Evaluating Wild Germplasm Introgression into Autotetraploid Blueberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotypic Evaluations
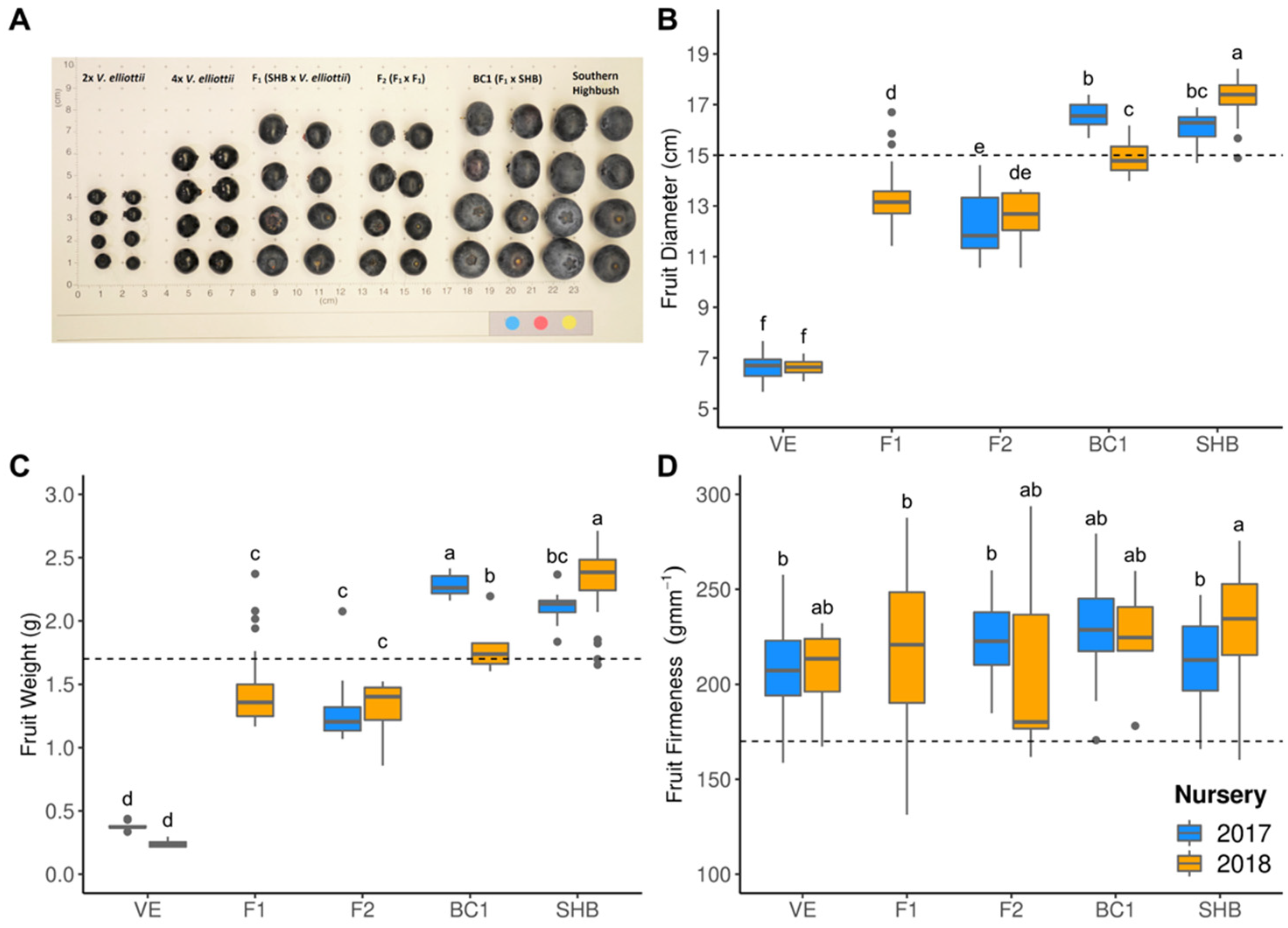
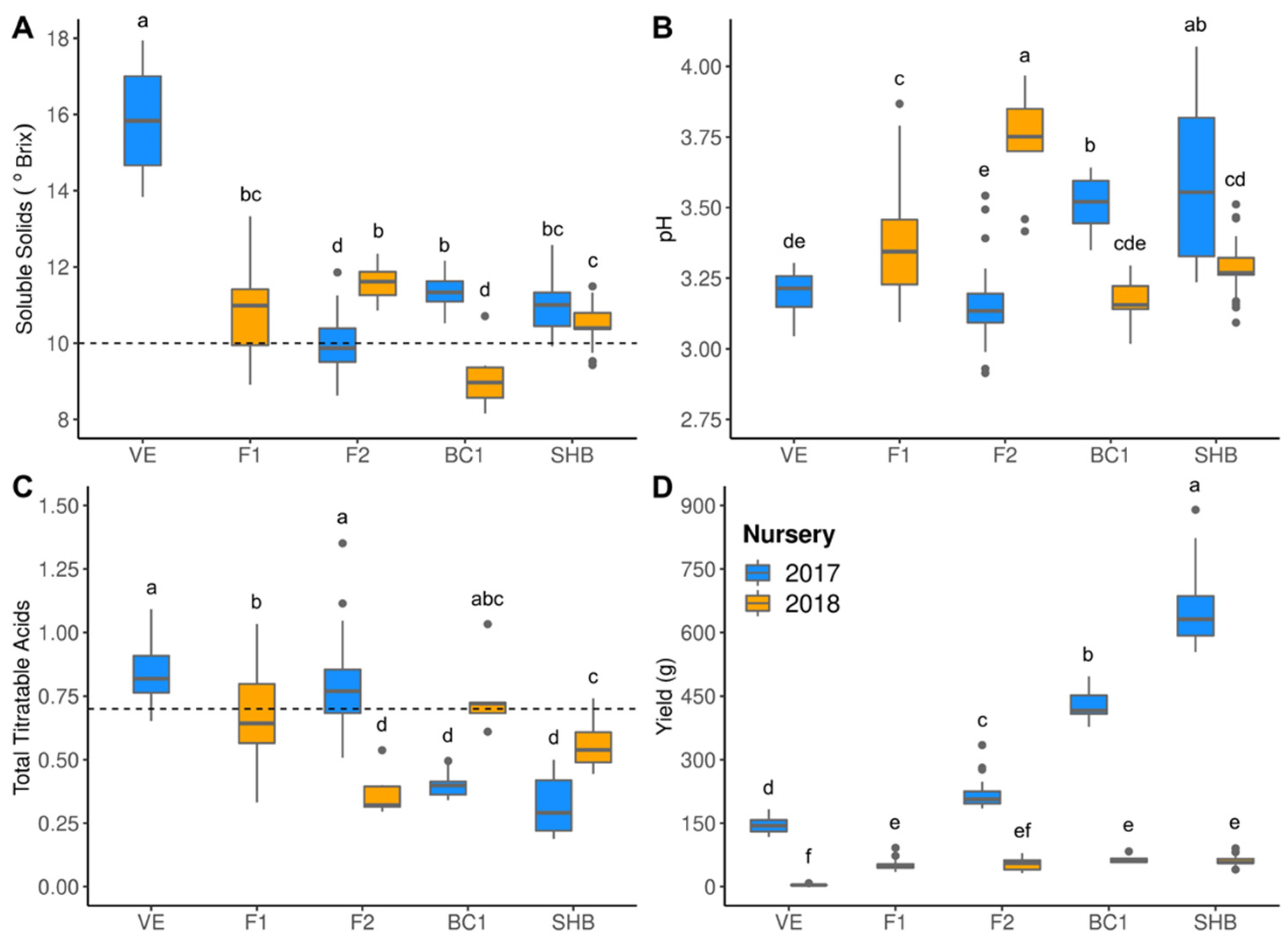
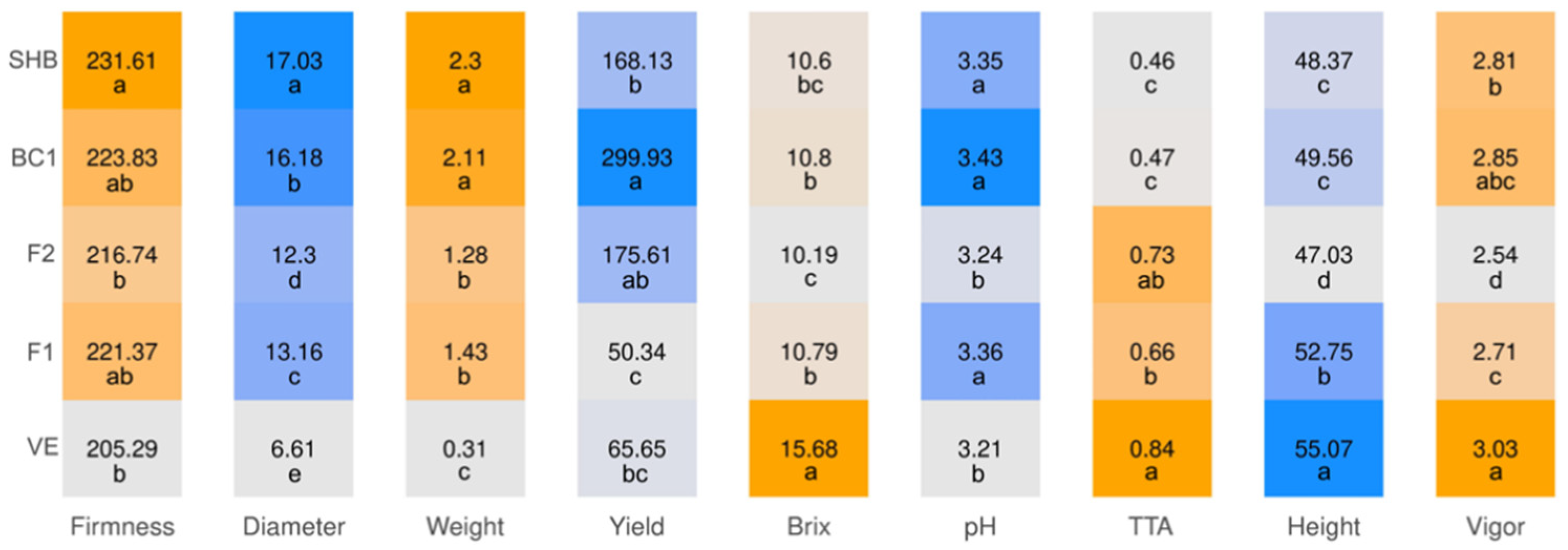
3. Results
3.1. Population Genetics
3.2. Hybrid Performance
4. Discussion
4.1. Interspecific Hybridization in Blueberry
4.2. The Use of Vaccinium elliottii as a Genetical Resource
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 May 2020).
- Finn, C.E.; Hancock, J.F.; Olmstead, J.W.; Brazelton, D.M. Welcome to the party! Blueberry breeding mixes private and public with traditional and molecular to create a vibrant new cocktail. Acta Hortic. 2014, 1017, 51–62. [Google Scholar] [CrossRef]
- Fang, Y.; Nunez, G.H.; da Silva, M.N.; Phillips, D.A.; Munoz, P.R. A Review for Southern Highbush Blueberry Alternative Production Systems. Agronomy 2020, 10, 1531. [Google Scholar] [CrossRef]
- Lyrene, P.M. Low-chill highbush blueberries. Fruit Var. J. 1990, 44, 82–86. [Google Scholar]
- Cellon, C.; Amadeu, R.R.; Olmstead, J.W.; Mattia, M.R.; Ferrao, L.F.V.; Munoz, P.R. Estimation of genetic parameters and prediction of breeding values in an autotetraploid blueberry breeding population with extensive pedigree data. Euphytica 2018, 214, 1–13. [Google Scholar] [CrossRef]
- Esquinas-Alcázar, J. Protecting crop genetic diversity for food security: Political, ethical and technical challenges. Nat. Rev. Genet. 2005, 6, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Lyrene, P.M. Value of various taxa in breeding tetraploid blueberries in Florida. Euphytica 1997, 94, 15–22. [Google Scholar] [CrossRef]
- Sharpe, R. Horticultural Development of Florida Blueberries. Proc. Florida State Hort. Soc. 1954, 66, 188–190. [Google Scholar]
- Galletta, G.J. Blueberries and Cranberries. In Advances in Fruit Breeding; Purdue University Press: West Lafayette, IN, USA, 1975; pp. 154–196. [Google Scholar]
- Retamales, J.B.; Hancock, J.F. Blueberries—Jorge B Retamales, James F Hancock—Google Livros, 2nd ed.; CABI: Boston, MA, USA, 2018; ISBN 9781780647265. [Google Scholar]
- Norden, E.H.; Lyrene, P.M.; Chaparro, J.X. Ploidy, Fertility, and Phenotypes of F1 Hybrids between Tetraploid Highbush Blueberry Cultivars and Diploid Vaccinium elliottii. HortScience 2020, 55, 281–286. [Google Scholar] [CrossRef]
- Cappai, F.; Benevenuto, J.; Ferrão, L.; Munoz, P. Molecular and Genetic Bases of Fruit Firmness Variation in Blueberry—A Review. Agronomy 2018, 8, 174. [Google Scholar] [CrossRef]
- Fellers, J.P.; Matthews, A.; Fritz, A.K.; Rouse, M.N.; Grewal, S.; Hubbart-Edwards, S.; King, I.P.; King, J. Resistance to wheat rusts identified in wheat/Amblyopyrum muticum chromosome introgressions. Crop Sci. 2020, 1957–1964. [Google Scholar] [CrossRef]
- Brar, D.S.; Khush, G.S. Alien introgression in rice. Plant Mol. Biol. 1997, 35, 35–47. [Google Scholar] [CrossRef]
- Wang, L.; Yang, A.; He, C.; Qu, M.; Zhang, J. Creation of new maize germplasm using alien introgression from Zea mays ssp. mexicana. Euphytica 2008, 164, 789–801. [Google Scholar] [CrossRef]
- Dweikat, I.M.; Lyrene, P.M. Induced Tetraploidy in a Vaccinium elliottii Facilitates Crossing with Cultivated Highbush Blueberry. J. Am. Soc. Hortic. Sci. 1991, 116, 1063–1066. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Sherman, W.B. Mitotic Instability and 2n Gamete Production in Vaccinium corymbosum X Vaccinium elliottii Hybrids. J. Am. Soc. Hortic. Sci. 1983, 108, 339–342. [Google Scholar]
- Brevis, P.A.; Bassil, N.V.; Ballington, J.R.; Hancock, J.F. Impact of Wide Hybridization on Highbush Blueberry Breeding. J. Am. Soc. Hortic. Sci. 2008, 133, 427–437. [Google Scholar] [CrossRef]
- Lyrene, P. The Use of Vaccinium elliottii Chapmn. in Breeding Highbush Blueberry. In Proceedings of the North American Blueberry Research and Extension Workers Conference; Rutgers University: New Brunswick, NJ, USA, 2014. [Google Scholar]
- NCGR-Corvallis Vaccinium Catalog: Blueberry Cultivars and Selections. Available online: http://www.xn--arsgrin-706c.gov/cor/catalogs/vactc.html (accessed on 1 March 2021).
- Brooks, R.M.; Olmo, H.P. Register of New Fruit and Nut Varieties; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1952. [Google Scholar]
- Brooks, R.M.; Olmo, H.P. Register of New Fruit and Nut Varieties. HortScience 1997, 32, 785–805. [Google Scholar] [CrossRef]
- Amadeu, R.R.; Cellon, C.; Olmstead, J.W.; Garcia, A.A.F.; Resende, M.F.R.; Muñoz, P.R. AGHmatrix: R Package to Construct Relationship Matrices for Autotetraploid and Diploid Species: A Blueberry Example. Plant Genome 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Kerr, R.J.; Li, L.; Tier, B.; Dutkowski, G.W.; McRae, T.A. Use of the numerator relationship matrix in genetic analysis of autopolyploid species. Theor. Appl. Genet. 2012, 124, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- De los Campos, G.; Rodriguez, P.P. The Comprehensive R Archive Network. BGLR: Bayesian Generalized Linear Regression 2016. Software User Manual. Available online: https://cran.r-project.org/web/packages/BGLR/BGLR.pdf (accessed on 22 May 2020).
- Pérez, P.; De Los Campos, G. Genome-wide regression and prediction with the BGLR statistical package. Genetics 2014, 198, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Least-Squares Means: The {R} Package {lsmeans}. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Ag, N.P.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis—Hadley Wickham—Google Books; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319242774. [Google Scholar]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/index.html (accessed on 2 December 2020).
- D’Hont, A. Unraveling the genome structure of polyploids using FISH and GISH; examples of sugarcane and banana. Cytogenet. Genome Res. 2005, 109, 27–33. [Google Scholar] [CrossRef] [PubMed]
- D’hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Fang, L.; Zhang, Z.; Ma, W.; Niu, Y.; Ju, L.; Deng, J.; Zhao, T.; Lian, J.; et al. Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat. Genet. 2019, 51, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, Z.; Hu, H.; Ye, X.; Zheng, Z.; Wei, Y.; Zheng, Y.; Wang, Y.; Liu, C. Differentiating homoploid hybridization from ancestral subdivision in evaluating the origin of the D lineage in wheat. New Phytol. 2020, 228, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.Q.; Qian, K.X.; Xue, G.P.; Wu, Z.C.; Chen, Y.L.; Yan, Q.S.; Zhang, X.Q.; Wu, P. Production of bacterial blight resistant lines from somatic hybridization between Oryza saliva L. and Oryza meyeriana L. J. Zhejiang Univ. Sci. 2004, 5, 1199–1205. [Google Scholar] [CrossRef][Green Version]
- Snowdon, R.J. Cytogenetics and genome analysis in Brassica crops. Chromosom. Res. 2007, 15, 85–95. [Google Scholar] [CrossRef]
- Louwes, K.M.; Hoekstra, R.; Mattheij, W.M. Interspecific hybridization between the cultivated potato Solanum tuberosum subspecies tuberosum L. and the wild species S. circaeifolium subsp. circaeifolium Bitter exhibiting resistance to Phytophthora infestans (Mont.) de Bary and Globodera pallida (Stone) Behrens—2. Sexual hybrids. Theor. Appl. Genet. 1992, 84, 362–370. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Figlan, S.; Shimelis, H.; Mondal, S.; Tsilo, T.J. Genetic resources and breeding methodologies for improving drought tolerance in wheat. J. Crop Improv. 2017, 31, 648–672. [Google Scholar] [CrossRef]
- Gaston, A.; Osorio, S.; Denoyes, B.; Rothan, C. Applying the Solanaceae Strategies to Strawberry Crop Improvement. Trends Plant Sci. 2020, 25, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Peris, E.; Cortés-Olmos, C.; Díez-Díaz, M.; González-Mas, M.C.; de Luis-Margarit, A.; Fita, A.; Rodríguez-Burruezo, A. Hybridization in Peppers (Capsicum spp.) to Improve the Volatile Composition in Fully Ripe Fruits: The Effects of Parent Combinations and Fruit Tissues. Agronomy 2020, 10, 751. [Google Scholar] [CrossRef]
- Coville, F.V. Improving the wild blueberry. Improv. Wild Blueberry 1937, 559–574. [Google Scholar]
- Ballington, J.R. The deerberry [Vaccinium starnineurn L. Vaccinium Section Polycodium (Raf.) Sleumer]: A potential new small fruit crop. J. Small Fruit Vitic. 1996, 3, 21–28. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Olmstead, J.W. The Use of Inter-Sectional Hybrids in Blueberry Breeding. Int. J. Fruit Sci. 2012, 12, 269–275. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Ballington, J.R. Wide hybridization in vaccinium. HortScience 1986, 21, 52–57. [Google Scholar]
- Hancock, J.F.; Lyrene, P.; Finn, C.E.; Vorsa, N.; Lobos, G.A. Blueberries and Cranberries. In Temperate Fruit Crop Breeding; Springer: Cham, Switzerland, 2008; p. 149. [Google Scholar]
- Retamales, J.B.; Hancock, J.F. Blueberries; (Crop Production Science in Horticulture Book 21); Atherton, J., Ed.; CABI: Cambridge, UK, 2012; ISBN 1845938267. [Google Scholar]
- Sharpe, R.H.; Darrow, G.M. Breeding blueberries for the Florida climate. Proc. Florida State Hortic. Soc. 1960, 72, 308–311. [Google Scholar]
- Ballington, J.R. Germplasm resources available to meet future needs for blueberry cultivar improvement. Fruit Var. J. 1990, 44, 54–62. [Google Scholar]
- Lyrene, P.M. Breeding Southern Highbush Blueberries in Florida. Acta Hortic. 2002, 149–152. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Stafne, E.T.; DeVetter, L.W.; Zhang, Q.; Li, C.; Takeda, F.; Williamson, J.; Yang, W.Q.; Cline, W.O.; Beaudry, R.; et al. Blueberry Producers’ Attitudes toward Harvest Mechanization for Fresh Market. Horttechnology 2018, 28, 10–16. [Google Scholar] [CrossRef]
- Moggia, C.; Graell, J.; Lara, I.; González, G.; Lobos, G.A. Firmness at Harvest Impacts Postharvest Fruit Softening and Internal Browning Development in Mechanically Damaged and Non-damaged Highbush Blueberries (Vaccinium corymbosum L.). Front. Plant Sci. 2017, 8, 535. [Google Scholar] [CrossRef]
- Hanson, E.J.; Beggs, J.L.; Beaudry, R.M. Applying Calcium Chloride Postharvest to Improve Highbush Blueberry Firmness. HortScience 1993, 28, 1033–1034. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Moggia, C.E.; Retamales, J.B.; Hancock, J.F. Quality of Ivanhoe and Bluecrop blueberry fruit transported by air and sea from Chile to North America. HortScience 1998, 32, 313–317. [Google Scholar]
- Gilbert, J.L.; Guthart, M.J.; Gezan, S.A.; de Carvalho, M.P.; Schwieterman, M.L.; Colquhoun, T.A.; Bartoshuk, L.M.; Sims, C.A.; Clark, D.G.; Olmstead, J.W. Identifying Breeding Priorities for Blueberry Flavor Using Biochemical, Sensory, and Genotype by Environment Analyses. PLoS ONE 2015, 10, e0138494. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, S.R.; Harker, R.; Triggs, C.M.; Gunson, A.; Campbell, R.L.; Jackman, R.; Requejo-Jackman, C. Determining Consumer Purchase Intentions: The Importance of Dry Matter, Size, and Price of Kiwifruit. J. Food Sci. 2011, 76, S177–S184. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Benevenuto, J.; Oliveira, I.d.B.; Cellon, C.; Olmstead, J.; Kirst, M.; Resende, M.F.R.; Munoz, P. Insights Into the Genetic Basis of Blueberry Fruit-Related Traits Using Diploid and Polyploid Models in a GWAS Context. Front. Ecol. Evol. 2018, 6, 107. [Google Scholar] [CrossRef]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Magazin, N.; Miodragović, M.; Popara, G. Bioregulators can improve fruit size, yield and plant growth of northern highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2018, 235, 214–220. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A Review of the Fruit Volatiles Found in Blueberry and Other Vaccinium Species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef] [PubMed]
- Ferrão, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef] [PubMed]

| Trait | h2 | ||||
|---|---|---|---|---|---|
| Fruit firmness (g mm−1) | 1118.55 (±345.90) | 357.22 (±209.13) | 768.34 (±202.15) | 0.66 | 0.50 |
| Fruit diameter (mm) | 1.86 (±0.44) | 9.91 (±6.01) | 1.77 (±0.28) | 0.87 | 0.14 |
| Fruit weight (g) | 0.14 (±0.05) | 0.33 (±0.22) | 0.17 (±0.03) | 0.74 | 0.22 |
| Total yield (g) | 7080.91 (±2816.68) | 30494.15 (±19,257.27) | 14211.86 (±2282.682) | 0.73 | 0.14 |
| °Brix | 2.03 (±0.69) | 2.98 (±1.90) | 1.47 (±0.40) | 0.77 | 0.31 |
| pH | 0.05 (±0.01) | 0.04 (±0.03) | 0.03 (±0.01) | 0.77 | 0.41 |
| TTA | 0.05 (±0.01) | 0.04 (±0.02) | 0.04 (±0.01) | 0.70 | 0.38 |
| Plant height (cm) | 122.30 (±38.86) | 143.15 (±95.22) | 235.30 (±22.27) | 0.53 | 0.24 |
| Plant vigor (1–5 scale) | 1.04 (±0.27) | 0.69 (±0.46) | 0.67 (±0.14) | 0.72 | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabezas, D.; de Bem Oliveira, I.; Acker, M.; Lyrene, P.; Munoz, P.R. Evaluating Wild Germplasm Introgression into Autotetraploid Blueberry. Agronomy 2021, 11, 614. https://doi.org/10.3390/agronomy11040614
Cabezas D, de Bem Oliveira I, Acker M, Lyrene P, Munoz PR. Evaluating Wild Germplasm Introgression into Autotetraploid Blueberry. Agronomy. 2021; 11(4):614. https://doi.org/10.3390/agronomy11040614
Chicago/Turabian StyleCabezas, Diego, Ivone de Bem Oliveira, Mia Acker, Paul Lyrene, and Patricio R. Munoz. 2021. "Evaluating Wild Germplasm Introgression into Autotetraploid Blueberry" Agronomy 11, no. 4: 614. https://doi.org/10.3390/agronomy11040614
APA StyleCabezas, D., de Bem Oliveira, I., Acker, M., Lyrene, P., & Munoz, P. R. (2021). Evaluating Wild Germplasm Introgression into Autotetraploid Blueberry. Agronomy, 11(4), 614. https://doi.org/10.3390/agronomy11040614







