NaCl- and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Plant Material and Experimental Design
2.3. Soil Analysis
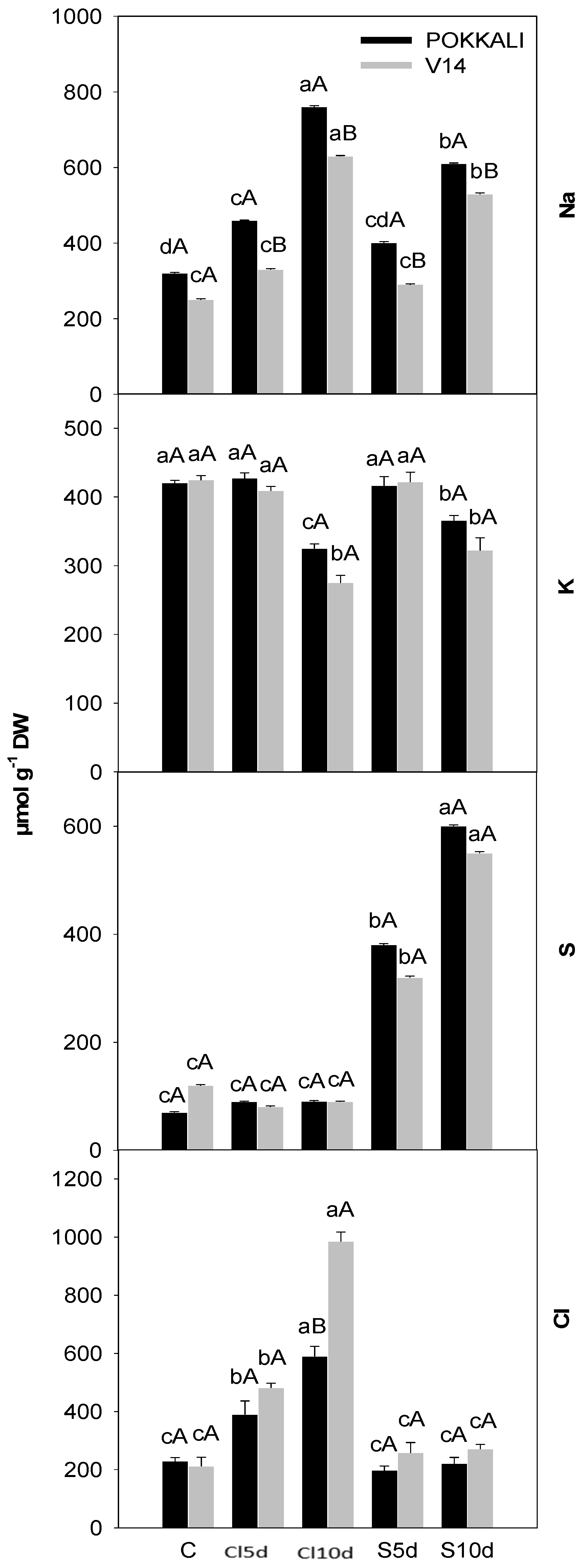
2.4. Estimation of Plant Ion Content
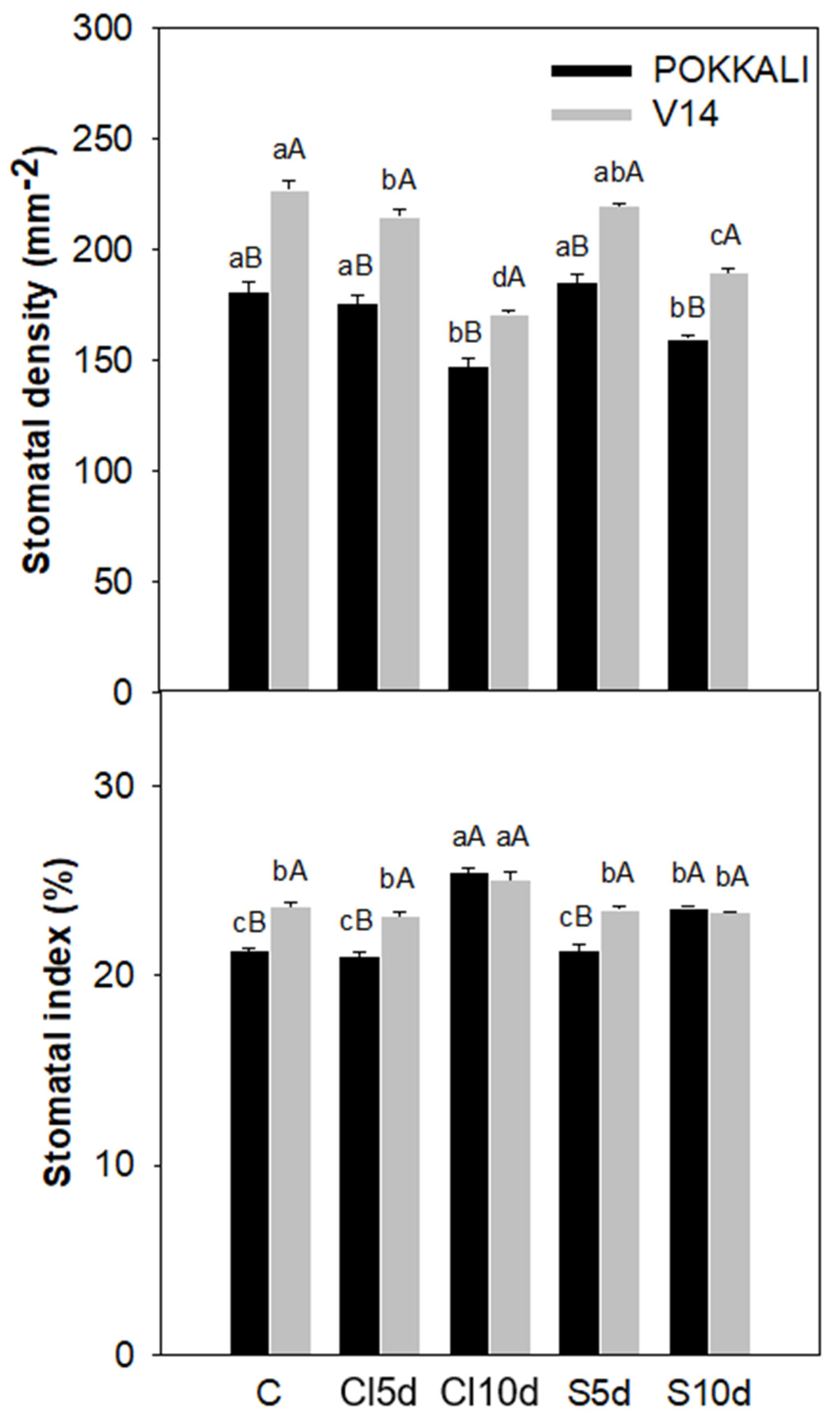
2.5. Measurement of Stomatal Density and Stomatal Index
2.6. Total Grain Protein Content
2.7. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Physiological- and Yield-Related Parameters
4. Discussion
4.1. Soil Properties
4.2. Plant Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. How to Feed the World in 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 28 November 2019).
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pol. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell. Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Nijimbere, S. Physico-Chimie de Sols Rizicultivés Affectés par la Salinité Dans la Basse Vallée de la Rusizi au Burundi [Physico-Chemistry of Salinity-Affected Rice Soils in Burundi’s Lower Rusizi Valley]; Thèse Présentée en vue de L’obtention du Grade de Docteur en Sciences Agronomiques et Ingénierie Biologique; UCLouvain: Ottignies-Louvain-la-Neuve, Belgium, 2014. [Google Scholar]
- Gahiro, L. Compétitivité des Filières Rizicoles Burundaises: Le riz de l’Imbo et le riz des Marais [Competitiveness of Burundian Rice Fields: Imbo Rice and Marsh Rice]; Thèse Présentée en vue de L’obtention du Grade de Docteur en Socio-économie Rurale; Université de Liège: Liège, Belgium, 2011. [Google Scholar]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Front. Plant Sci. 2020, 11, 559876. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Ndayiragije, A.; Lutts, S. Long term exogenous putrescine application improves grain yield of a salt-sensitive rice cultivar exposed to NaCl. Plant Soil 2007, 29, 225–238. [Google Scholar] [CrossRef]
- Zhu, G.; Kinet, J.; Lutts, S. Characterisation of rice (Oryza sativa) F3 populations selected for salt resistance. 2. Relationships between yield-related parameters and physiological properties. Austral. J. Exp. Agric. 2004, 44, 333–342. [Google Scholar] [CrossRef]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Rufyikiri, G.; Lutts, S. NaCl and Na2SO4 salinities have different impact on photosynthesis and yield-related parameters in Rice (Oryza sativa L.). Agronomy 2020, 10, 864. [Google Scholar] [CrossRef]
- Irakoze, W.; Vanpee, B.; Rufyikiri, G.; Dailly, H.; Nijimbere, S.; Lutts, S. Comparative effects of chloride and sulfate salinities on two contrasting rice cultivars (Oryza sativa L.) at the seedling stage. J. Plant. Nut. 2019, 42, 1001–1015. [Google Scholar] [CrossRef]
- Baxter, G.; Zhao, J.; Blanchard, C. Salinity alters the protein composition of rice endosperm and the physicochemical properties of rice flour. J. Sci. Food Agric. 2011, 91, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.Q.; Liu, Y.F.; Liu, P.; Lei, G.; He, S.J.; Ma, B.; Zhang, W.K.; Zhang, J.S.; Chen, S.Y. Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J. 2010, 62, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Apte, S.K. Transient expression of multiple genes in salinity-stressed young seedlings of rice (Oryza sativa L.) cv. Bura Rata. Biochem. Biophys. Res. Commun. 1997, 233, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Yunita, R.; Khumaida, N.; Sopandie, D.; Marisha, I. Somaclonal putative mutants of rice tolerant to salinity. Indon. J. Agric. Sci. 2018, 19, 67–74. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulture 2017, 3, 30. [Google Scholar] [CrossRef]
- Rengasamy, P.; Chittleborough, D.; Helyar, K. Root-zone constraints and plant-based solutions for dryland salinity. Plant Soil 2003, 257, 249–260. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Cieleski, H.; Sterckeman, T.A. Comparison between three methods for the determination of cation exchange capacity and exchangeable cations in soils. Agronomie 1997, 17, 9–16. [Google Scholar] [CrossRef]
- Rodier, J. L’Analyse de l’eau: Eaux Naturelles, Eaux Résiduaires, Eau de Mer, 8th ed.; Dunod: Malakoff, France, 2005; p. 1381. [Google Scholar]
- Hamrouni, L.; Hanana, M.; Abdelly, C.; Ghorbel, A. Exclusion du chlorure et inclusion du sodium: Deux mécanismes concomitants de tolérance à la salinité chez la vigne sauvage Vitis vinifera subsp. sylvestris (var.‘Séjnène’) [Exclusion of chloride and inclusion of sodium: Two concomitant mechanisms of salinity tolerance in wild vine Vitis vinifera subsp. sylvestris (var.‘Sejnène’)]. Biotech. Agron. Soc. Environ. 2011, 15, 387–400. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do All the Numbers Mean? CSIRO Publishing: Clayton South, VIC, Australia, 2016; p. 185. [Google Scholar]
- HillLab. Cation Exchange Capacity & Base Saturation. Available online: https://www.hill-laboratories.com/assets/Documents/Technical-Notes/Agriculture/CATION-EXCHANGE-CAPACITY-BASE-SATURATION.pdf (accessed on 28 November 2019).
- Skaggs, T.H.; Arya, L.M.; Shouse, P.J.; Mohanty, B.P. Estimating particle-size distribution from limited soil texture data. Soil Sci. Soc. Am. J. 2001, 65, 1038–1044. [Google Scholar] [CrossRef]
- Suarez, D.L. Sodic soil reclamation: Modelling and field study. Soil Res. 2001, 39, 1225–1246. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. Soil Sci. 1954, 78, 54. [Google Scholar] [CrossRef]
- Lebron, I.; Suarez, D.; Yoshida, T. Gypsum effect on the aggregate size and geometry of three sodic soils under reclamation. Soil Sci. Soc. Am. J. 2002, 66, 92–98. [Google Scholar] [CrossRef]
- Shainberg, I.; Letey, J. Response of soils to sodic and saline conditions. Hilgardia 1984, 52, 1–57. [Google Scholar] [CrossRef]
- McIntyre, D. Exchangeable sodium, subplasticity and hydraulic conductivity of some Australian soils. Soil Res. 1979, 17, 115–120. [Google Scholar] [CrossRef]
- Saidi, D.; Le Bissonnais, Y.; Duval, O.; Daoud, Y.; Halitim, A. Effet du sodium échangeable et de la concentration saline sur les propriétés physiques des sols de la plaine du Cheliff (Algérie) [Effect of exchangeable sodium and saline concentration on the physical properties of the soils of the Cheliff plain (Algeria)]. Etud. Gest. Sols. 2004, 11, 137–148. [Google Scholar]
- Alves, M.; Lavorenti, A. Sulfate adsorption and its relationships with properties of representative soils of the São Paulo State, Brazil. Geoderma 2004, 118, 89–99. [Google Scholar] [CrossRef]
- Bolan, N.; Syers, J.; Tillman, R. Ionic strength effects on surface charge and adsorption of phosphate and sulphate by soils. J. Soil Sci. 1986, 37, 379–388. [Google Scholar] [CrossRef]
- Kitadai, N.; Nishiuchi, K.; Tanaka, M. A comprehensive predictive model for sulfate adsorption on oxide minerals. Geochim. Cosmochim. Acta 2018, 238, 150–168. [Google Scholar] [CrossRef]
- Sokolova, T.; Alekseeva, S. Adsorption of sulfate ions by soils (a review). Euras. Soil Sci. 2008, 41, 140–148. [Google Scholar] [CrossRef]
- Marlet, S.; Job, J.O. Processus et Gestion de la Salinité des Sols [Soil Salinity Process and Management], 1st ed.; Tec & Doc—Lavoisier: Paris, France, 2006; pp. 797–822. Available online: http://www.documentation.ird.fr/hor/fdi:010067448 (accessed on 14 April 2019).
- Qadir, M.; Noble, A.D.; Schubert, S.; Thomas, R.J.; Arslan, A. Sodicity-induced land degradation and its sustainable management: Problems and prospects. Land Degrad. Dev. 2006, 17, 661–676. [Google Scholar] [CrossRef]
- Rengasamy, P. Osmotic and ionic effects of various electrolytes on the growth of wheat. Soil Res. 2010, 48, 120–124. [Google Scholar] [CrossRef]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Compared effects of sudden and progressive impositions of salt stress in three durum wheat (Triticum durum Desf.) cultivars. J. Plant Physiol. 1999, 154, 743–752. [Google Scholar] [CrossRef]
- Mizanur, R.B.; Roxana, A. Effect of Salinity on Physical and Chemical Properties of Soils of Khulna Region; Khulna University: Khulna, Bangladesh, 2005; 17p. [Google Scholar]
- Li, M.; Chai, S.; Du, H.; Wang, C. Effect of chlorine salt on the physical and mechanical properties of inshore saline soil treated with lime. Soils Found. 2016, 56, 327–335. [Google Scholar] [CrossRef]
- Naher, N.; Uddin, M.K.; Alam, A. Impacts of salinity on soil properties of coastal areas in Bangladesh. J. Agric. Sci. 2011, 33, 161–173. [Google Scholar] [CrossRef]
- Lutts, S.; Bouharmont, J.; Kinet, J.M. Physiological characterisation of salt-resistant rice (Oryza sativa) somaclones. Aust. J. Bot. 1999, 47, 835–849. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. The Importance of Cl- exclusion and vacuolar Cl− sequestration: Revisiting the role of Cl− transport in plant salt tolerance. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Pate, J.S. Cation and chloride partitioning through xylem and phloem within the whole plant of Ricinus communis L. under conditions of salt stress. J. Exp. Bot. 1991, 42, 1105–1116. [Google Scholar] [CrossRef]
- Kretschmer, A.; Toth, S.; Bear, F. Effect of chloride versus sulfate ions on nutrient-ion absorption by plants. Soil Sci. 1953, 76, 193–200. [Google Scholar] [CrossRef]
- Gauch, H.G.; Wadleigh, C.H. Effect of high concentrations of sodium, calcium, chloride, and sulfate on ionic absorption by bean plants. Soil Sci. 1945, 59, 139–155. [Google Scholar] [CrossRef]
- Messerli, M.A.; Smith, P.J.S.; Lewis, R.C.; Robinson, K.R. Chloride fluxes in lily pollen tubes: A critical reevaluation. Plant J. 2004, 40, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Mateu, I.; Sanz, A.; Cornejo, M.J. Common and distinctive responses of rice seedlings to saline-and osmotically-generated stress. Plant Growth Regul. 2002, 38, 83–94. [Google Scholar] [CrossRef]
- Lestari, E.G. The relation between stomata index and drought resistant at rice somaclones of Gajahmungkur, Towuti, and IR 64. Biodivers. J. Biol. Divers. 2006, 7, 44–48. [Google Scholar] [CrossRef]
- Wankhade, S.D.; Bahaji, A.; Mateu-Andrés, I.; Cornejo, M.J. Phenotypic indicators of NaCl tolerance levels in rice seedlings: Variations in development and leaf anatomy. Acta Physiol. Plant. 2010, 32, 1161–1169. [Google Scholar] [CrossRef][Green Version]
- Yokas, İ.; Tuna, A.L.; Bürün, B.; Altunlu, H.; Altan, F.; Kaya, C. Responses of the tomato (Lycopersicon esculentum Mill.) plant to exposure to different salt forms and rates. Turk. J. Agric. For. 2008, 32, 319–329. [Google Scholar]
- Boccalandro, H.E.; Rugnone, M.L.; Moreno, J.E.; Ploschuk, E.L.; Serna, L.; Yanovsky, M.J.; Casal, J.J. Phytochrome B enhances photosynthesis at the expense of water-use efficiency in Arabidopsis. Plant Physiol. 2009, 150, 1083–1092. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B. Molecular and environmental factors determining grain quality in rice. Food Energy Sec. 2012, 1, 111–132. [Google Scholar] [CrossRef]
- Thitisaksakul, M.; Tananuwong, K.; Shoemaker, C.F.; Chun, A.; Tanadul, O.; Labavitch, J.M.; Beckles, D.M. Effects of timing and severity of salinity stress on rice (Oryza sativa L.) yield, grain composition, and starch functionality. J. Agric. Food Chem. 2015, 63, 2296–2304. [Google Scholar] [CrossRef]
- Wu, G.; Peterson, A.J.; Morris, C.F.; Murphy, K.M. Quinoa seed quality response to sodium chloride and sodium sulfate salinity. Front. Plant Sci. 2016, 7, 790. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Bhowmik, P.C.; Hossain, M.; Rahman, M.M.; Prasad, M.N.V.; Ozturk, M.; Fujita, M.H. Performance of four irrigated rice varieties under different levels of salinity stress. Int. J. Integr. Biol. 2009, 6, 85–90. [Google Scholar]
- Khan, M.A.; Abdullah, Z. Salinity–sodicity induced changes in reproductive physiology of rice (Oryza sativa) under dense soil conditions. Environ. Exp. Bot. 2003, 49, 145–157. [Google Scholar] [CrossRef]
- Abdullah, Z.; Khan, M.A.; Flowers, T. Causes of sterility in seed set of rice under salinity stress. J. Agron. Crop. Sci. 2001, 187, 25–32. [Google Scholar] [CrossRef]
- Grieve, C.; Lesch, S.M.; Maas, E.V.; François, L.E. Leaf and spikelet primordia initiation in salt-stressed wheat. Crop Sci. 1993, 33, 1286–1294. [Google Scholar] [CrossRef]
- Khatun, S.; Rizzo, C.; Flowers, T. Genotypic variation in the effect of salinity on fertility in rice. Plant Soil 1995, 173, 239–250. [Google Scholar] [CrossRef]

| Cultivar | Treatment | pH | EC (dS/m) | CEC (meq/100 g Soil) |
|---|---|---|---|---|
| Pokkali | Soil | 6.9 ± 0.09 bA | 0.17 ± 0.003 cA | 22.67 ± 1.16 aA |
| C | 7.0 ± 0.05 bA | 0.21 ± 0.022 cA | 21.62 ± 0.13 aA | |
| Cl 5 | 7.4 ± 0.2 bA | 0.72 ± 0.116 bA | 21.14 ± 0.15 aA | |
| Cl10d | 7.5 ± 0.2 bA | 0.97 ± 0.027 aA | 19.34 ± 0.68 aA | |
| S5d | 8.2 ± 0.04 aA | 0.69 ± 0.019 bA | 22.34 ± 1.05 aA | |
| S10d | 8.2 ± 0.01 aA | 1.03 ± 0.017 aA | 15.27 ± 1.14 bB | |
| V14 | Soil | 6.9 ± 0.09 bA | 0.17 ± 0.003 cA | 22.67 ± 1.16 aA |
| C | 7.1 ± 0.03 bA | 0.25 ± 0.050 cA | 18.50 ± 0.60 aA | |
| Cl5d | 7.3 ± 0.07 bA | 0.65 ± 0.038 bA | 17.19 ± 1.15 aA | |
| Cl10d | 7.0 ± 0.14 bA | 0.98 ± 0.033 aA | 17.60 ± 0.84 aA | |
| S5d | 8.1 ± 0.06 aA | 0.61 ± 0.078 bA | 19.32 ± 1.10 aA | |
| S10d | 8.0 ± 0.15 aA | 0.91 ± 0.021 aA | 20.53 ± 6.14 aA |
| Cultivar | Treatment | Exchangeable Cations (meq/100g Soil) | ||||
|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | ESP (%) | ||
| Pokkali | Soil | 0.8 ± 0.02 cA | 0.9 ± 0.05 bA | 20.3 ± 0.4 aA | 10 ± 0.2 cA | 3.5 ± 0.2 cdA |
| C | 0.3 ± 0.06 cA | 1.8 ± 0.05a bA | 20.4 ± 0.3 aA | 15.7 ± 0.4 aA | 1.4 ± 0.3 dA | |
| Cl5d | 1.3 ± 0.5 cA | 2.8 ± 0.5 aA | 19.9 ± 0.6 aA | 12.9 ± 0.9 bA | 6.1 ± 2.1 cdA | |
| Cl10d | 3.4 ± 0.3 bA | 2.4 ± 0.2 aA | 19.3 ± 0.3 aA | 12 ± 0.4 bcA | 17.6 ± 1.7 bA | |
| S5d | 3.0 ± 0.15 bA | 2.0 ± 0.02 abA | 20.0 ± 0.3 aA | 14 ± 0.5 abA | 13.4 ± 1.3 bcA | |
| S10d | 5.5 ± 0.2 aA | 2.3 ± 0.04 aA | 20.2 ± 0.4 aA | 13.9 ± 0.8 abA | 36 ± 5 aA | |
| V14 | Soil | 0.8 ± 0.02 dA | 0.9 ± 0.05 cA | 20.3 ± 0.4 aA | 10 ± 0.2 cA | 3.5 ± 0.2 dA |
| C | 1 ± 0.009 dA | 2.4 ± 0.09 aA | 18.3 ± 1.3 abA | 15 ± 0.4 cA | 5.4 ± 0.2 dA | |
| Cl5d | 2 ± 0.2 cA | 2.0 ± 0.24 abA | 18.4 ± 0.6 abA | 12 ± 0.2 bA | 11.6 ± 1.2 bcA | |
| Cl10d | 1.4 ± 0.2 cdb | 2.2 ± 0.1 abA | 16.9 ± 0.18 abA | 9.9 ± 0.2 cA | 8 ± 1.4 cdB | |
| S5d | 3.1 ± 0.3 bA | 1.7 ± 0.13 bA | 16.7 ± 0.7 bB | 13 ± 0.3 abA | 16.1 ± 1.4 abA | |
| S10d | 4.2 ± 0.9 aA | 2.1 ± 0.06 abA | 18 ± 0.8 abA | 13 ± 0.6 bA | 20.5 ± 1 aB | |
| Cultivar | Treatment | Water Soluble Ions (mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | Cl | S | CO32− | SAR | ||
| Pokkali | Soil | 4 ± 0.03 dA | 0.5 ± 0.02 bA | 1.9 ± 0.005 bcA | 0.8 ± 0.007 dA | 1.5 ± 0.03 cA | 1.4 ± 0.06 cA | 3.7 ± 0.2 cA | 3.4 ± 0.02 dA |
| C | 2.2 ± 0.2 dA | 1.3 ± 0.06a bA | 1.6 ± 0.03 cA | 1.1 ± 0.04c dA | 1.7 ± 0.7 cA | 0.8 ± 0.04 cA | 9.1±0.5 abA | 1.9 ± 0.1 dA | |
| Cl5d | 7.5 ± 1.3 cA | 3.3 ± 1.23 aA | 2.8 ± 0.7a bcA | 1.6 ± 0.3 abcA | 16 ± 4.9 bA | 1.2 ± 0.16 cA | 6.6±1.4 bcA | 5.3 ± 1 cA | |
| Cl10d | 17 ± 0.8 bA | 2.8 ± 0.3 abA | 3.2 ± 0.28 abA | 1.8 ± 0.06a dA | 27 ± 1.6a A | 1.2 ± 0.02 cA | 6.8±0.7 abcA | 11 ± 0.8 bA | |
| S5d | 14 ± 0.6 bA | 1.7 ± 0.02 abA | 2.4 ± 0.16a bcA | 1.5 ± 0.02 bcA | 2.8 ± 0.5 cA | 7.4 ± 0.3 bA | 10.5±0.6 aA | 10.5 ± 0.7 bA | |
| S10d | 27 ± 0.3 aA | 2.3 ± 0.14 abA | 3.4 ± 0.15 aA | 2.2 ± 0.03 aA | 2.7 ± 0.5 cA | 17 ± 0.6 aA | 10.1±0.7 abA | 16.4 ± 0.3 aA | |
| V14 | Soil | 4 ± 0.03 dA | 0.5 ± 0.02 cA | 1.9 ± 0.005 bA | 0.8 ± 0.007 cA | 1.5 ± 0.03 cA | 1.4 ± 0.06 cA | 3.7±0.2 cA | 3.4 ± 0.02 dA |
| C | 3 ± 0.07 dA | 1.7 ± 0.37 abA | 1.8 ± 0.09 bA | 1.1 ± 0.04 bcA | 1.2 ± 0.1 cA | 0.8 ± 0.07 cA | 9.5±0.3 aA | 2.5 ± 0.02 dA | |
| Cl5d | 8 ± 0.7 cA | 1.6 ± 0.3 bcA | 1.7 ± 0.1 bA | 0.9 ± 0.02 cB | 13 ± 2.4 bA | 0.8 ± 0.03 cA | 7.8±0.05 bA | 7.3 ± 0.5 cA | |
| Cl10d | 10 ± 1.1 bcB | 2.8 ± 0.3 aA | 3.4 ± 0.3 aA | 1.7 ± 0.12 aA | 27 ± 1.3 aA | 1.4 ± 0.03 cA | 2.6±0.48 cB | 6.3 ± 0.9 cB | |
| S5d | 13 ± 0.7 bA | 1.5 ± 0.1 bcA | 1.7 ± 0.1 bA | 1.1 ± 0.06 bcA | 1.5 ± 0.3 cA | 7.5 ± 1.5 bA | 8.4±0.3 abA | 10.5 ± 0.5 bA | |
| S10d | 19 ± 0.7 aB | 1.9 ± 0.07 abA | 2.4 ± 0.17 bA | 1.4 ± 0.14 abB | 1.1 ± 0.09 cA | 14 ± 0.6 aB | 8.4±0.2 abA | 13.7 ± 0.1 aA | |
| Yield Parameters Measured | Cultivar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pokkali | V14 | |||||||||
| C | Cl5d | Cl10d | S5d | S10d | C | Cl5d | Cl10d | S5d | S10d | |
| Plant height | 157 ± 3.5 aA | 154 ± 4.6 aA | 124 ± 3.2 bA | 154 ± 3 aA | 136 ± 3 bA | 73 ± 1.7 aB | 70 ± 1.2abB | 60 ± 0.58 dB | 68 ± 1.15 bcB | 64 ± 0.7 cdB |
| Number tiller/plant | 25 ± 0.7 aB | 16 ± 2.6 abB | 7 ± 3.3 bA | 20 ± 2 aB | 16 ± 1.2a bA | 45 ± 1.5 aA | 38 ± 0.6bA | 6 ± 1.9 dA | 45 ± 1.5 aA | 30 ± 1.5c A |
| Number panicle/plant | 25 ± 0.7 aB | 16 ± 2.6 abB | 5 ± 2.3 cA | 20 ± 2 abB | 14 ± 0.9 bcB | 45 ± 1.5 aA | 35 ± 1.2bA | 3 ± 1 dA | 39 ± 2 abA | 24 ± 0.9 cA |
| Days to heading time | 110 ± 1.7 aA | 107 ± 0.1 abA | 106 ± 1.2a bA | 109 ± 0.7 aA | 104 ± 0.1 bA | 107 ± 2 aA | 108 ± 2aA | 95 ± 0.9b B | 107 ± 0.3 aA | 96 ± 1.9b B |
| Panicle length (cm) | 28 ± 0.6 aA | 26.7 ± 0.9 aA | 20 ± 0.6 bA | 27.7 ± 0.9 aA | 22.3 ± 0.9 bA | 21 ± 0.09 aB | 18.5 ± 1aB | 14 ± 0.5 bB | 19.3 ± 0.3 aB | 15.2 ± 0.8 bB |
| Straw fresh weight g/plant | 87 ± 2 aA | 74.7 ± 1.9 bA | 38 ± 3.2 cA | 76.3 ± 1.9 bA | 67.7 ± 0.9 bA | 76 ± 0.7 aB | 69 ± 1 bA | 22.7 ± 1.5 dB | 71 ± 0.6 bA | 37 ± 1.5 bA |
| Grains number/panicle | 195 ± 4.7 aA | 186 ± 3.8a A | 72.7 ± 8 cA | 192 ± 5 aA | 128 ± 10 bA | 160 ± 5 aB | 139 ± 2 bB | 92.3 ± 4.4 dA | 146 ± 1.5ab B | 118 ± 1.9 cA |
| Filled grains/panicle (%) | 85 ± 1.8 aA | 55.4 ± 5 bA | 35.6 ± 3.7 cA | 84.3 ± 1.8 aA | 58.7 ± 3.1 bA | 81.3 ± 3a A | 51 ± 0.6 cA | 12.6 ± 1.2 bB | 64.3 ± 1.2 bB | 41.7 ± 1.5 dB |
| 1000-grains weight (g) | 19.5 ± 1 aA | 18.4 ± 0.4 aA | 12.6 ± 0.5 bA | 17.4 ± 0.4 aA | 14 ± 0.6 bA | 21.6 ± 0.8 aA | 16.4 ± 0.5 bcA | 11.4 ± 0.9 dA | 19 ± 0.6 abA | 14.3 ± 0.9 cdA |
| Grain yield/plant (g) | 23 ± 1.2 aA | 17 ± 0.58 bA | 10.7 ± 0.9 bA | 18.3 ± 0.9 bA | 15.3 ± 0.7 bA | 24.7 ± 0.3aA | 16.3 ± 0.3 cA | 6.3 ± 0.3e B | 19.3 ± 0.3 bA | 10.7 ± 0.9 dB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Bizimana, J.B.; Bigirimana, J.; Rufyikiri, G.; Lutts, S. NaCl- and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi. Agronomy 2021, 11, 571. https://doi.org/10.3390/agronomy11030571
Irakoze W, Prodjinoto H, Nijimbere S, Bizimana JB, Bigirimana J, Rufyikiri G, Lutts S. NaCl- and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi. Agronomy. 2021; 11(3):571. https://doi.org/10.3390/agronomy11030571
Chicago/Turabian StyleIrakoze, Willy, Hermann Prodjinoto, Séverin Nijimbere, Jean Berchmans Bizimana, Joseph Bigirimana, Gervais Rufyikiri, and Stanley Lutts. 2021. "NaCl- and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi" Agronomy 11, no. 3: 571. https://doi.org/10.3390/agronomy11030571
APA StyleIrakoze, W., Prodjinoto, H., Nijimbere, S., Bizimana, J. B., Bigirimana, J., Rufyikiri, G., & Lutts, S. (2021). NaCl- and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi. Agronomy, 11(3), 571. https://doi.org/10.3390/agronomy11030571






