Influences of Priming on Selected Physiological Attributes and Protein Pattern Responses of Salinized Wheat with Extracts of Hormophysa cuneiformis and Actinotrichia fragilis
Abstract
1. Introduction
2. Materials and methods
2.1. Sampling and Extract Preparation
2.2. Physico-Chemical and Biochemical Analysis of Seaweeds and Resulted Extracts
| Parameters | H. cuneiformis Extract | A. fragilis Extract |
| Color | Brown | Red |
| PH | 6.8 | 6.8 |
| Magnesium | 100.24 mg L−1 | 120.00 mg L−1 |
| Calcium | 110.00 mg L−1 | 180.22 mg L−1 |
| Sodium | 176.00 mg L−1 | 206.00 mg L−1 |
| Potassium | 150.00 mg L−1 | 230.00 mg L−1 |
| Chloride | 200.50 mg L−1 | 350.00 mg L−1 |
| Phosphorus | 43.42 mg L−1 | 74.00 mg L−1 |
| Protein | 221.11 mg L−1 | 146.74 mg L−1 |
| Carbohydrate content | 352.77 mg L−1 | 305.82 mg L−1 |
| Lipid | 53.00 mg L−1 | 32.55 mg L−1 |
2.3. Seaweed Liquid Fertilizers and GC-MS Conditions
2.4. Plant Materials, Growth Conditions and Treatments
2.5. Plant Growth Parameters and Pigment Contents
2.6. Content Organic Solutes and Phenolic Compounds
2.7. Measurement of the Content of Na+, K+, Ca2+ and Mg2+
2.8. Assay of Malondialdehyde and Activities of Enzymatic Antioxidants
2.9. Protein Extraction and SDS-PAGE Electrophoresis
2.10. Statistical Analyses
3. Results
3.1. GC-MS Analysis of the Seaweed Extracts
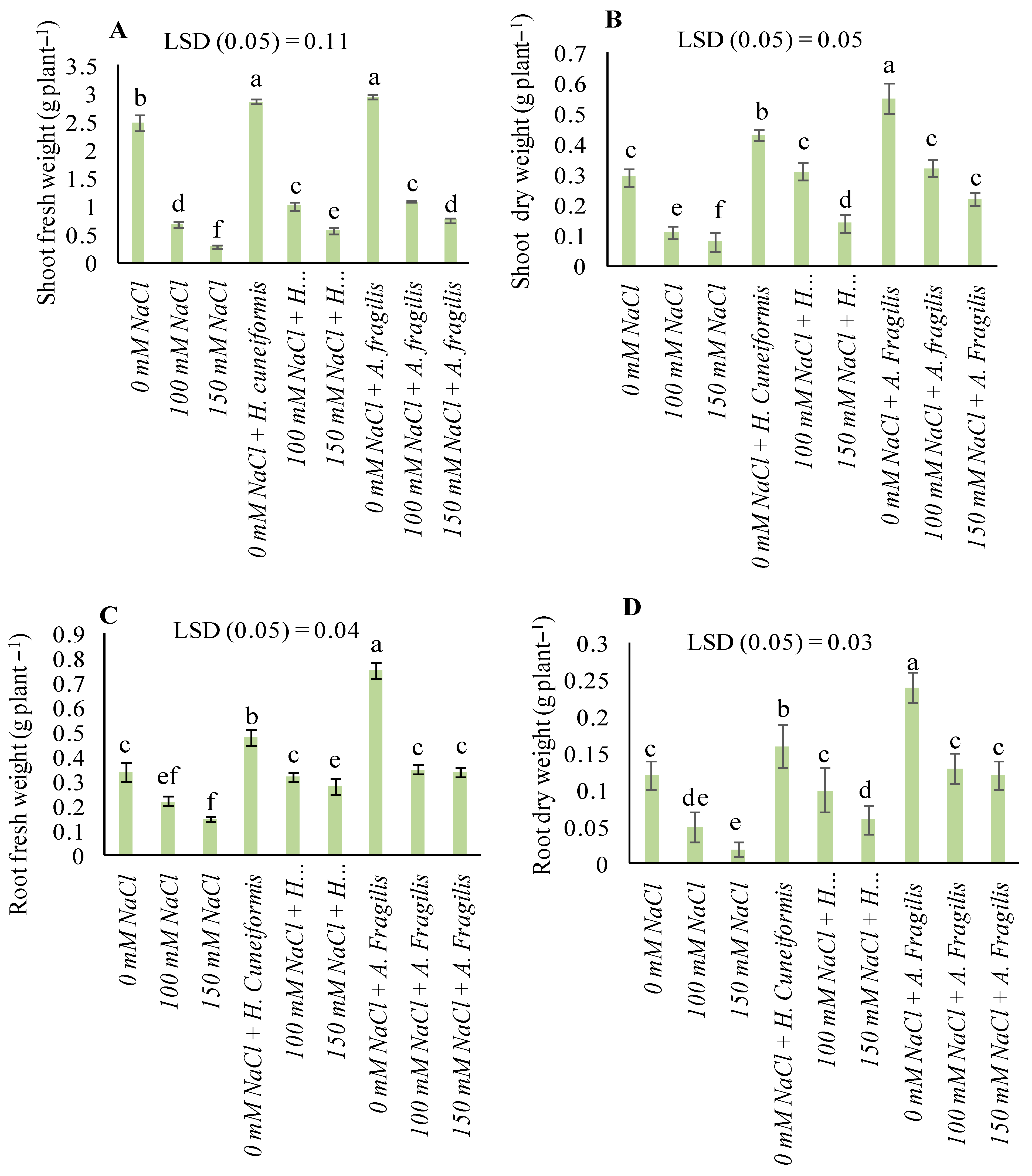
3.2. Plant Growth Parameters
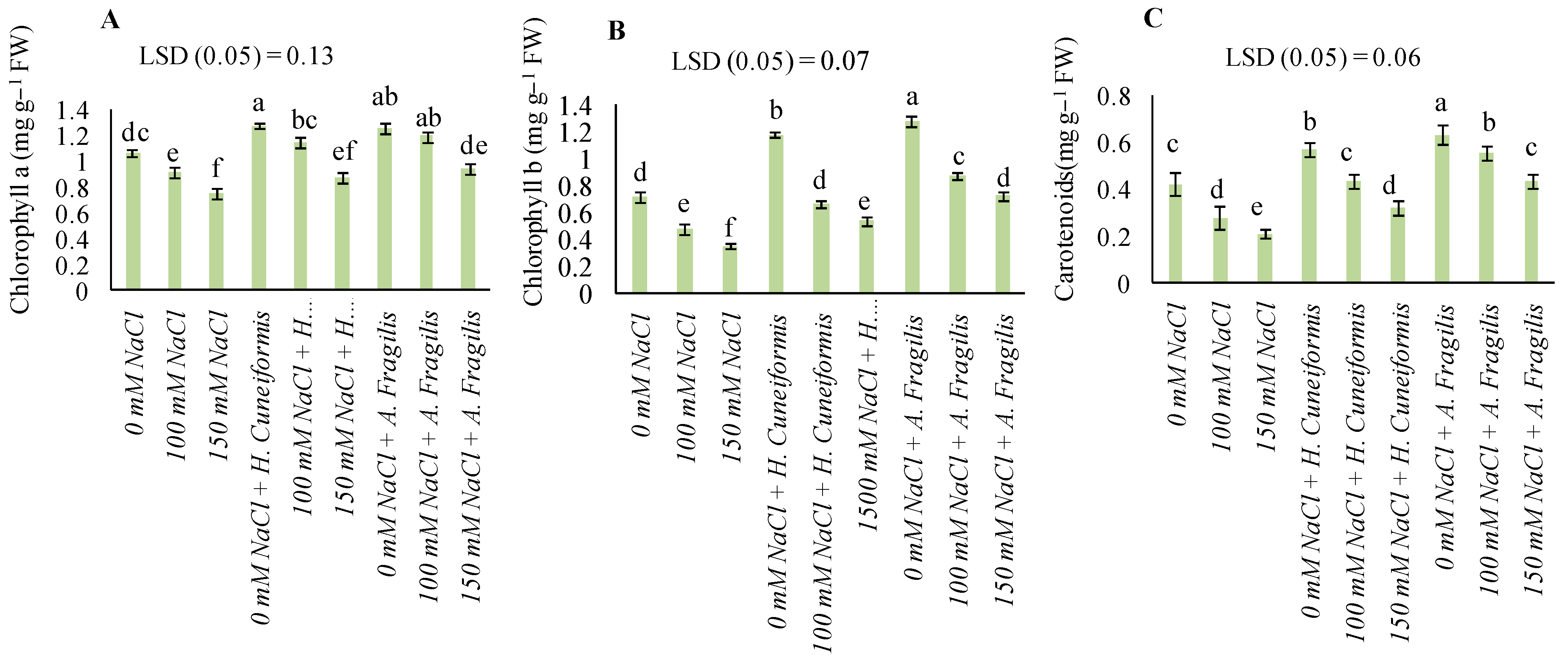
3.3. Content of Pigments
3.4. Osmolytes and Phenolic Contents
3.5. Na+, K+, Ca2+ and Mg2+ Uptake
3.6. Oxidative Damage and Antioxidant Enzyme Activities
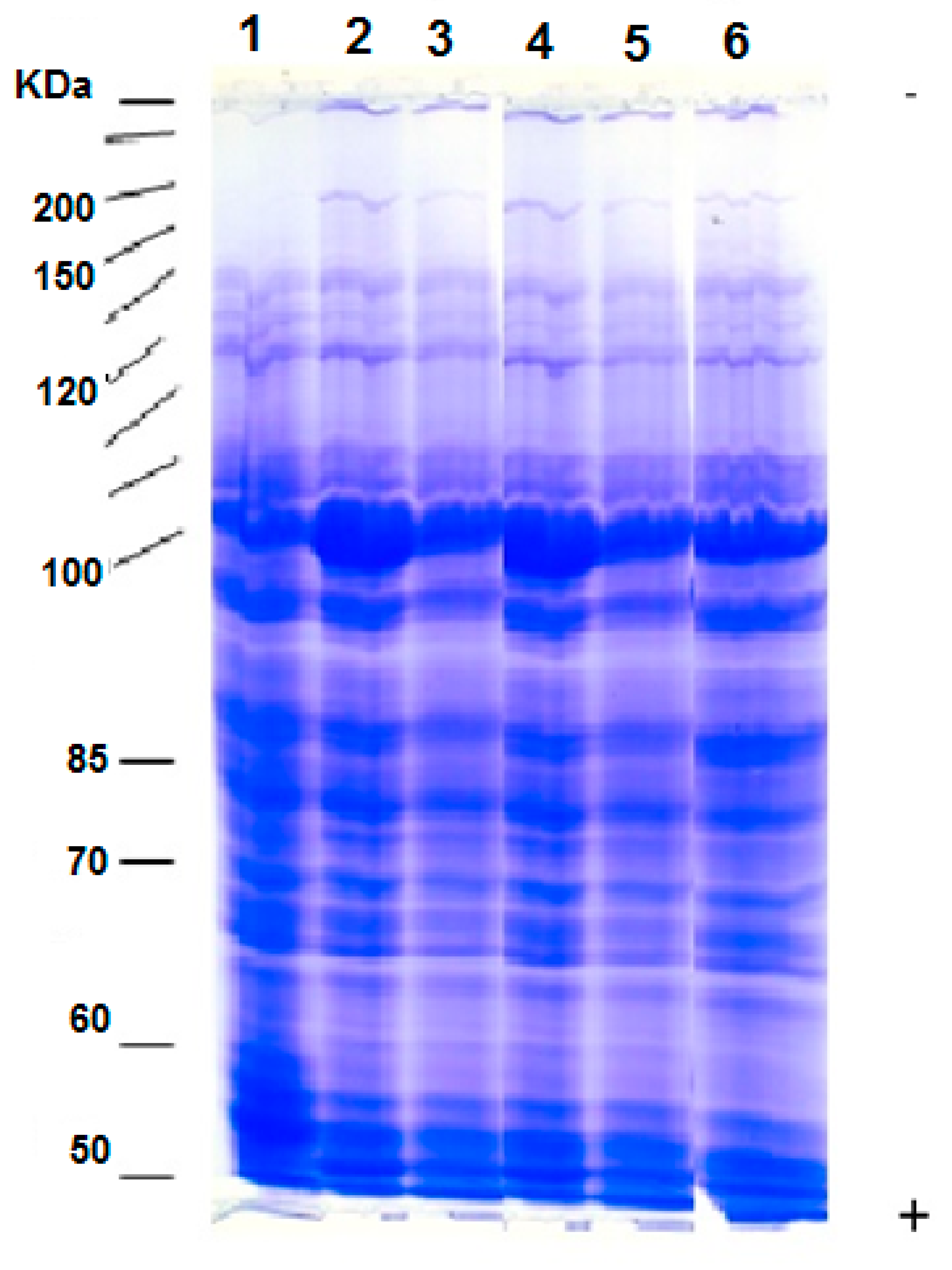
3.7. Protein Patterns
4. Discussion
4.1. Both H. cuneiformis and A. fragilis Extracts Mediated Amelioration of Salt Stress in Wheat Plants by Regulating Shoot and Root Growth
4.2. H. cuneiformis and A. fragilis Extracts Mediated Amelioration of Salt Stress in Wheat Plants by Regulating Physiological and Biochemical Attributes
4.3. H. cuneiformis and A. fragilis Extracts Mediated Amelioration of Salt Stress in Wheat Plants by Regulating Ionic Balance
4.4. H. cuneiformis and A. fragilis Extracts Mediated the Amelioration of Salt Stress in Wheat by Preventing Oxidative Damage and Accelerating Antioxidant Enzyme Activities
4.5. Differential Changes Were Observed in the Expression Patterns of Proteins in Wheat Plants Grown under Salinity Stress and Seaweed Extract Treatments
4.6. Possibility of Correlation between the Bioactive Compounds Detected in Extracts and Elevated Salinity Tolerance in Wheat Plants Based on GC-MS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roychoudhury, A.; Singh, A.; Aftab, T.; Ghosal, P.; Banik, N. Seedling Priming with Sodium Nitroprusside Rescues Vigna radiata from Salinity Stress-Induced Oxidative Damages. J. Plant Growth Regul. 2021. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Zaid, A.; Mohammad, F. Plant Growth Regulators and Salt Stress: Mechanism of Tolerance Trade-Off. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Springer: Singapore, 2019; pp. 91–111. [Google Scholar]
- Osman, M.S.; Badawy, A.A.; Osman, A.I.; Abdel Latef, A.A.H. Ameliorative Impact of an Extract of the Halophyte Arthrocnemum macrostachyum on Growth and Biochemical Parameters of Soybean Under Salinity Stress. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Hernández, J.A. Salinity tolerance in plants: Trends and perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 facilitates plant growth by improving tolerance to salt stress in two wheat cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Mostofa, M.G.; Rahman, M.; Abdel-Farid, I.B.; Tran, L.-S.P. Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physiobiochemical characteristics of stressed plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Kordrostami, M.; Abo-Baker, A.-B.A.-E.; Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 2020, 39, 1–14. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Comp. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mahajan, M.M.; Singh, N.K.; Kumar, D.; Kumar, K. Physiological and molecular response under salinity stress in bread wheat (Triticum aestivum L.). J. Plant Biochem. Biotechnol. 2020, 29, 125–133. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Liu, W.; Jing, Q.; Cao, W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H. Changes of antioxidative enzymes in salinity tolerance among different wheat cultivars. Cereal Res. Commun. 2010, 38, 43–55. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kishor, P.B.K. Engineering salinity tolerance in plants: Progress and prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Dutta, T.; Neelapu, N.R.R.; Surekha, C. Transgenic approaches to enhance salt and drought tolerance in plants. Plant Gene 2017, 11, 219–231. [Google Scholar] [CrossRef]
- Wani, S.H.; Tripathi, P.; Zaid, A.; Challa, G.S.; Kumar, A.; Kumar, V.; Upadhyay, J.; Joshi, R.; Bhatt, M. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol. Biol. 2018, 97, 469–487. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Mancuso, S.; Briand, X.; Mugnai, S.; Azzarello, E. Marine bioactive substances (IPA extract) improve ion fluxes and water stress tolerance in potted Vitis vinifera plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar]
- El-Sharkawy, M.; El-Beshsbeshy, T.; Al-Shal, R.; Missaoui, A. Effect of plant growth stimulants on alfalfa response to salt stress. Agric. Sci. 2017, 8, 267–291. [Google Scholar] [CrossRef][Green Version]
- Abdel Latef, A.A.H.; Srivastava, A.K.; Saber, H.; Alwaleed, E.A.; Tran, L.-S.P. Sargassum muticum and Jania rubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- El-Sharouny, H.M.; El-Tayeb, M.A.; Ismail, M.S. Macroalgae associated with mangroves at Hurghada and Safaga of the Egyptian Red Sea coast. J. King Abdulaziz Univ. Mar. Sci. 2001, 12, 241–251. [Google Scholar] [CrossRef]
- Sayed, A.A.; Sadek, S.A.; Soliman, A.M.; Marzouk, M. Prospective effect of red algae, Actinotrichia fragilis, against some osteoarthritis aetiology. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 231–241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salem, W.M.; Galal, H.; Nasr El-deen, F. Screening for antibacterial activities in some marine algae from the red sea (Hurghada, Egypt). Afr. J. Microbiol. Res. 2011, 5, 2160–2167. [Google Scholar] [CrossRef]
- Mohamed, S.S.; Saber, A.A. Antifungal potential of the bioactive constituents in extracts of the mostly untapped brown seaweed Hormophysa cuneiformis from the Egyptian coastal waters. Egypt. J. Bot. 2019, 59, 695–708. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein—Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Weidner, K.; Eggum, B. Protein hydrolysis: A description of the method used at the Department of Animal Physiology in Copenhagen. Acta Agric. Scand. 1966, 16, 115–119. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Williams, V.; Twine, S. Flame photometric method for sodium, potassium and calcium. Mod. Methods Plant Anal. 1960, 5, 3–5. [Google Scholar]
- Abdel Latef, A.A.H.; Tran, L.-S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdel Latef, A.A.H.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.-S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C.; Chance, B. The assay of catalase and peroxidase. Methods Biochem. Anal. 1959, 1, 357–425. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Studier, F.W. Analysis of Bacteriophage T4 early RNAs and proteins of slab gel. J. Mol. Biol. 1973, 79, 237–248. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Srivastava, A.K.; El-Sadek, M.S.A.; Kordrostami, M.; Tran, L.-S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline conditions. Land Degrad. Dev. 2018, 29, 1065–1073. [Google Scholar] [CrossRef]
- Aymen, E.M.; Salma, L.; Halima, C.; Cherif, H.; Mimoun, E. Effect of seaweed extract of Sargassum vulgare on germination behavior of two tomatoes cultivars (Solanum Lycopersıcum L.) under salt stress. Octa J. Environ. Res. 2014, 2, 203–210. [Google Scholar]
- Yildiztekin, K.; Tuna, A.L.; Kaya, C. Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol. Hung. 2018, 69, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Chernane, H.; Latique, S.; Mansori, M.; El Kaoua, M. Salt stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. IOSR J. Agric. Vet. Sci. 2015, 8, 36–44. [Google Scholar]
- Mostafa, M.E.; Zheekh, L. Effect of seaweed extracts on seed germination, seedling growth and some metabolic process of Vicia faba L. Cytobios 1999, 100, 23–25. [Google Scholar]
- Blunden, G.; Jenkins, T.; Liu, Y.W. Enhanced chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. [Google Scholar] [CrossRef]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betanines in the increased chlorophyll content of plants treated with seaweed extract. Appl. Physiol. 1993, 5, 231–234. [Google Scholar]
- Akila, N.; Jeyadoss, T. The potential of seaweed liquid fertilizer on the growth and antioxidant enhancement of Helianthus annuus L. Orient. J. Chem. 2010, 26, 1353–1360. [Google Scholar]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef]
- Howladar, S.M. A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Kasim, W.A.E.A.; Khalil, M.; Saad-Allah, K.M.; Hamouda, M. Seed Priming with Extracts of two Seaweeds Alleviates the Physiological and Molecular Impacts of Salinity Stress on Radish (Raphanus sativus). Int. J. Agric. Biol. 2016, 18, 653–660. [Google Scholar] [CrossRef]
- Azooz, M.M.; Shaddad, M.A.; Abdel-Latef, A.A.H. Leaf growth and K+/Na+ ratio as an indication of the salt tolerance of three sorghum cultivars grown under salinity stress and IAA treatment. Acta Agric. Hung. 2004, 52, 287–296. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Fei, H.; Crouse, M.; Papadopoulos, Y.; Vessey, J.K. Enhancing the productivity of hybrid poplar (Populus × hybrid) and switchgrass (Panicum virgatum L.) by the application of beneficial soil microbes and a seaweed extract. Biomass Bioenergy 2017, 107, 122–134. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Ferrante, A.; Magnani, G. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 3, 86–88. [Google Scholar]
- Latique, S.; Chernane, H.; Mansori, M.; El Kaoua, M. Biochemical modification and changes in antioxidant enzymes in Triticum durum L. by seaweed liquid extract of Ulva rigida macroalgae under salt stress condition. Adv. Environ. Res. 2016, 50, 35–54. [Google Scholar]
- Fike, J.H.; Allen, V.G.; Schmidt, R.E.; Zhang, X.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Coelho, R.W.; Wester, D.B. Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J. Anim. Sci. 2001, 79, 1011–1021. [Google Scholar] [CrossRef]
- Ibrahim, W.M. Potential Impact of Marine Algal Extracts on the Growth and Metabolic Activities of Salinity Stressed Wheat Seedlings. J. Appl. Sci. 2016, 16, 388–394. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R. Antioxidant response to hormone-containing product in Kentucky Bluegrass subjected to drought. Crop Sci. 1999, 39, 545–551. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Altunlu, H.; Ashraf, M. Mitigation effects of non-enzymatic antioxidants in maize (Zea mays L.) Plants under salinity stress. Aust. J. Crop Sci. 2013, 7, 1181–1188. [Google Scholar]
- Abdel Latef, A.A.H.; Abu Alhmad, M.F.; Hammad, S.A. Foliar application of fresh moringa leaf extract overcomes salt stress in Fenugreek (Trigonella foenum-graecum) plants. Egypt. J. Bot. 2017, 57, 157–179. [Google Scholar]
- Mohiuddin, M.; Das, A.K.; Ghosh, D.C. Growth and productivity of wheat as influenced by integrated use of chemical fertilizer, biofertilizer and growth regulator. Indian J. Plant Physiol. 2000, 5, 334–338. [Google Scholar]
- Kang, K.; Park, S.; Kim, Y.S.; Lee, S.; Back, K. Biosynthesis and biotechnological production of serotonin derivatives. Appl. Microbiol. Biotechnol. 2009, 83, 27–34. [Google Scholar] [CrossRef]
- Popova, L.P.; Stoinova, Z.G.; Maslenkova, L.T. Involvement of abscisic acid in photosynthetic process in Hordeum vulgare L. during salinity stress. J. Plant Growth Regul. 1995, 14, 211–218. [Google Scholar] [CrossRef]
- Davies, P.J. The Plant Hormones: Their Nature, Occurence, and Function; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.H.D.; Wu, R. Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef]
- Liu, X.; Ma, D.; Zhang, Z.; Wang, S.; Du, S.; Deng, X.; Yin, L. Plant lipid remodelling in response to abiotic stresses. Environ. Exp. Bot. 2019, 165, 174–184. [Google Scholar] [CrossRef]
- Kaur, H.; Mukherjee, S.; Baluska, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 2015, 10, e1049788. [Google Scholar] [CrossRef]
- Rahuman, A.; Gopalakrishnan, G.; Ghouse, B.; Arumugam, S.; Himalayan, B. Effect of Feronia limonia on mosquito larvae. Fitoterapia 2000, 71, 553–555. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kumaravel, S.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biochem. Res. 2010, 4, 191–195. [Google Scholar]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharm. Sci. 2011, 15, 775–780. [Google Scholar]
- Awa, E.P.; Ibrahim, S.; Ameh, D.A. GC/MS analysis and antimicrobial activity of diethyl ether fraction of methanolic extract from the stem bark of Annona senegalensis Pers. Int. J. Pharm. Sci. Res. 2012, 3, 4213–4218. [Google Scholar]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and genetic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef] [PubMed]


| No. | Compound Name | Molecular Weight (M.W.) | Formula | Retention Time (R.T.) | |||
|---|---|---|---|---|---|---|---|
| H. cuneiformis | A Fragilis | H. cuneiformis | A Fragilis | H. cuneiformis | A Fragilis | ||
| 1 | Dodecanoic acid, methyl ester (CAS) | 200 | 214 | C12H24O2 | C13H26O2 | 15.60 | 14.62 |
| 2 | Heptadecane | 240 | - | C17H36 | - | 17.22 | - |
| 3 | Tetradecanoic acid, methyl ester (CAS) | 242 | - | C15H30O2 | - | 17.77 | - |
| 4 | Oleic acid | 282 | - | C18H34O2 | - | 18.26 | - |
| 5 | octadecanoic acid 10 | 284 | - | C18H36O2 | - | 18.88 | - |
| 6 | Palmitic acid (n-hexadecanoic acid 1 | 256 | - | C16H32O2 | - | 19.23 | - |
| 7 | (E)-9-Octadecenoic acid ethyl ester | 310 | - | C20H38O2 | - | 19.75 | - |
| 8 | 2-Hexadecen-1-ol, 3,7,11,15-tetramethyl-, [R-[R*,R*-(E)]]- S) | 296 | - | C20H40O | - | 19.82 | - |
| 9 | Hexadecenoic acid, methyl ester | 270 | 270 | C17H34O2 | C17H34O2 | 20.53 | 20.56 |
| 10 | Phytol, acetate | 338 | - | C22H42O2 | - | 22.22 | - |
| 11 | Cholestan-3-ol, 2-methylene-, (3á,5à)- | 400 | - | C28H48O | - | 27.03 | - |
| 12 | Heptanoic acid, methyl ester (CAS) | - | 144 | - | C8H16O | - | 5.99 |
| 13 | Nonanoic acid, methyl ester (CAS) | - | 172 | - | C10H20O2 | - | 9.48 |
| 14 | 2-Methyltetracosane | - | 352 | - | C25H52 | - | 11.25 |
| 16 | Tetradecanoic acid, methyl ester (CAS) | - | 242 | - | C15H30O2 | - | 17.71 |
| 17 | Tetraneurin-A-diol | - | 280 | - | C18H20O5 | - | 19.18 |
| 18 | 2-[5-(2-Hydroxypropyl)-tetrahydrofuran-2-yl]-propionic acid, t-butyl ester | - | 258 | - | C14H26O4 | - | 19.18 |
| 19 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | - | 296 | - | C20H40O | - | 19.52 |
| 20 | Phytol, acetate | 338 | C22H42O2 | 19.58 | |||
| 21 | 13-Heptadecyn-1-ol | - | 252 | - | C17H32O | - | 19.77 |
| 22 | Ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)- | - | 310 | - | C20H38O2 | - | 20.08 |
| 24 | 9,12,15-Octadecatrienoic acid-2-phenyl-1,3-dioxan-5-yl ester | - | 440 | - | C28H40O4 | - | 20.36 |
| 25 | 11-Hexadecenoic acid, methyl ester | - | 268 | - | C22H42O2 | - | 20.22 |
| 26 | Hexadecanoic acid, 14-methyl-, methyl ester | - | 284 | - | C18H36O2 | - | 21.78 |
| 27 | 7,10,13-Eicosatrienoic acid, methyl ester (CAS) | - | 320 | - | C21H36O2 | - | 24.83 |
| Treatments | SS | SP | Pro | TFAA | Phenolic Compounds |
|---|---|---|---|---|---|
| 0 mM NaCl | 43.15 ± 0.82 d | 27.53 ± 3.25 h | 7.86 ± 0.97 bcd | 9.15 ± 1.12 e | 10.17 ± 0.38 h |
| 100 mM NaCl | 29.54 ± 1.59 e | 55.19 ± 4.83 ef | 11.02 ± 2.5 2 b | 12.13 ± 1.46 de | 20.54 ± 0.24 e |
| 150 mM NaCl | 29.31 ± 3.45 e | 62.41 ± 1.84 cd | 14.83 ± 3.58 a | 19.77 ± 1.76 b | 25.07 ± 1.10 d |
| 0 mM NaCl + H. cuneiformis | 53.00 ± 2.66 c | 30.08 ± 2.39 h | 4.41 ± 1.45 e | 15.16 ± 2.28 cd | 17.28 ± 0.25 f |
| 100 mM NaCl + H. cuneiformis | 50.26 ± 1.78 c | 58.43 ± 2.30 de | 8.44 ± 1.19 bcd | 19.69 ± 2.39 b | 24.66 ± 0.80 d |
| 150 mM NaCl + H. cuneiformis | 43.81 ± 1.35 d | 65.90 ± 4.61 bc | 10.07 ± 1.67 bc | 20.54 ± 1.67 b | 28.35 ± 0.73 c |
| 0 mM NaCl + A. fragilis | 58.59 ± 1.65 a | 31.53 ± 2.41 h | 3.92 ± 1.87 e | 14.14 ± 4.62 d | 19.92 ± 0.95 f |
| 100 mM NaCl + A. fragilis | 53.22 ± 2.68 bc | 68.04 ± 2.82 ab | 6.78 ± 2.22 cde | 20.16 ± 2.08 b | 30.60 ± 0.61 b |
| 150 mM NaCl + A. fragilis | 40.70 ± 2.40 d | 72.04 ± 4.09 a | 10.45 ± 1.46 b | 28.21 ± 2.27 a | 38.44 ± 0.50 a |
| LSD | 3.90 | 5.21 | 3.32 | 3.92 | 1.04 |
| Treatments | Na+ | K+ | K+/Na+ | Ca2+ | Mg2+ |
|---|---|---|---|---|---|
| 0 mM NaCl | 21.99 ± 4.57 de | 23.65 ± 3.54 de | 1.12 ± 0.38 de | 13.96 ± 0.56 g | 14.48 ± 0.50 e |
| 100 mM NaCl | 32.66 ± 2.31 b | 14.74 ± 1.86 f | 0.45 ± 0.06 fg | 12.22 ± 0.47 h | 7.92 ± 0.34 f |
| 150 mM NaCl | 38.99 ± 7.53 a | 12.67 ± 2.46 f | 0.33 ± 0.08 g | 8.22 ± 0.29 i | 7.14 ± 0.65 f |
| 0 mM NaCl + H. cuneiformis | 10.73 ± 2.10 g | 26.59 ± 4.22 bcd | 2.49 ± 0.11 b | 20.52 ± 0.50 b | 39.37 ± 0.94 a |
| 100 mM NaCl + H. cuneiformis | 22.32 ± 3.20 de | 22.74 ± 2.99 de | 1.04 ± 0.23 def | 17.30 ± 0.43 d | 29.00 ± 1.0 d |
| 150 mM NaCl + H. cuneiformis | 34.37 ± 2.73 ab | 21.56 ± 1.46 de | 0.63 ± 0.02 efg | 15.52 ± 0.46 f | 30.18 ± 1.05 cd |
| 0 mM NaCl + A. fragilis | 11.02 ± 1.68 g | 39.03 ± 2.53 a | 3.60 ± 0.68 a | 23.53 ± 0.34 a | 36.95 ± 6.04 ab |
| 100 mM NaCl + A. fragilis | 19.69 ± 1.85 ef | 31.39 ± 2.61 b | 1.61 ± 0.28 cd | 16.51 ± 0.49 e | 33.55 ± 1.50 bc |
| 150 mM NaCl + A. fragilis | 28.88 ± 2.68 bc | 29.59 ± 4.07 bc | 1.06 ± 0.11 def | 15.41 ± 0.52 f | 32.48 ± 0.17 c |
| LSD | 5.99 | 5.47 | 0.65 | 0.77 | 3.46 |
| Treatments | MDA | POD | CAT | SOD |
|---|---|---|---|---|
| 0 mM NaCl | 41.25 ± 0.54 f | 3.22 ± 0.22 f | 5.42 ± 0.52 i | 4.44 ± 0.51 f |
| 100 mM NaCl | 81.26 ± 0.78 b | 4.66 ± 0.41 d | 7.20 ± 0.27 fg | 5.25 ± 0.27 e |
| 150 mM NaCl | 111.11 ± 1.17 a | 4.93 ± 0.06 cd | 9.00 ± 0.11 d | 6.17 ± 0.21 d |
| 0 mM NaCl + H. cuneiformis | 27.59 ± 0.52 i | 3.99 ± 0.11 e | 5.99 ± 0.11 h | 4.87 ± 0.14 ef |
| 100 mM NaCl + H. cuneiformis | 58.63 ± 1.19 d | 5.20 ± 0.26 c | 8.45 ± 0.45 de | 7.26 ± 0.31 c |
| 150 mM NaCl + H. cuneiformis | 66.22 ± 0.70 c | 5.34 ± 0.15 c | 10.37 ± 0.39 b | 8.47 ± 0.50 b |
| 0 mM NaCl + A. fragilis | 29.07 ± 0.89 h | 5.89 ± 0.11 b | 7.65 ± 0.27 f | 5.13 ± 0.23 e |
| 100 mM NaCl + A. fragilis | 58.53 ± 0.81 d | 6.55 ± 0.29 a | 9.67 ± 0.29 c | 7.41 ± 0.09 c |
| 150 mM NaCl + A. fragilis | 59.00 ± 0.00 d | 6.81 ± 0.23 a | 11.40 ± 0.44 a | 9.33 ± 0.42 a |
| LSD | 1.37 | 0.41 | 0.56 | 0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Latef, A.A.H.; Zaid, A.; Alwaleed, E.A. Influences of Priming on Selected Physiological Attributes and Protein Pattern Responses of Salinized Wheat with Extracts of Hormophysa cuneiformis and Actinotrichia fragilis. Agronomy 2021, 11, 545. https://doi.org/10.3390/agronomy11030545
Abdel Latef AAH, Zaid A, Alwaleed EA. Influences of Priming on Selected Physiological Attributes and Protein Pattern Responses of Salinized Wheat with Extracts of Hormophysa cuneiformis and Actinotrichia fragilis. Agronomy. 2021; 11(3):545. https://doi.org/10.3390/agronomy11030545
Chicago/Turabian StyleAbdel Latef, Arafat Abdel Hamed, Abbu Zaid, and Eman A. Alwaleed. 2021. "Influences of Priming on Selected Physiological Attributes and Protein Pattern Responses of Salinized Wheat with Extracts of Hormophysa cuneiformis and Actinotrichia fragilis" Agronomy 11, no. 3: 545. https://doi.org/10.3390/agronomy11030545
APA StyleAbdel Latef, A. A. H., Zaid, A., & Alwaleed, E. A. (2021). Influences of Priming on Selected Physiological Attributes and Protein Pattern Responses of Salinized Wheat with Extracts of Hormophysa cuneiformis and Actinotrichia fragilis. Agronomy, 11(3), 545. https://doi.org/10.3390/agronomy11030545








