Abstract
Sweet basil (Ocimum basilicum), a highly valuable medicinal crop, is extremely susceptible to Meloidogyne incognita infestation, leading to severe losses in yield and chemo-pharmaceutical quality. Currently, chemical nematicides are the only effective option for the disease management. However, high toxicity to non-target organisms and adverse impact on soil health motivated the look for ecofriendly alternatives. Here, bioinoculants (Bacillus megatarium (“BM”), B. subtilis (“BS”)) were isolated from the rhizosphere of various medicinal and aromatic plants growing in Lucknow region, India. Their biocontrol effects were studied in O. basilicum plants that were invaded by M. incognita either in single or in consortium with Trichoderma harzianum (“TH”) and their efficiency compared to chemical nematicide (carbofuran) and a microbial reference strain (Pseudomonas fluorescens). The results show that all bioinoculants enhanced the growth and oil yield production and increased the nutrient content of O. basilicum by significantly reducing M. incognita infestation by 46 to 72%. Among the strains, a consortium of BM and TH was the most potent treatment. The efficiency of these bioinoculants was not restricted to sterile soil condition but remained high also in natural soil conditions, indicating that enriching soils with rhizospheric microbes can be an effective alternative to chemical nematicides.
1. Introduction
Nematode management strategies are long-sought goals of plant pathologists. Due to the unavailability and high cost of efficient biological nematicides, farmers in developing countries such as in India often use synthetic chemical nematicides [1,2,3]. However, wide use of chemical nematicides impacts non-target organisms and is hazardous to the environment [1]. Long-term use of such chemical nematicides alters the soil microbial flora and impacts soil health, resulting in declining productivity. Thus, identification and development of effective non-synthetic nematicide is highly desired. Ocimum basilicum (CIM Soumya), commonly known as sweet basil, is a potent therapeutic plant which bears large variety of medicinal and aromatic compounds [4,5]. Sweet basil has a long history in traditional systems of medicine and has found regular usages as a flavoring agent, and as supplementary treatment for stress, aches, inflammations, asthma and diabetes [6]. Basil is also part of many kinds of food preparations in Mediterranean countries [7]. Ocimum species contains economically valuable essential oil rich in phenolic compounds and a wide array polyphenols [8]. Some of which act as antiviral, antimicrobial and anti-cancer agents [9,10] or used in the toiletry, perfumery and cosmetics industries [7]. The major active components of the oil produced by O. basilicum are the phenylpropanoids methyl chavicol (MC, chemically known as 1-methoxy-4-prop-2-enylbenzene, estragole, or p-allylanisole) [10,11]. Lower concentrations of other hydrophobic molecules can also be found in the oil (1,8 cineole, linalool, eugenol, methyl cinnamate, β-caryophyllene and methyl isoeugenol), adding to its economic importance [5,12]. Because of its high economic value and industrial importance, sweet basil has been introduced as a commercial crop in many countries, including India [13].
The commercial yield of sweet basil is threatened by infestation of root knot nematode, Meloidogyne incognita (Kofoid and White) Chitwood [3,14]. The infestation of the parasitic nematodes causes a drastic reduction in the herb and oil yield by influencing host nutrient content, and eventually compromises the growth of the plants [15]. Currently, there are no effective eco-friendly nematicides for basil, and the only easily available options to growers remains the chemical nematicides. Thus, the development of eco-friendly nematicides safe to the environment receives a lot of attention [3,16]. One promising direction is the development of biological nematicides that are based on antagonist rhizospheric microbes. In the last few decades, researchers have shown that rhizospheric bioinoculants which are based on them are highly potent against several soil borne plant pathogens such as root knot nematodes on a variety of agricultural crops, including medicinal and aromatic plants [1,5,16,17]. However, biocontrol activity often exhibits high inconsistency, since a potent microbe from one agro-climatic zone may not work effectively under other agro-climatic conditions due to the differences in the physical and chemical properties between soil environments. Thus, characterization and use of indigenous microbes from local suppressive soils is required for an effective and reliable management of root knot nematode [18].
Here, a greenhouse experiment was conducted to study the effects of various indigenous rhizospheric microbial strains on sweet basil plants growing in Northern India that were invaded by M. incognita.
To this end, 165 bacterial isolates were isolated from the rhizospheres of local medicinal plants and picked the most potent isolates based on biochemical screening. Then, basil plants were inoculated with these indigenous rhizospheric microbes, and nematode activity, plant growth, nutrient content and oil yield were measured. The efficiency of these strains was compared to that of carbofuran, a commercial chemical nematicide, and to a common microbial biocontrol agent as a reference strain Pseudomonas fluorescens (“PF”).
Here we hypothesized that indigenous rhizospheric microbes in consortium would reduce the activity of root knot nematodes and enhance plant growth and oil yield in comparison to non-treated plants, but also to plants that were inoculated with a non-indigenous reference strain and or plants treated with chemical nematicides.
2. Material and Methods
Overview of methodological approach for nematode management on sweet basil.
Figure 1 presents a summary of the principle methodological steps used to isolate, screen and identify potent indigenous microbial bioinoculants and examine their management of root knot nematode in basil plants. A detailed description of each of the steps is given below.
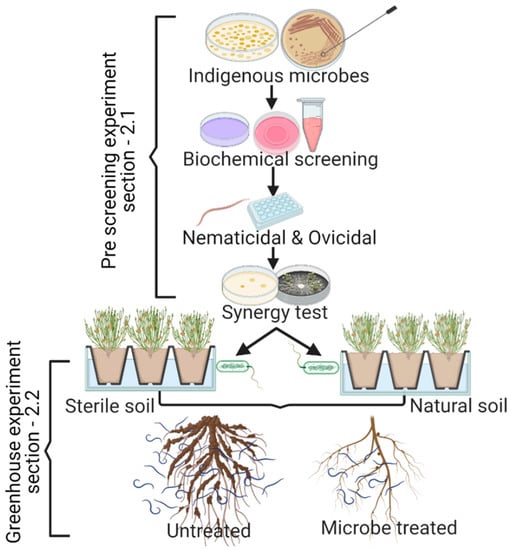
Figure 1.
Schematic diagram of the microbial management of root knot nematode via indigenous microbes isolated from suppressive soils. The diagram shows the methodological steps we used in this study. In the first steps we isolated indigenous microbes, selected the most potent ones based on biochemical screening, and identified the gnomically. Then, in vitro nematicidal activity and synergy tests were done. In the second step we inoculated the selected microbes into the rhizosphere of basil plants in a greenhouse to examine the management of root knot nematode. The figure was created with the help of online illustration app BioRender.com.
2.1. Prescreening Experiments
2.1.1. Isolation of Bioagents
Plant growth promoting rhizobacteria (“PGPR”) strains were isolated from the root vicinity of medicinal and aromatic plants (“MAP’s”) cultivated in 12 agricultural sites around the city of Lucknow (Banthra, Barabanki, Bachhrawan, Goshainganj, Hardoi, Itaunja, Kukrial, Mahmudabad, Malihabad, Mohanlalganj, Purwa and Sidhauli), a major basil growing area in the state of Uttar Pradesh, India and from Council of Scientific and Industrial Research–Central Institute of Medicinal and Aromatic Plants (CSIR-CIAMP) field study center (Table S1). The sites are located 131 m elevation above sea level and the region has semiarid sub-tropical climate with an average annual rainfall of 1000 mm (https://en.climate-data.org/asia/india/uttar-pradesh/Lucknow). Most of the strains were isolated from the rhizosphere of Ocimum spp. (Ocimum americanum, O. basilicum, O. gratissimum, O. kilimandscharicum, O. sanctum, Table S1).
2.1.2. Biochemical Characterization, Plant Growth Promoting (“PGP”) Activity and Selection of Bioinoculants
In total, 165 distinctly looking colonies were picked and purified from the total isolated strains of rhizospheric microbes. The picked isolates were subjected to biochemical and PGP screening based on their ability to produce hydrolytic enzymes such as chitinases, cellulases, phosphatases, pectinases, gelatinases, L-asparaginase, siderophore, etc. The production of these enzymes is indicative of a beneficial relationship between bacteria and plants [19] and may reflect their ability to effectively manage root knot nematodes [1]. The qualitative screening of extracellular enzymes was performed by following the methods described by Guerrieri et al. [20] with modifications applicable for conducting assays on petriplates.
Cellulose and Protease Activity
The cellulase activity was examined by inoculating the overnight grown culture on carboxymethyl cellulose (“CMC”) agar (Himedia, India). After 3 days of incubation at 28 ± 2 °C, the culture plates were stained with 2% of congo red solution for 5 min followed by de-staining through washing them with 1 M Sodium chloride solution. The presence of a clear holo zone around the colony indicates the positive cellulase activity. For estimating protease activity, freshly overnight grown cultures were inoculated on Glycerol Casein Agar (“GCA”) and TriButyrin (“TB”) agar medium (Himedia, India), respectively, and incubated for 4 days at 28 ± 2 °C. The presence of a clear holo zone around the colony indicates the positive protease activity.
Estimation of Chitinases and Pectinases Activity
To estimate chitinases and pectinases activity, the culture media was prepared as per the protocols of Tran et al. [21] with pectin and chitin as substrates. The overnight grown cultures on nutrient agar plates were spot inoculated on the prepared pectin and chitin plates and incubated for 4 days at 28 ± 2 °C. For assessment of pectinase and chitinases activity, the pectin cultures plates were flooded with 5.0 mL of 1% (w/v) CTAB (Hexadecyl Trimethyl Ammonium Bromide) solution and incubated at room temperature for 15 min and further decanted. The clearance of halo zone indicates positive activity for pectinase and chitinases enzymes.
Gelatinase and Asparaginase Activity
Gelatinase activity was assessed on agar plates with gelatin as a substrate (1%). The cavity was prepared in the medium through the help of cork borer. Furthermore, the prepared cavity was filled with 0.2 mL of bacterial suspension and incubated for 4 days at 28 ± 2 °C. After 4 days the gelatinase activity was determined by pouring of 5.0 mL of saturated solution of ammonium sulfate [(NH4)2SO4] and decanting. The halo zone of clearance was indicative of positive activity. The L-asparaginase activity was assessed on modified czapek dox agar supplemented with L-asparagine. Spot inoculation was performed on the prepared plates and incubated for 4 days at 28 ± 2 °C. The Asparaginase production was observed with formation of a pink color change of the media from the initial yellow.
PGP Activity
The qualitative screening of PGP activities, such as indole production (“IAA”), phosphate solubilization and siderophore production, was carried out for the isolated bacteria as per the protocols described by Guerrieri et al. [20]. The production of IAA activity was evaluated by transferring overnight grown cultures to culture tubes and which were incubated at 28 ± 2 °C on a rotary shaker at 200 rpm. At the end of the 3rd day of incubation, 1 mL supernatant was separated by centrifugation and mixed with 1 mL of Salkowski reagent. The appearance of stable pink color showed positive IAA production [20,22]. For phosphate solubilizing (phosphatase activity) and sidrophore production activity freshly grown cultures of isolates were spot transferred on the culture plates containing Pikovskaya’s (“PVK”) agar medium (HiMedia, Bengaluru, India) and CAS (Chrome Azurol Sulphonat) agar plates prepared as per the methods of Schwyn and Neilands [23]. The plates were incubated at 28 ± 2 °C for 7 days. The appearance of clear zone around the growing colony in PVK indicates positive phosphate solubilization activity [20,24], whereas siderophore production was examined by observing the development of deep blue to yellow or orange color zone around the colony [23]. On the basis of biochemical and in vitro PGP screening, 10 strains (RPN 1, RPN 2, RPN 3, RPN 4, RPN 5, MTN 2, MTN 100, MTN 101, MTN K4, and MTN 121) were selected (Tables S2 and S3, Figure S1) for further prescreening of nematode management potentials in basil plants (see—Section 3.1.2).
2.1.3. Prescreening of Bioinoculants for the Management of M. incognita in Basil Plants
For conducting the prescreening experiment for the 10 selected strains, root knot nematodes (M. incognita, “Mi”) were obtained from pure cultures of Mi and maintained on the roots of Brinjal (Solanum melongena L.) at CSIR-CIMAP research farm center until the initiation of the experiment. The plants that showed infested symptoms were uprooted from the soil with their root systems intact and dipped in water to remove adhering soil and dirt particles. Nematode eggs were extracted from severely galled roots, using a modified extraction technique of Hussey and Barker [25]. The eggs obtained were incubated for hatching following similar procedure as reported by Saikia et al. [1]. The population density of hatched second stage juveniles (J2) was determined from three replications of 1 mL aliquots of inoculum suspension counted under stereoscopic microscope [2].
To assess the efficacy of the microbial bioagents for management of root knot nematode and plant growth promotion activity the strains were first prepared for inoculation. A single colony of each of the selected strains was picked from a plate cultured on nutrient agar, inoculated in the flasks containing nutrient broth and incubated at 28 ± 2 °C on an orbital shaker at 200 rpm. After 48 h, the culture was harvested and centrifuged at 7168 RCF for 5 min [5]. For priming and inoculation of bacterial strains, the CFU/mL of overnight grown bacterial cultures was adjusted to 1.2 × 106 (CFU) mL−1 [5] in normal saline using spectrophotometer (Spectra Max M2 multimode micro plate reader Molecular Devices) at 610 nm [1]. Then, small pots (12 cm × 15 cm) were filled with 1.5 kg of sterile soil (autoclaved two times) obtained from CIMAP research farm and mixed with vermicompost in a 3:1 ratio. The pots were treated in single inoculations with each of the selected strains (RPN 1, RPN 2, RPN 3, RPN 4, RPN 5, MTN 2, MTN 100, MTN 101, MTN K4, MTN 121). Three control treatments without inoculation of the selected bacterial strains were used: untreated control, CL), untreated Mi inoculated control (CL + Mi) and untreated Mi inoculated control treated with the chemical nematicide carbofuran (Carbofuran + Mi, Table 1). O. basilicum (CIM-Soumya, Gene bank no. CIMAP-1133) was provided by farm management of CSIR-CIAMP, India. Healthy looking basil seeds were sorted and incubated for priming with the microbial strains for 1 h with adjusted bacterial density at 1.2 × 106 (CFU) mL−1 [5] in normal saline. Three primed seeds were sown at each pot and were grown in a greenhouse under natural light conditions, an average temperature of about 28 ± 2 °C and relative humidity of 65–70%. On emergence of seedlings, the pots were thinned to one plant per pot and treated again with the same bioagents to maintain effective bacterial population (bacterial density at 1.2 × 106 (CFU) mL−1). One week after microbial inoculation, the pure culture of root knot nematode (J2 stage) was inoculated to all of the pots (1000 Mi/pot) except the untreated control (CL). The experiment was terminated 60 days after the inoculation of Mi. All plant growth parameters (plant height, canopy, root knot index, plant dry and fresh weight) were recorded. Each treatment was replicated seven times.

Table 1.
Description of the treatments and abbreviations used in the study.
2.1.4. Molecular Identification of the Selected Strains
Genomic DNA were extracted from two bacterial strains which showed most potent biopotentials in basil plants under the prescreening experiment (MTN K4 and RPN-3, see results). The DNA was extracted from overnight grown culture using ZR fungal/Bacterial DNA kit (Zymo Research, Irvine, CA, USA). The forward primer (5′AGAGTTTGATCCTGGCTCAG-3′) and reverse (5′AAGGAGGTGATCCAGCCGCA-3′) primers were used to amplify the 16S rRNA gene fragment [26]. For PCR amplification, 2 μL (approximately 25 ng) of the genomic DNA was added to 10 pmol of each primer, 50 μmol dNTPs, 0.7 U of TaqDNA polymerase (Bangalore Genie, Bengaluru, India) and 10 μL of 10×buffer (Bangalore Genie, Bengaluru, India); reaction volume was made up to 100 μL with nuclease-free water. The amplified PCR product was analyzed by electrophoresis (15 V/cm; 60 min) on 0.8% horizontal agarose gels. Then, the PCR products were cleaned using a QIAquick spin column (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The purified PCR products were sequenced by ABI 3130xl analyzer based on Sangers Dideoxy termination method [27]. The gene sequences obtained were subjected to homology search using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) to identify and download the nearest neighbor sequences from the NCBI database. All the sequences were aligned using ClustalW alignment tool accessed through the MEGA 5.0 software [28]. The phylogenetic tree was constructed using the UPGMA method from MEGA5 [27]. The BLAST analysis showed MTN K4 and RPN-3 belongs to the genus Bacillus and exhibited 100%, and 99% similarity with Bacillus subtilis (MTN K4), and Bacillus megaterium (RPN-3, details are given in the result Section 3.1.3).
2.1.5. In Vitro Nematicidal and Ovicidal Activity of Bioinoculants
The ovicidal and nematicidal activity was assessed for cell free culture filtrates [29] of B. subtilis (BS), Bacillus megatarium (BM), along with Trichoderma harzianum (TH, a well-established fungal bioinoculants for nematode) and P. fluorescens (PF, a common biocontrol agent which we have used as a bacterial reference strain [1]). TH (KF157964) was isolated form the rhizosphere of Mentha (Table S3) and preserved in our department at CSIR-CIMAP [2], while PF strains ((ATCC (13525)) were gifted by the Microbial repository, Biological central facility CSIR-CIMAP (please see Acknowledgments) [1,30]. The protocols of Saikia et al. [1] and Tiwari et al. [5] were followed for the assessment ovicidal and nematicidal activity. The pure culture of Mi was obtained from roots of Solanum melongena L. by manually picking egg masses and were either directly used for ovicidal activity or allowed to hatch for performing nematicidal activity [1]. The freshly isolated eggs were counted under stereoscopic microscope (Leica S8AP0), adjusting eggs concentration to 5000/mL using distill water. 10 μL (i.e., ~50 eggs) from the adjusted egg solution were added to 250 μL of cell free culture filtrates (100% crude metabolites) in a 24-well microtiter (TCP-24T-ST) Tissue Culture Plate (Axygen Life sciences India). The plates were then incubated under humidified condition at ambient temperature of 28 °C for 72 h and observed under a stereo microscope (Leica S8AP0) to calculate the % of egg mortality [1]. On reaching J2 stage, the hatched nematode cultures (Mi) were used for assessment of nematicidal activity by adding 10 μL of J2 nematodes to 250 μL of cell free culture filtrates containing 100% crude metabolites in a 24-well microtiter Tissue Culture Plate. The uninoculated media and distill water served as controls. The plates were then incubated under humidified condition at ambient temperature of 28 °C for 72 h. The plate was visualized under a stereo microscope (LeicaS8AP0) at different time intervals (12 h, 24 h, 36 h, 48, h, 60 h and 72 h) to calculate the mortality percentage [5]. Each treatment was repeated three times. Nematodes that were considered dead after not responding when probed with a fine needle [29] were not counted.
2.1.6. In Vitro Synergy Test
To test the in vitro synergistic and antagonistic interactions of the bacterial bioagents (PF, BS, and BM) with TH the method of Lee et al. was followed [31]. In short, both bacterial and fungal bioagents were juxtaposed at a distance from each other on petri dishes containing Potato dextrose agar (“PDA” solid medium with 15% (w/v) agar. The bacteria culture was placed at two equidistant sites while the fungal bioinoculants was inoculated in the center of the petri plate. The inhibitory activity was calculated using the formula: %inhibition = (C − S)/C × 100, where C represents the average of replicates of mycelium extension (cm) in the control and S is the mycelium extension (cm) towards the bacterial colony [31]. The interaction studies revealed that there was no inhibitory zone formation in any of the fungal vs. bacterial interactions (Figure 2) indicating that these microbes are fit to be used in the consortium.
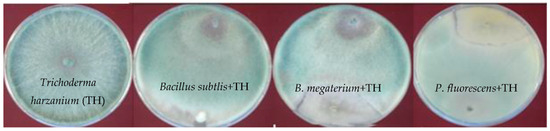
Figure 2.
Photographs showing the synergistic interaction between fungi (T. harzanium) and the bacterial strains. The strains Bacillus subtlis (BS), B. megaterium (BM) and Pseudomonas fluorescens (PF) were juxtaposed against T. harzanium (TH). The bioinoculants assayed for in vitro interactions showed no significant antagonistic activity against each other.
2.2. Greenhouse Experiments
2.2.1. Preparation of Fungal and Bacterial Strains for Rhizospheric Inoculation
T. harzanium (TH) was subcultured from preserved slants on potato dextrose agar plates. After attaining the proper growth, plates were further used for mass multiplication. The fungal inoculants were mass-multiplied by inoculating the Potato dextrose broth (“PDB”) media with small discs, from freshly grown TH plates, incubated in an incubator for 96 h at 28 ± 1 °C in orbital shaking conditions, to allow proper multiplication. After incubation, the mycelial mat with conidia was separated from the broth (using muslin cheese cloth), homogenized and suspended in 500 mL of 0.1 M phosphate buffer (K2HPO4− pH 7.4). The CFU was estimated through hemocytometer [32] and adjusted to CFU (mL−1) density of 1.2 × 106 (5) for inoculation of different treatments. The bacterial inoculations were prepared as described previously in the prescreening process.
2.2.2. Testing the Effects of Microbial Bioinoculants on Nematode Population and Growth Parameters of Basil Plants in a Greenhouse
Basil seeds were primed (in the same manner as described for the prescreening experiment) with the following seven inoculums of bacterial and fungal strains: PF + Mi, BS + Mi, BM + Mi, TH + Mi, PF + TH + Mi, BS + TH + Mi, BM + TH + Mi (Table 1). The treated seeds were transplanted into pots with either sterile or natural soil (24 cm × dia. 30 cm) containing 5 kg soil–vermicompost mixture (3:1) with seven replications (a total of 49 pots in sterile soil and 49 pots in natural soil) along with seven replication of three controls for each soil conditions: untreated control (CL), untreated Mi inoculated control (CL + Mi) and chemical control (Carbofuran + Mi, in a concentration of 2 kg active ingredient (a.i.) per ha; [33]). Two weeks after seeding, the same microbial bioagents were added to the seedlings (as explained for the prescreening experiment). Three weeks after seeding, plants were inoculated with pure culture of M. incognita (10,000 (Mi/pot) by making holes in the rhizospheric vicinity. Pots were kept in a complete randomized block design pattern [34]. Ninety-one days after seeding (or seventy days after Mi inoculation), when plants were at full flowering stage, the experiment was terminated and the data for different growth parameters (plant height, leaf weight, stem weight, number of branches, nematode population, nutrient uptake and oil yield) was recorded.
2.2.3. Estimation of Nematode Management Potentials of Bioinoculants
For the assessment of populations of Mi under the different treatments, soil and root samples from each treatment were taken at the end of the greenhouse experiment and processed by Cobb’s sieving and decanting technique followed by Baermann funnel method [35]. Mi infestation was recorded by counting the number of galls in three random samples out of the 7 replicates of each treatment [5]. Root knot indices (“RKI”) were calculated according to Krusberg and Nielson on a scale of 0–4 [35]. The reproduction factor was assessed by estimating the final populations of nematodes in the infested roots with the same protocols described in our previous work [1]. The root samples from the terminated experiments were gently washed to free the adhered soil and chopped into 1 cm small pieces by scissors. The chopped roots were immersed in a sufficient amount of distilled water and macerated by a waring blender for 1–2 min, for freeing the nematodes (Mi) from the roots. The whole macerated solution was allowed to pass through series of U.S standard sieves with mesh sizes of 100, 200, and 325, and with openings of 149, 74 and 44 μm, respectively. The extracted nematodes were collected and further diluted with distilled water (100 mL). A 1 mL aliquot was taken by micropipette for Mi counting using stereoscopic microscope. The numbers of Mi were determined from mean of three counts. For the estimation of actual frequency and relative density of Mi in pot soils, 250 g of soil sample from each of the replicative pots of all the treatments was collected. The composite soil samples were properly mixed, and subsamples of 50 g were extracted by means of modified Cobb’s sieving and decanting technique followed by the Baermann funnel method [36]. The suspension was passed through nested sieves with meshes size openings of 149, 65 and 45 μM. The final volume of each of the extracted samples was made up to 50 mL, and nematode numbers were counted in a 1 mL capacity counting on a slide under stereoscopic binocular microscope. The mean of three independent counts was taken in each case. The reproduction factor (“Rf”) was calculated by the using the formula, Rf = Pf/Pi, where Pf = final Mi population and Pi = initial Mi population [1].
2.2.4. Plant Yield and Essential Oil Extraction
At the end of the experiment, the plants were harvested manually with sharp sickles. Immediately after harvest, yield attributes such as leaf area, stem weight, shoot height, number of branches, plant canopy etc. were recorded for all the plants from each replication. Aerial plant biomass was then subjected to hydro distillation in a Clevenger type glass apparatus for 4 h for essential oil extraction [5]. The oil was collected, measured and dried over anhydrous sodium sulphate (Na2SO4) and stored in sealed glass vials at 4 °C for further analysis. Percentage essential oil concentration was calculated as follows:
Essential oil obtained (mL)/Weight of loaded biomass (g) × 100.
2.2.5. Gas Chromatography Analysis of the Essential Oil
To analyze the chemical content of the extracted essential oils a gas chromatographic (GC) analysis was used following the same procedure as described by Tiwari et al. [5]. An Agilent Perkin Elmer GC Instrument fitted with “FID” (flame ionization detector) and electronic integrator (30 m × 32 mm i.d., 0.25 μm film thickness) with fused-silica capillary column was used. Nitrogen (at a flow rate of 0.4 mL/min) was used as a carrier gas. Essential oil samples were injected in the split mode at a ratio of 1:10–1:100. The injector was kept at 250 °C and the transfer line at 280 °C. The column was maintained at 50 °C for 2 min and then programmed to 220 °C at 5 °C/min and held for 10 min at 300 °C. The relative amounts of individual components of essential oil were calculated on the basis of software-computed GC peak area percentage without applying correction for FID response factor. The identification of the compounds was performed by comparing their retention indices (“RI”), determined with reference to a homologous series of n-alkanes (C9–C24, Polyscience Corp.) under identical experimental conditions in both polar and nonpolar columns, coinjection with standards. The relative amounts of the individual components were calculated based on computer calculated GC peak areas without correction for flame ionization detection response factors. Retention indices of “neutral” components were compared with those earlier reported values by Adams [37].
2.2.6. Nutrient Uptake Analysis
To assess how active bioinoculants affect plant nutrient uptake, plant total nitrogen (N), phosphorus (P) and potassium (K) content in the various treatments were determined. For N and P extraction and estimation, dry plant biomass was digested with Kjeldahl’s method (H2SO4 acid digestion) as suggested by Jackson [38]. P was estimated by the Molybdenum blue method, and K by following methods of Jackson [39], where 0.5 g plant dry matter was extracted with 2 M HCl and read in flame photometer 128, (Cistronic model).
2.3. Statistical Analysis
For all parameters, data from treatments were statistically analyzed by the analysis of variance method (ANOVA). Significant differences among treatments were based on Duncan’s test in ANOVA, and treatment means were compared using least significant difference at p ≤ 0.01. The statistical analyses were performed using ASSISTAT software (version 7.6, Brazil). The graphs were drawn using Sigma plot 14.0 version.
3. Results
3.1. Prescreening Experiments
3.1.1. Biochemical Characterization and PGP Activity of Isolates and Selection of Bioinoculants
The results obtained from the biochemical screening showed that the isolated bacteria were bestowed with different plant beneficial activities. A total of 63% of the isolates showed cellulase activity, 52% of the isolates showed proteolytic activity and IAA production, 20% showed L-asparaginase activity, 12% showed phosphatase activity and 6% showed siderophore activity (Table S2 and Figure S1). However, no isolates with chitinases and pectinases activity were obtained (Table S2, Figure S1). In total, we picked 10 isolates (RPN 1, RPN 2, RPN 3, RPN 4, RPN 5, MTN 2, MTN 100, MTN 101, MTN K4, MTN 121) based on their potential to produce maximal extracellular enzymes under in vitro conditions, which were further used for biopotential screenings in basil plants (Table S3).
3.1.2. Prescreening of Bioinoculants for the Management of M. incognita in Basil Plants
The 10 selected bioinoculants (Table S3) from 165 initial isolates having different biochemical and PGP properties were examined for the biocontrol potentials in prescreening experiment in basil plants. Root Knot Index (“RKI”), production of plant beneficial enzymes and increased herb yield were considered to be the most important parameters for the selection of potential bioinoculants for nematode management [1,2,5]. The two strains that showed the best overall growth promotion traits—RPN-3, which showed the maximal total herb yield at 16.27 g pot−1, and MTN K4, which showed the maximal Root Knot Index (RKI of 1.33, Table 2)—were selected. These strains were used for experimentation in consortium with TH.

Table 2.
Preliminary screening and impact of selected bacterial isolates on growth parameters of O. basilicum.
3.1.3. Molecular Identification of Bioinoculants
Homology search and Blast analysis revealed that the newly isolated strains (MTN K4 and RPN-3) belong to the genus Bacillus and exhibited 100%, and 99% similarity with Bacillus subtilis (BS) and Bacillus megaterium (BM), respectively. The gene sequences were also submitted to the NCBI GenBank to procure accession numbers for BS (JQ 713565) and BM (KF157961) for future reference (Table S4). The phylogenetic tree was constructed using the UPGMA method from MEGA5 (Figure S2).
3.1.4. In Vitro Nematicidal and Ovicidal Activity of Bioinoculants
The nematicidal and ovicidal potentials of all the cell free culture filtrates of the selected bioinoculants (TH, PF, BM and BS) showed significant nematicidal and ovicidal activity (Figure 3a,b) in comparison to controls (control water and control medium). The maximal nematicidal activity was recorded after 72 h in cell free culture filtrates of BM (75.3%) followed by BS (69.3%), PF (50.7%) and TH (34.0% (Figure 3a). Ovicidal activity of cell free culture filtrates also showed higher % egg mortality after 72 h among all the treatments. The cell free culture filtrates of BM showed 78.0% egg mortality in comparison to the control, followed by BS (74.0%), PF (72.0%) and TH (36.0% (Figure 3b).
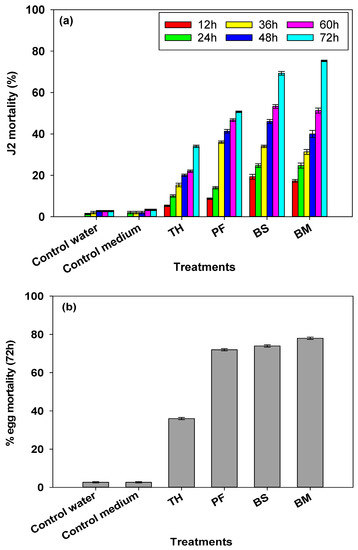
Figure 3.
(a) Impact of cell free culture filtrates of Trichoderma harzanium (TH), Pseudomonas fluorescens (PF) Bacillus subtlis (BS) and B. megaterium (BM) on larvae (J2 stage) mortality of Mi (in %) at different time intervals. (b) Impact of cell free culture filtrates of. TH, PF, BS and BM on egg mortality of Mi at 72 h (in %). Distilled water and the medium used for bacterial culture were used as controls. The vertical bar represents mean of three replicates and standard error of mean (SE).
3.2. Greenhouse Experiments
3.2.1. Effects of Microbial Bioinoculants on Growth Parameters of Basil Plants
The growth parameters (plant height, canopy, number of branches, inflorescence and plant weight) showed significant differences between treatments when compared to uninoculated control CL + Mi (Figures S4 and S5a, p ≤ 0.01). The increase in plant height (the sum of root and shoot length) in comparison to CL + Mi was generally higher for plants treated with consortium inoculation (such as BM + TH, BS + TH, PF + TH, ranging from 67.6 to 74.5% in sterile soil condition and from 66.2 to 73.2% in natural soil conditions) than for single inoculation such as BM, BS, PF, TH (increase ranging from 29.7% to 53.3% in sterile soil conditions and from 31.8% to 55.0% in natural soil conditions). The number of branches was higher in in all the inoculated treatments in comparison to CL + Mi in both soil conditions (increase ranging from 60% to 140%, Figure S4a,b). The number of inflorescence and canopy spreads were found to be higher in all consortium treatments in comparison to CL + Mi (Figure S5a,b) under both natural soil conditions (increase of 61.5 to 107.7%) and sterile soil conditions (increase of 67.2 to 98.5%). Similar increasing trends were found for plant leaf area under both natural (1.3 to 3.1-fold) and sterile (1.3 to 2.6-fold) conditions (Figure S4c).
3.2.2. Effects of Bioinoculants on Biomass Yield of Basil Plants
Total biomass yield of O. basilicum (in terms of dry weight) was significantly higher (p < 0.05) in all inoculated treatments as compared to CL + Mi in both soil conditions (Table 3, Figure S3). The increase in dry above ground biomass ranged from 77 to 129% in natural soil conditions and from 78% to 105% in sterile soil conditions with maximal increase for the consortium treatment of BM + TH + Mi in both soil conditions. A lower, but significant, increase was found for all treatments in comparison to the chemical control (Carbofuran + Mi) under both soil conditions. Similar pattern was found for root biomass, which increased significantly under both natural and sterile soil conditions (from 44.5 to 208.1% in comparison for CL + Mi (p ≤ 0.05). The highest increase was found for BS + TH + Mi (208.1% in natural soil conditions and 110.6% in sterile conditions, Table 3).

Table 3.
Dry biomass of O. basilicum (g pot−1) treated with different bioinoculants and grown under different soil conditions.
3.2.3. Estimation of Nematode Management Potentials of Bioinoculants
RKI decreased significantly (p ≤ 0.05) for all treatments in comparison to CL + Mi (decrease ranged from 49.6% to 74.8% in natural soil conditions and from 45.5% to 72.7% in sterile soil conditions, Figure 4a). Overall, the RKI values in all treatment were statistically similar to the values measured for the Carbofuran + Mi treatment and ranged from 1 to 2. The lowest RKI value was found for consortium of BM + TH + Mi in both natural and sterile soil conditions. Overall BS + TH + Mi and BM + TH + Mi were the most effective treatments in lowering the RKI values. The treatments with single and co-inoculations significantly reduced the reproduction factor (Rf) of the nematode in comparison to CL + Mi under both soil conditions (Figure 4b), with reduction percentage ranging from 46.4 to 59.1% in natural soil conditions and from 49.4 to 72.75% under sterile soil conditions. The reduction in Rf values of consortium treatments (BS + TH + Mi and BM + TH + Mi) was almost comparable to the reduction measured for plants treated with carbofuran (78.7% in sterile soil and 64.1% in natural soil). The maximal reduction under sterile conditions was found for BM + TH + Mi (72.7%) and for BM + TH + Mi and BM + Mi under natural soil conditions (58.3, and 59.2%, Figure 4b).
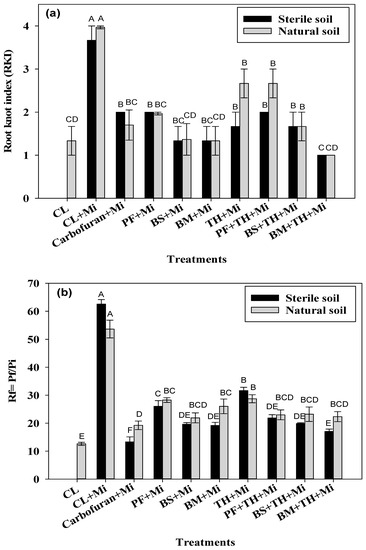
Figure 4.
(a) Impact of different bioinoculants in single inoculations (BM + Mi, TH + Mi, BS + Mi, PF + Mi) and co-inoculations (BM + TH + Mi, BS + TH + Mi, PF + TH + Mi) on root knot index (RKI) in the rhizosphere of O. basilicum grown in sterile (black) and natural (grey) soil conditions. The comparisons were made for all of the treatments of the same experiment, either in sterile soil or natural soil conditions. Error bars (mean ± SE) with different letters represent statistically significant values (at p ≤ 0.001) in comparison to untreated inoculated control (CL +Mi). (b) Impact of different bioinoculants in single inoculations (BM + Mi, TH + Mi, BS + Mi, PF + Mi) and co-inoculations (BM + TH + Mi, BS + TH + Mi, PF + TH + Mi) on reproduction factor (Rf) in sterile (black) and natural (grey) soil conditions.
3.2.4. Effect of Bioinoculants on Essential Oil
The essential oil yield significantly increased for all inoculated treatments in comparison to CL + Mi (p ≤ 0.05, increase ranging from 43.1 to 80.7% in sterile soil conditions and from 42.3 to 112.4% in natural soil conditions). The oil yield in the inoculated treatment ranged from 0.36 to 0.66% in sterile soils and from 0.32 to 0.69% in natural soils (Figure 5, Table S4). The maximal oil yield was observed in plants treated with the consortium of BM + TH + Mi under both soil conditions. A lower, but statistically significant increase (p ≤ 0.05) was found for plants treated with single inoculants, ranging from 64.9 to 100.0% under natural soil conditions and from 51.4 to 71.6% under sterile soil conditions. The oil yield in all of the bioinoculants treated plants was higher than the yield found for Carbofuran + Mi under both soil conditions (Figure 5, Table S5).
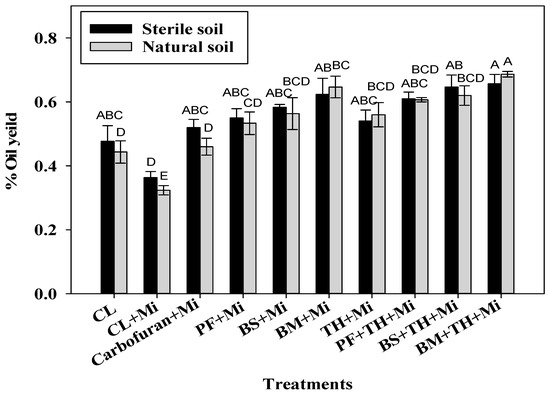
Figure 5.
Oil yield of O. basilicum (%) plants grown in sterile (black) and natural soil (grey) conditions under different microbial treatments in single inoculations (BM + Mi, TH + Mi, BS + Mi, PF + Mi) and consortium inoculations (BM + TH + Mi, BS + TH + Mi, PF + TH + Mi). The comparisons were made for all of the treatments of the same experiment, either in sterile soil or natural soil conditions. The vertical bars (mean ± SE) with different letters represent statistically significant values (at p ≤ 0.001) in comparison to untreated inoculated control (CL +Mi).
The quality of the essential oil mainly depends on the concentrations of the major constituents like methyl chavicol, methyl eugenol, linalool and caryophyllene etc. The gas chromatography results showed large differences between CL + Mi and all bioinoculants treated plants (Table 4a,b). Among all metabolites, only methyl chavicol showed a constant significant increase (p ≤ 0.05) in the plants treated with single or consortium treatments in comparison to CL + Mi (increase ranging from 62.2 to 78.1% under sterile soil conditions and from 63.7 to 71.16% under natural soil conditions, Table 4a,b). The maximal increase in methyl chavicol was found for plants treated with BM + TH + Mi (78.1% under sterile conditions and 71.2% under natural conditions).

Table 4.
(a) Essential oil chemical constituent profiling (relative percent from total constituent) of Ocimum basilicum grown under sterile soil conditions and treated with different bioinoculants in single or in consortium. (b) Essential oil chemical constituent profiling (relative chemical constituent percent from total constitutes) of Ocimum basilicum grown under natural soil conditions and treated with different bioinoculants in single or in consortium.
3.2.5. Effect of bioinoculants on plant nutrient uptake.
N uptake was significantly enhanced in all of the inoculated plants in comparison to CL + Mi under sterile soil conditions except for the individual PF, BM and carbofuran + Mi treatments (Table 5).

Table 5.
Nutrient uptake (N, P and K, in %weight) under sterile and natural soil conditions.
Overall, a larger increase was found for the consortium treated plants (24.7 to 74.2% under natural conditions and 87.5 to 196.9% in sterile conditions), with not much difference among single and consortium treatments. No significant increase was found for P uptake in comparison to CL + Mi under both soil conditions, with no apparent difference between consortium and single inoculants (Table 5). K uptake was significantly higher in consortium treatments under sterile soil conditions (increase in comparison to controls ranging from 43.3 to 46.6%) and for both single and consortium treatment under natural soil conditions (increase in comparison to CL + Mi ranging from 51.8 to 63.8%).
4. Discussion
Application of indigenous microbes isolated from agricultural soils in the Lucknow region (B. megaterium, B. subtilis, and T. harzianum) was found to reduce root knot nematode activity in the rhizosphere of O. basilicum and increase all the auxiliary plant growth parameters, directly impacting overall plant health and essential oil yield. A significant increase in biomass growth and oil yield was found in plants that were treated with single inoculations of the native strains BS and BM in comparison to the chemical control carbofuran, showing their potential as alternative, biological nematode management agents.
However, in comparison to the reference strain PF, single inoculations of BS resulted in almost the same biocontrol potentials (Figure 3a,b). More pronounced differences were found in consortium treatment with TH, where BM emerged as the most efficient bioinoculant, followed by BS and PF, yielding the highest percent of Methyl chavicol, the major chemical constituent of basil oil. These findings are in accordance with previous studies which reported that the use of Trichoderma spp. and different strains of pseudomonas (P. fluorescens, P. monteilii) and Bacillus genera in consortium improved plant growth parameters on different medicinal and aromatic plants such as Coleus forskohlii, Pogostemon cablin, Withania somnifera [16,40,41,42,43]. Bacillus spp. (BM and BS) is considered an effective root colonizer that can sporulate under stress conditions [44], and thus acts as a potent bioinoculant, which decreases nematode activity by producing anti-nematodal lytic extracellular enzymes [45,46]. Trichoderma spp. has the ability to parasitize plant pathogens by extending hyphal branches towards the target hosts [47]. They secrete enzymes such as proteases, chitinases, and glucanases with antifungal activity [48], and also has potentials for J2 motility (nematicidal), ovicidal activity (egg hatching) and adversely impacting nematode development and reproduction [49,50,51]. These mechanisms are somewhat different from those of Pseudomonas spp. (which was used a reference strain in our study), a well-established bioinoculant for root knot nematodes that secretes hydrogen cyanide (HCN), ammonia (NH3), siderophores and antibiotics in order to manage nematode activity [52].
Our in vitro experimental results (Figure 3a,b) also showed potential nematicidal and ovicidal activity in different cell free culture filtrates of BS, BM and TH, which is considered one of the contributing factors for low reproduction factor and root knot index [53,54]. Although all bioinoculant treatments reduced nematode activity and improved plant growth parameters in comparison to untreated inoculated control, the effects of consortium treatments (BS + TH, BM + TH, PF + TH) were usually more potent. We attribute these benefits to the additive contributing factors from two potent microbes in synergy [55], as no antagonistic activity was found under in vitro synergistic–antagonistic assay, predicting that they would work in synergy for the inoculated crop. Overall, maximal biocontrol effect was found for the consortium of BM and TH under both natural and sterile soil conditions, which might have been achieved by the competition for colonization sites and nutrients secreted by roots, hyperparatism, production of lytic enzymes, secondary metabolites or induced systemic resistance, etc. [1,56]. In addition to the anti-nematode activity, consortium bioinoculants were found to significantly enhance leaf area as compared to untreated inoculated control, which strongly correlates with essential oil metabolism and oil yield [57]. Furthermore, based on our in vitro pre-screening experiment, we predict that potentials of microbes to secrete plant growth promoting enzymes in the inoculated treatments in our experimental system reflects and additional benefit for plant health, which further enhanced essential oil yields, showcasing the positive effect of synergism. The obtained result is in agreement with other reports on biocontrol agents which suggest that indigenous bioinoculants in consortium have better potentials to stimulate plant growth [5,42]. They adapt different mechanisms (improvement of plant nutrition, production and regulation of phyto-hormones) and biocontrol strategies such as suppression of disease-causing organisms by adopting different approaches either cidal or static in nature [1,58,59]. A possible set of mechanisms of disease protection by a consortium of strains could be attributed to direct effects of lytic metabolites that induce mortality in J2 (larvae), or ovicidal activity that may have enhanced host defense mechanism in roots resisting invasion and consequent infection by pathogens [60,61]. Results reported for consortium treatments of bioinoculants on tomato crops also showed potentials for both PGPR and root knot nematode management [62]. Furthermore, biological controls of single microbes were shown to be inconsistent, especially in natural soil conditions [63,64,65], emphasizing that synergetic use has far greater nematode management potential [66].
Usually, strains performed better under sterile soil conditions, probably because the inoculated microbes do not have to compete for nutrients and other resources or for colonization sites on roots with other microorganisms [55]. In natural soils, on the other hand, the biocontrol potentials depend on the interactions of the new strains with the natural microbial community. Only if no resource competitions exist, can the colonization of bioinoculants successfully enhance efficacy in nematode management [67]. Interestingly, the similarity in RKI and RF values under both soil conditions indicates that the performance of the bioinoculants used in this study in natural soil is almost the same as that in sterile soil conditions. The high potency of the microbial inoculants under natural soil conditions is attributed to pre-existing antagonistic microbes and additive impact of indigenous PGPR strains (BS, BM) that might have managed the impact of externally added M. incognita to the treatments, along with already-existing pathogens in natural soils (overall population) [68,69]. Therefore, we suggest that in natural soil conditions, the inoculated bioinoculants act together with the indigenous microbial community in the soil to enhance anti root knot nematode activities [70] and reduce adverse impact on plant growth probably through a set of mechanisms such as parasitism and antibiosis production of toxins and traps, etc. [69,71].
There are a number of reports depicting an inverse relationship between nematode population and reduced uptake of macro and micro nutrients such as N, P, K, Zinc, Manganese and Copper [72,73]. Previous studies conducted on the same bacterial lines used in this study reported that inoculation of bioinoculants in soil enriches nutrients, which, in turn, enhanced the growth and essential oil yields in medicinal and aromatic plants [16,41]. No significant differences in P uptake were observed, either by single or by consortium treatments. Yet, a significant increase in N and K was found under most treatments. Thus, in addition to their anti-nematode activity, the increase in plant P and K uptake may be associated with the P [74] and K solubilization [75] activity of Bacillus spp. and Pseudomonas spp. The increase in plant N uptake was also observed under both soil conditions, which may be due to N2 fixing potentials of Bacillus spp. [76]. Furthermore, much better responses in natural soil conditions were found, probably due to the presence of naturally N2 fixing microbes colonizing rhizosphere.
5. Conclusions
Overall, the present experiment indicates that indigenous microbes isolated from rhizosphere of basil growing in suppressive soils are highly efficient in suppressing nematode and increase oil yield in O.basilicum in both sterile and natural soil conditions. The synergy of bioinoculants with different biocontrol and growth promoting features provided effective eco-friendly management of root knot nematode in O. basilicum, and maybe in other plants of same family (Lamiaceae), and could be an alternative for the chemical nematicides. With this experiment as a lead, further study is needed to develop a prospective PGPR’s consortium with nematicidal traits for sustainable cultivation of commercially grown medicinal and aromatic plants.
Supplementary Materials
The following are available online https://www.mdpi.com/2073-4395/11/3/570/s1—alongside the manuscript. Table S1: List of isolated bacteria from rhizosphere of different medicinal and aromatic plants in Lucknow region, Uttar Pradesh state, India. Table S2: Preliminary screening of in vitro biochemical and PGP screening. Table S3: list of identified isolates and their biochemical properties. Table S4: Identification of bacterial isolates through BLAST search and homology. Table S5: Comparative graph of the oil yield production of Ocimum basilicum under different microbial treatment. Figure S1: The biochemical activity of different bacterial isolates on different mediums. Figure S2: Results of identification of bacteria through 16S rRNA gene.). Figure S3: A representative picture of O.basilicum plants that were either untreated, untreated inoculated (CL) or treated with bioinoculants. Figure S4a–c: Effect of different microbial treatments in single (BM + Mi, TH + Mi, BS + Mi, PF + Mi) and co-inoculation (BM + TH + Mi, BS + TH + Mi, PF + TH + Mi) on different growth parameters of the O. basilicum under natural soil conditions.
Author Contributions
Conceptualization, S.T.; methodology, S.T.; software, S.T., R.P. and A.G.; validation; S.T., R.P. and A.G.; formal analysis, S.T., R.P. and A.G.; investigation, S.T.; resources, S.T. and R.P. and A.G.; data curation, S.T. and A.G.; writing—original draft preparation, S.T., and A.G.; writing—review and editing, A.G. & R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on reasonable request.
Acknowledgments
The authors ST and RP are grateful to the Director of CSIR-CIMAP, Lucknow, India for providing all necessary facilities. The authors are thankful to Alok Kalra and R.P. Patel and Virendra Shukla for their support and help during the entire tenure of experimentation. Special thanks are also due to R.P. Singh for providing ATCC bacterial strains and helping in maintenance of other strains in the repository.
Conflicts of Interest
The authors declare that there is no conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Saikia, K.S.; Tiwari, S.; Pandey, R. Rhizospheric biological weapons for growth enhancement and Meloidogyne incognita management in Withania somnifera cv. Poshita. Biol. Control. 2013, 65, 225–234. [Google Scholar] [CrossRef]
- Tiwari, S.; Saikia, K.S.; Singh, R.; Singh, S.P.; Pandey, R. Native microbial inoculants for the management of Meloidogyne incognita in Withania somnifera cv. Poshita. Proc. Natl. Acad. Sci. India. Sect B Biol. Sci. 2014, 86, 55–63. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, R. Microbial interference ameliorates essential oil yield and diminishes root-knot infestation in sweet basil under field conditions. Biocontrol Sci. Technol. 2015, 25, 1165–1179. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Sreeramu, B.S. Cultivation of Medicinal and Aromatic Crops; University Press: Hyderabad, India, 2001. [Google Scholar]
- Tiwari, S.; Pandey, S.; Singh, P.C.; Pandey, R. Biocontrol agents in co-inoculation manages root knot nematode Meloidogyne incognita (Kofoid & White) Chitwood and enhances essential oil content in Ocimum basilicum L. Ind. Crops Prod. 2017, 97, 292–301. [Google Scholar] [CrossRef]
- Telci, I.; Bayram, E.; Yilmaz, G.; Avci, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochem. Syst. Ecol. 2006, 34, 489–497. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Patel, R.K.; Kalra, A. Technology for improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind. Crop. Prod. 2013, 45, 335–342. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Shoot regeneration of young leaf explants from basil (Ocimum basilicum L.). In Vitro Cell. Dev. Biol. Plant 2000, 36, 250–254. [Google Scholar] [CrossRef]
- Paula, J.P.de.; Gomes-Carneiro, M.R.; Paumgartten, F.J.R. Chemical composition, toxicity and mosquito repellency of Ocimum selloi oil. J. Ethnopharmacol. 2003, 88, 253–260. [Google Scholar] [CrossRef]
- Santos, B.C.S.; Pires, A.S.; Yamamoto, C.H.; Couri, M.R.C.; Taranto, A.G.; Alves, M.S.; de Matos Araujo, A.L.D.S.; de Sousa, O.V. Methyl Chavicol and Its Synthetic Analogue as Possible Antioxidant and Antilipase Agents Based on the In Vitro and In Silico Assays. Oxidative Med. Cell. Longev. 2018, 2018, 2189348. [Google Scholar] [CrossRef] [PubMed]
- Jnanesha, A.C.; Kumar, A.; Kumar, V.M. Effect of seasonal variation on growth and oil yield in Ocimum africanum Lour. J. Pharmacogn. Phytochem. 2018, 7, 73–77. [Google Scholar]
- Sajjadi, S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru J. Pharm. Sci. 2006, 14, 128–130. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Upadhyay, R.K.; Chauhan, A.; Singh, V.R. Productivity and essential oil quality assessment of promising accessions of Ocimum basilicum L. from north India. Ind. Crops. Prod. 2017, 97, 79–86. [Google Scholar] [CrossRef]
- Pandey, R.; Kalra, A.; Gupta, M.L. Evaluation of bio-agents and pesticide on root-knot nematode development and oil yield of patchouli. Arch. Phytopathol. Plant Prot. 2007, 42, 419–423. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Webster, J.M.; Brooke, R.C. Improved techniques for measuring the CO2 exchange rate of Meloidogyne nematode bean plants. Nematologica 1984, 30, 213–221. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, S.; Patel, R.P.; Soni, S.; Kalra, A. Bioinoculants and AM fungus colonized nursery improved management of complex root disease of Coleus forskohlii Briq. under field conditions. Biol. Control. 2018, 122, 11–17. [Google Scholar] [CrossRef]
- Saikia, S.K.; Tiwari, S.; Pandey, R. Rhizospheric innovations for growth enhancement and Meloidogyne incognita management in Mentha arvensis cv. Kosi. Int. J. Environ. Sci. Eng. Res. 2012, 3, 26–34. [Google Scholar]
- Adam, M.; Westphal, A.; Hallmann, J.; Heuer, H. Specific Microbial Attachment to Root Knot Nematodes in Suppressive Soil. Appl. Environ. Microbiol. 2014, 80, 2679–2686. [Google Scholar] [CrossRef]
- Goswami, D.; Dhandhukia, P.; Patel, P.; Thakker, J.N. Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol. Res. 2014, 169, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, M.C.; Fanfoni, E.; Fiorini, A.; Trevisan, M.; Puglisi, E. Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops. Plants 2020, 9, 668. [Google Scholar] [CrossRef]
- Tran, D.M.; Sugimoto, H.; Nguyen, D.A.; Watanabe, T.; Suzuki, K. Identification and characterization of chitinolytic bacteria isolated from a freshwater lake. Biosci. Biotechnol. Biochem. 2018, 82, 343–355. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Pikovaskya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Edwards, U.; Rogall, T.; Blocker, H.; Emde, M.; Bottger, E.C. Isolation and direct nucleotide determination of entire genes: Characterization of a gene encoding for 16S ribosomal RNA. Nucl. Acids Res. 1989, 17, 7843–7850. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, S.; Pandey, P.; Saikia, S.K.; Negi, A.S.; Gupta, S.K.; Pandey, R.; Banerjee, S. Isolation, structure determination and anti-aging effects of 2, 3-pentanediol from endophytic fungus of Curcuma amada and docking studies. Protoplasma 2014. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Cayrol, J.C.; Djian, C.; Pijarowski, L. Study of the nematicidal properties of the culture filtrate of the nematophagous fungus Paecilomyces lilacinus. Rev. Nematol. 1989, 12, 331–336. [Google Scholar]
- Katiyar, V.; Goel, R. Improved Plant Growth from Seed Bacterization Using Siderophore Overproducing Cold Resistant Mutant of Pseudomonas fluorescens. J. Microbiol. Biotechnol. 2004, 14, 653–657. [Google Scholar]
- Lee, Y.S.; Kim, J.; Shin, S.C.; Lee, S.G.; Park, I.K. Antifungal activity of Myrtaceae essential oils and their components against three phytopathogenic fungi. Flavour Fragr. J. 2008, 23, 23–28. [Google Scholar] [CrossRef]
- Singh, R.; Divya, S.; Ashutosh, A.; Kalra, A. Technology for efficient and successful delivery of vermicompost colonized bioinoculants in Pogostemon cablin (patchouli) Benth. World. J. Microbiol. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Pandey, R. Management of Meloidogyne incognita in Artemisia pallens with bio-organics. Phytoparasitica 2005, 33, 304–308. [Google Scholar] [CrossRef]
- Pandey, R. Chemical activators: A novel and sustainable management approach for Meloidogyne incognita (Kofoid and White) Chitwood in Chamomilla recutita L. Arch. Phytopathol. Plant Prot. 2005, 38, 107–111. [Google Scholar] [CrossRef]
- Krusberg, L.R.; Nielsen, L.W. Pathogenesis of root-knot nematodes to PortoRico variety of sweet potato. Phytopathology 1958, 48, 30–39. [Google Scholar]
- Goodey, T. Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.F., Ed.; Reference Book; Ministry of Agriculture, Fisheries and Food: London, UK, 1986.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of Englewood Cliffs: Englewood Cliffs, NJ, USA, 1967. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of Englewood Cliffs: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Abd-El-Khair, H.; El-Nagdi, W.M.; Youssef, M.M.; Abd-Elgawad, M.M.; Dawood, M.G. Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root knot nematode Meloidogyne incognita on cowpea. Bull. Nat. Res. Cent. 2019, 43, 1–7. [Google Scholar] [CrossRef]
- Singh, R.; Paramaeswarn, T.N.; Rao, P.E.V.S.; Puttanna, K.; Kalra, A.; Srinivas, K.V.N.S.; Bagyaraj, D.J.; Divya, S. Effect of arbuscular mycorrhizal fungi and Pseudomonas fluorescens on root-rot/wilt, growth and yield of Coleus forskohlii. Biocontrol. Sci. Technol. 2009, 19, 835–841. [Google Scholar] [CrossRef]
- Ordookhani, K.; Sharafzadeh, S.H.; Zare, M. Influence of PGPR on growth, essential oil and nutrients uptake of Sweet basil. Adv. Environ. Biol. 2011, 5, 672–677. [Google Scholar]
- Pandey, R.; Mishra, A.K.; Tiwari, S.; Kalra, A. Nematode inhibiting organic materials and a strain of Trichoderma harzianum effectively manages Meloidogyne incognita in Withania somnifera fields. Biocontrol. Sci. Tech. 2011, 21, 1495–1499. [Google Scholar] [CrossRef]
- Kavitha, P.G.; Jonathan, E.I.; Nakkeeran, S. Effects of crude antibiotic of Bacillus subtilis on hatching of eggs and mortality of juveniles of Meloidogyne incognita. Nematol Medit. 2012, 40, 203–206. [Google Scholar]
- Gupta, R.; Tiwari, S.; Saikia, K.S.; Shukla, V.; Singh, R.; Singh, S.P.; Kumar, A.P.V.; Pandey, R. Exploitation of microbes for enhancing bacoside content and reduction of Meloidogyne incognita infestation in Bacopa monnieri L. Protoplasma 2014. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; El-Nagdi, W.M.A.; Ameen, H.H. Antagonistic effects of rhizobacteria isolates against Meloidogyne incognita infecting tomato plants under greenhouse conditions. Int. J. Pharm. Tech. Res. 2016, 9, 97–107. [Google Scholar]
- Goldman, G.H.; Hayes, C.H.; Harman, G.E. Molecular and cellular biology of biocontrol by Trichoderma spp. Trends Biotechnol. 1994, 12, 478–482. [Google Scholar] [CrossRef]
- Lorito, M.; Peterbauer, C.; Hayes, C.K.; Harman, G.E. Synergistic interaction between fungal cell-wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 1994, 140, 623–629. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Spiegel, Y. Improved attachment and parasitism of Trichoderma on Meloidogyne javanica in vitro. Eur. J. Plant Pathol. 2009, 123, 291–299. [Google Scholar] [CrossRef]
- Wang, M.; De Deyn, G.B.; Bezemer, T.M. Separating effects of soil microorganisms and nematodes on plant community dynamics. Plant Soil. 2019, 441, 455–467. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Timper, P.; Koné, D.; Yin, J.; Ji, P.; Gardener, B.B.M. Evaluation of an antibiotic-producing strain of Pseudomonas fluorescens for suppression of plant-parasitic nematodes. J. Nematol. 2009, 41, 234–240. [Google Scholar] [PubMed]
- Lu, H.; Wang, X.; Zhang, K.; Xu, Y.; Zhou, L.; Li, G. Identification and nematicidal activity of bacteria isolated from cow dung. Ann. Microbiol. 2014, 64, 407–411. [Google Scholar] [CrossRef]
- Liu, G.; Lin, X.; Xu, S.; Liu, G.; Liu, F.; Mu, W. Screening, identification and application of soil bacteria with nematicidal activity against root-knot nematode (Meloidogyne incognita) on tomato. Pest. Manag. Sci. 2020, 76, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, S.; Sharma, S.; Kumar, V. Synergistic effect of bio-inoculants on yield, nodulation and nutrient uptake of chickpea (Cicer arietinum L.) under rainfed conditions. J. Plant Nutr. 2019, 42, 374–383. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Lemberkovics, E.; Petri, G.; Nguyen, H.; Mathe, L. Relationships between essential oil and flavonoid biosynthesis in basil. Acta Hort. 1995, 426, 647–655. [Google Scholar] [CrossRef]
- Tian, B.; Yang, J.; Zhang, K.Q. Bacteria used in the biological control of plant-parasitic nematodes: Populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 2007, 61, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant growth promoting rhizobacteria. Annu. Rev Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Keneni, A.; Assefa, F.; Parbu, P.C. Isolation of phosphate solubilizing bacteria from the rhizosphere of faba bean of Ethiopia and their abilities on solubilizing insoluble phosphates. J. Agric. Sci. Technol. 2010, 12, 79–89. [Google Scholar]
- Norabadia, M.T.; Sahebania, N.; Etebarianb, H.R. Biological control of root-knot nematode (Meloidogyne javanica) disease by Pseudomonas fluorescens (Chao). Arch. Phytopathol. Plant Prot. 2014, 47, 615–621. [Google Scholar] [CrossRef]
- Rika, A.I.; Nyoman, P.A.; Tati, S.S. Role of Indigenous Rhizosphere Bacteria in Suppressing Root-knot Nematode and Improve Plant Growth Tomato. Plant Pathol. J. 2017, 16, 25–32. [Google Scholar]
- Pandey, R.; Gupta, A.; Haider, R.; Kalra, A. Management of root-knot nematode in Ocimum basilicum through bio-organics. J. Mycol. Plant Pathol. 2009, 39, 86–89. [Google Scholar]
- Pandey, R.; Gupta, A.; Singh, H.N.; Kalra, A. Phytonematode management through bacteria: An underground battle for existence. In Recent Advances in Biopesticides Biotechnological Applications; Johri, J.K., Ed.; NBRI: Lucknow, India, 2009; pp. 1–26. [Google Scholar]
- Singh, R.; Patel, R.P.; Singh, D.; Soni, S.K.; Tiwari, S. Bioinoculant coated seed improved the growth and yield of Withania somnifera (L.) Dunal. Medicinal Plants. Int. J. Phytomedicines Relat. Ind. 2018, 10, 191–195. [Google Scholar] [CrossRef]
- Reimann, S.; Hauschild, R.; Hildebrandt, U.; Sikora, R.A. Interrelationships between Rhizobium etli G12 and Glomus intraradices and multitrophic effects in the biological control of the root-knot nematode Meloidogyne incognita on tomato. J. Plant Dis. Prot. 2008, 115, 108–113. [Google Scholar] [CrossRef]
- Timper, P. Conserving and enhancing biological control of nematodes. J. Nematol. 2014, 46, 75–89. [Google Scholar]
- Chen, S.; Dickson, D.W.; Kimbrough, J.W.; McSorley, R.; Mitchell, D.J. Fungi associated with females and cysts of Heterodera glycines in a Florida soybean field. J. Nematol. 1994, 26, 296–303. [Google Scholar]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Feng, H.; Schuelke, T.; De Santiago, A.; Zhang, Q.; Zhang, J.; Luo, C.; Wei, L. Rhizosphere Microbiomes from Root Knot Nematode Non-infested Plants Suppress Nematode Infection. Microb. Ecol. 2019, 78, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Colagiero, M.; Rosso, L.C.; Ciancio, A. Diversity and biocontrol potential of bacterial consortia associated to root-knot nematodes. Biol. Control. 2017, 120, 11–16. [Google Scholar] [CrossRef]
- Rhoades, H.L. Effects of fallowing, summer cover crops, and fenamiphos on nematode populations and yields in a cabbage-field corn rotation in Florida. Nematropica 1984, 14, 131–138. [Google Scholar]
- Ferraz, H.B.; Bertolucci, P.H.F.; Pereira, J.S.; Lima, J.G.C.; Andrade, L.A.F. Chronic exposure to the fungicide maneb may produce symptoms and signs of CNS manganese intoxication. Neurology 1988, 38, 550–553. [Google Scholar] [CrossRef]
- Oteino, R.D.; Lally, S.; Kiwanuka, A.; Lloyd, D.; Ryan, K.J.; Germaine, D.N. Dowling Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- He, Y.; Pantigoso, H.; Wu, Z.; Vivanco, J. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).