 4: 627. https://doi.org/10.3390/agronomy11040627
APA StyleGómez-Bellot, M. J., Lorente, B., Ortuño, M. F., Medina, S., Gil-Izquierdo, Á., Bañón, S., & Sánchez-Blanco, M. J. (2021). Recycled Wastewater and Reverse Osmosis Brine Use for Halophytes Irrigation: Differences in Physiological, Nutritional and Hormonal Responses of Crithmum maritimum and Atriplex halimus Plants. Agronomy, 11(4), 627. https://doi.org/10.3390/agronomy11040627








