Application of Seaweed Organic Components Increases Tolerance to Fe Deficiency in Tomato Plants
Abstract
1. Introduction
2. Material and Methods
2.1. Organic Compounds
2.2. Germination Assay in Petri Dish
2.3. Plant Material and Growth Conditions
2.4. Plant Analysis
2.5. Statistical Analyses
3. Results
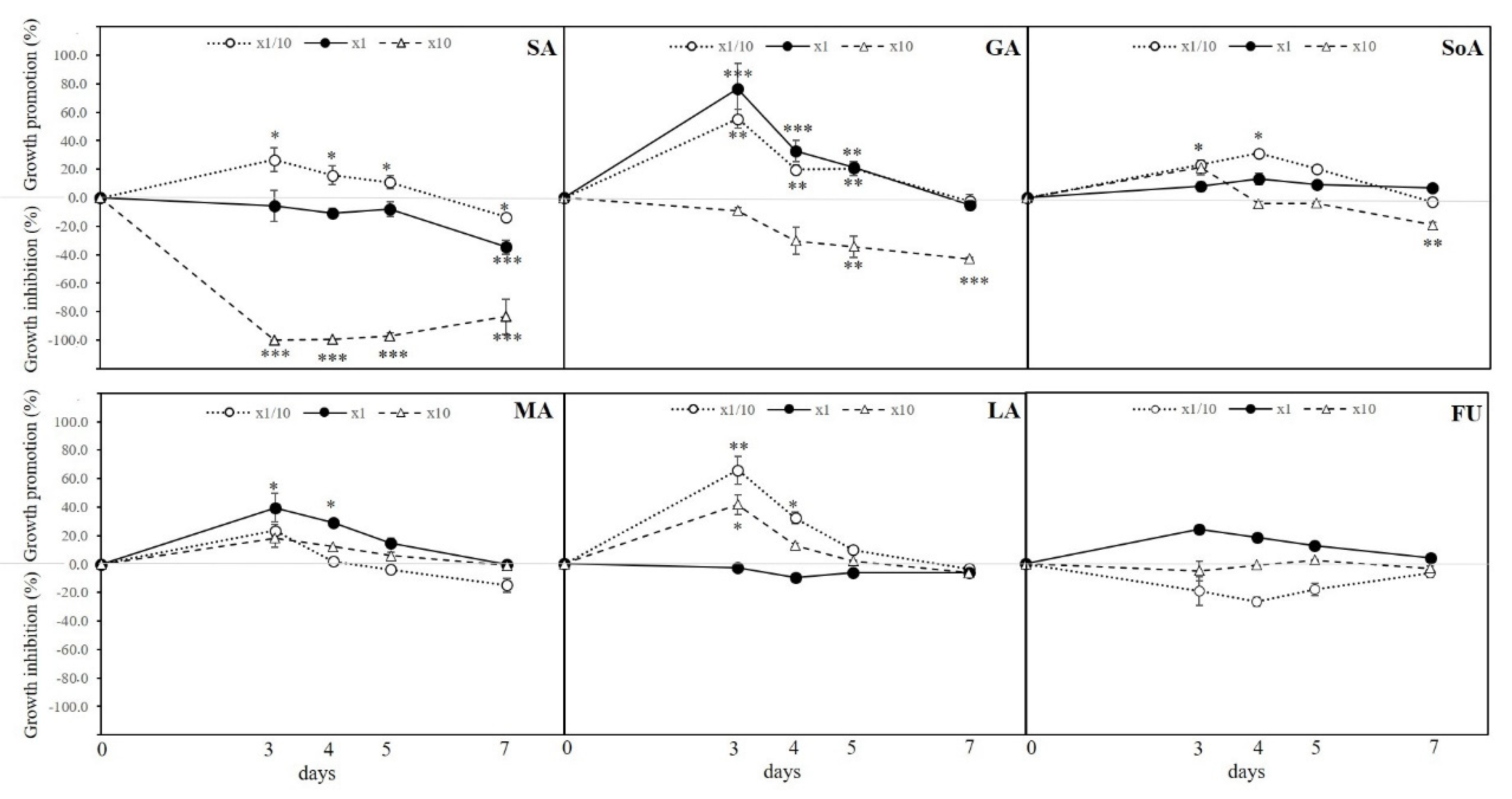
3.1. Germination Assay in Petri Dish
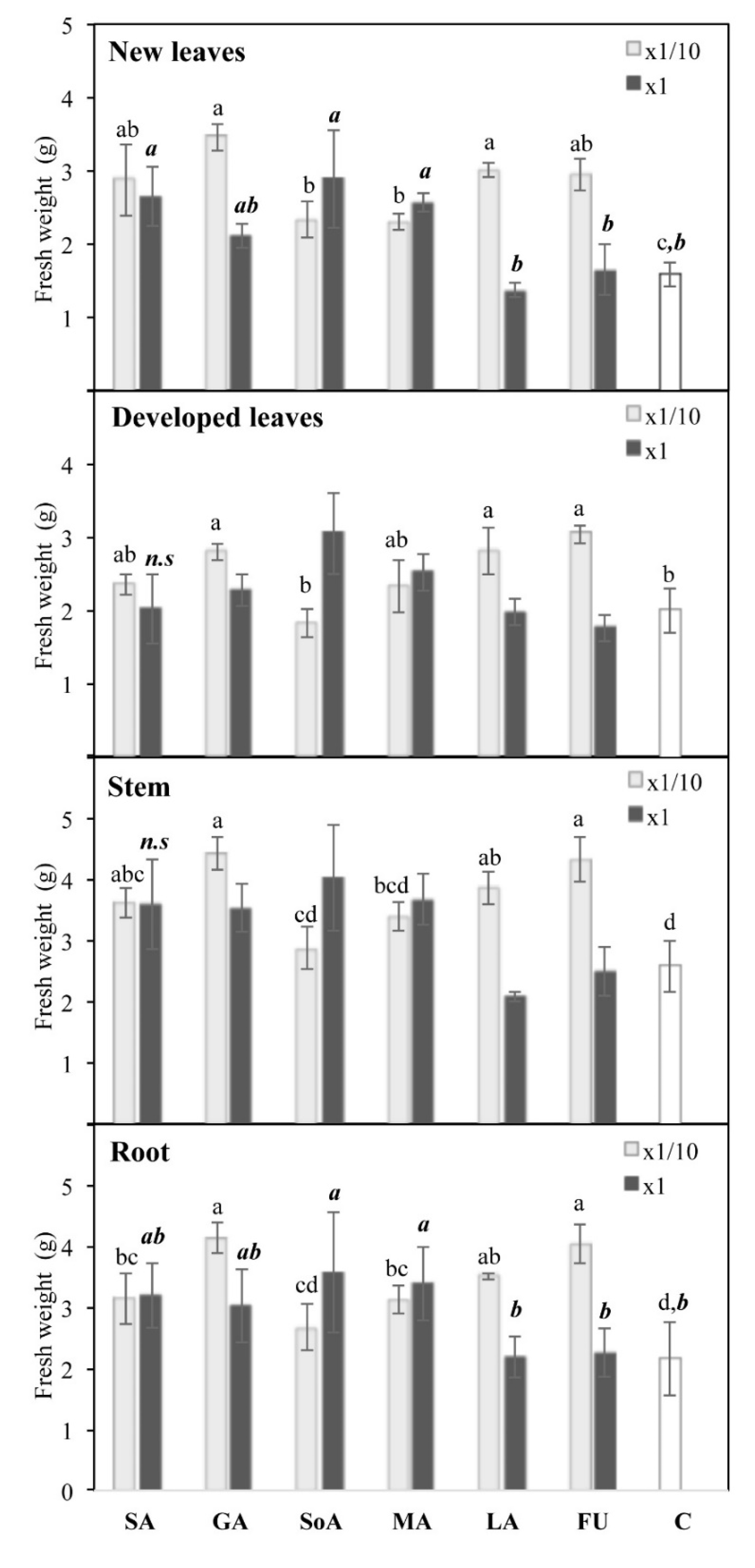
3.2. Fresh Weight
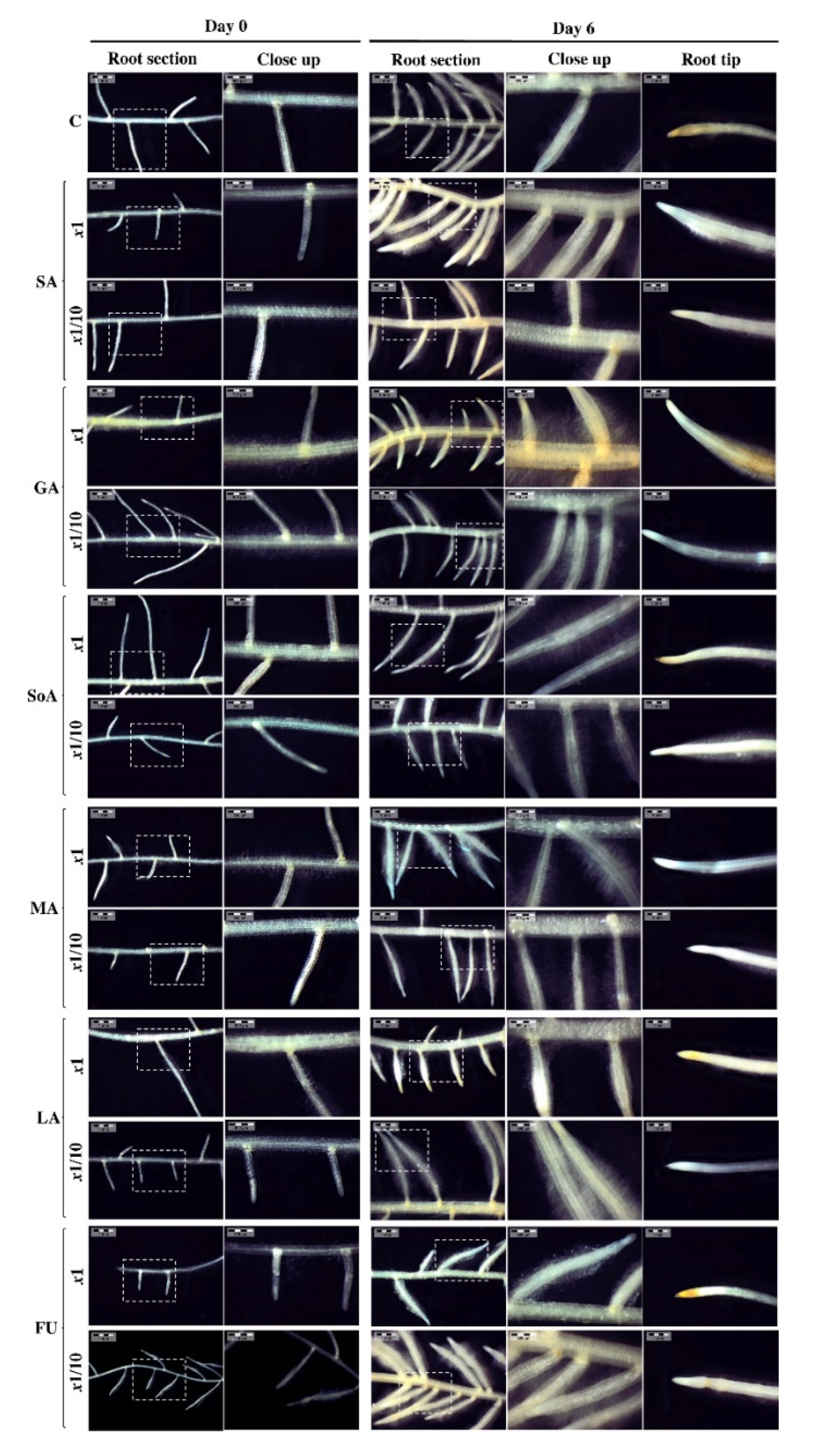
3.3. Morphology of Tomato Roots
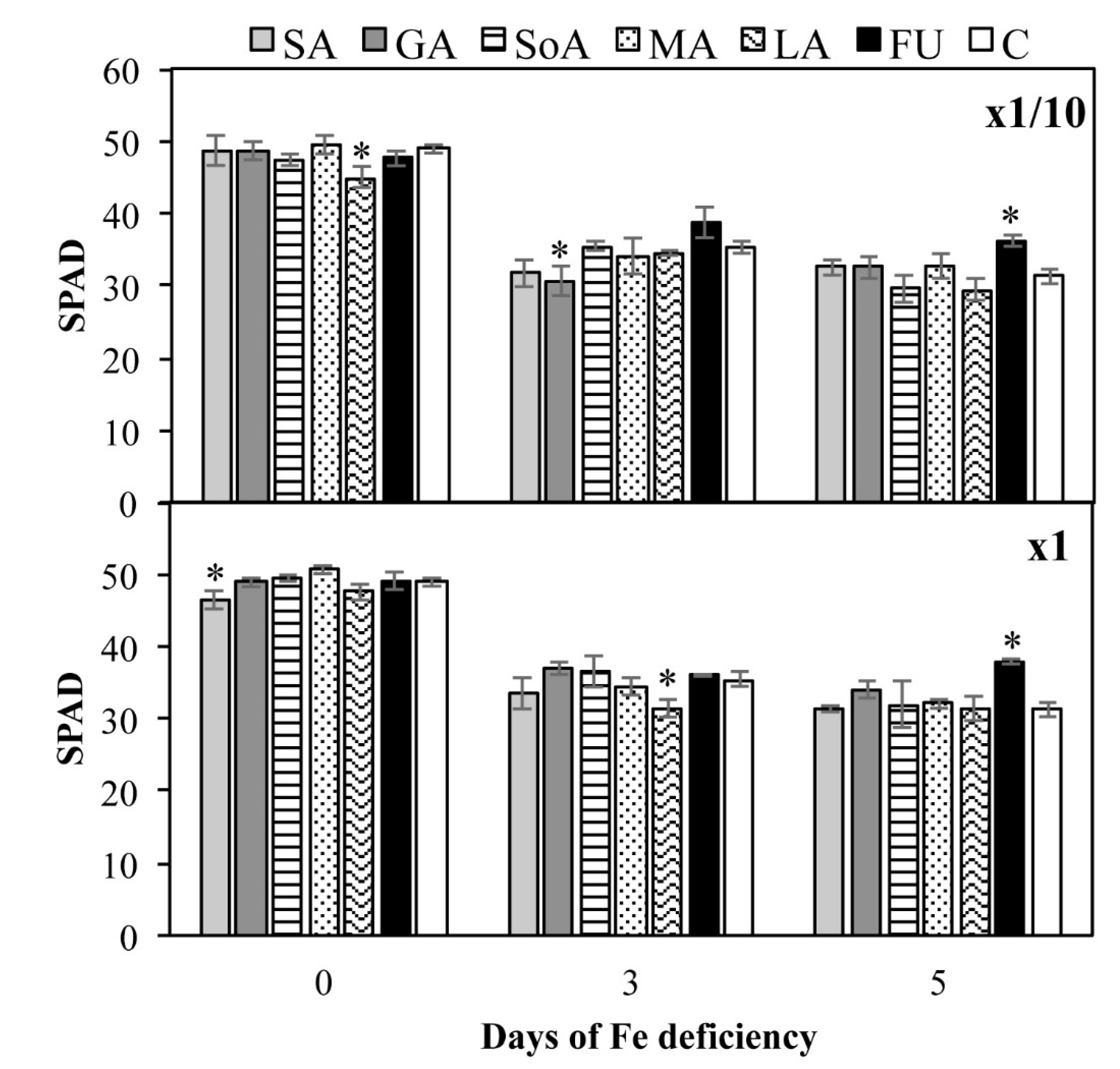
3.4. Leaf Chlorophyll Index
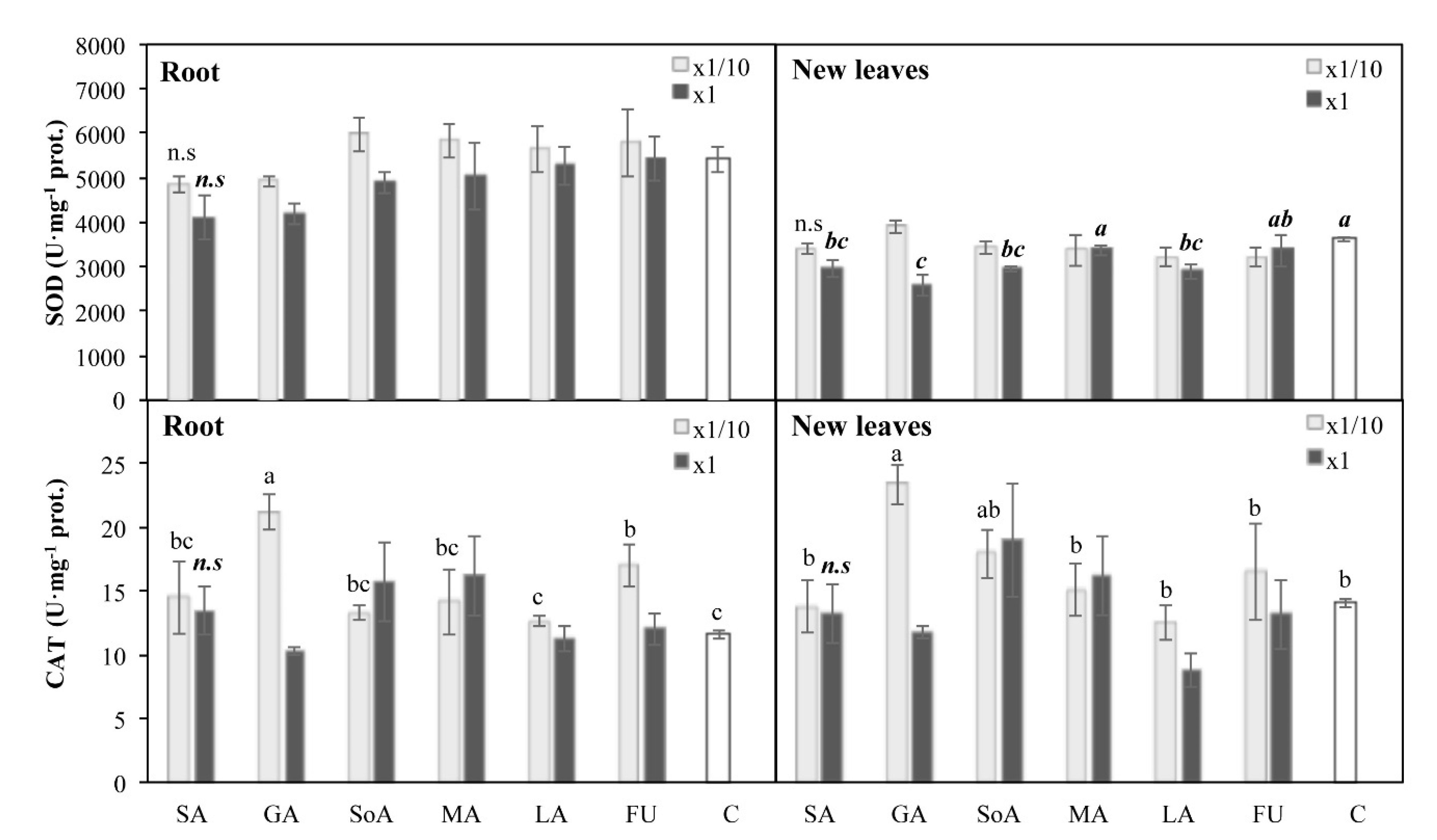
3.5. Oxidative Stress Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- E.F.S.A. Conclusion on the peer review of the pesticide risk assessment of the active substance sea-algae extract. EFSA J. 2012, 10, 2492. [Google Scholar] [CrossRef]
- Khan, W.; Zhai, R.; Souleimanov, A.; Critchley, A.T.; Smith, D.L.; Prithiviraj, B. Commercial extract of Ascophyllum nodosum improves root colonization of alfalfa by its bacterial symbiont Sinorhizobium meliloti. Commun. Soil Sci. Plant Anal. 2012, 43, 2425–2436. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Chen, S.K.; Edwards, C.A.; Subler, S. The influence of two agricultural biostimulants on nitrogen transformations, microbial activity, and plant growth in soil microcosms. Soil Biol. Biochem. 2003, 35, 9–19. [Google Scholar] [CrossRef]
- Boyer, C. Storage carbohydrates in vascular plants: Distribution, physiology and metabolism. Trends Biochem. Sci. 1985. [Google Scholar] [CrossRef]
- Seckin, B.; Sekmen, A.H.; Türkan, İ. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul. 2009, 28, 12–20. [Google Scholar] [CrossRef]
- Will, S.; Eichert, T.; Fernández, V.; Möhring, J.; Müller, T.; Römheld, V. Absorption and mobility of foliar-applied boron in soybean as affected by plant boron status and application as a polyol complex. Plant Soil 2011, 344, 283–293. [Google Scholar] [CrossRef]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll. 2016, 54, 77–88. [Google Scholar] [CrossRef]
- Zhang, C.X.; Feng, B.H.; Chen, T.T.; Zhang, X.F.; Tao, L.X.; Fu, G.F. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul. 2017, 83, 313–323. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, S.; Shim, I.S. Exogenous salicylic acid alleviates salt-stress damage in cucumber under moderate nitrogen conditions by controlling endogenous salicylic acid levels. Hortic. Environ. Biotechnol. 2017, 58, 247–253. [Google Scholar] [CrossRef]
- Silva, A.C.d.; Suassuna, J.F.; de Melo, A.S.; Costa, R.R.; Andrade, W.L.d.; da Silva, D.C. Salicylic acid as attenuator of drought stress on germination and initial development of sesame. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 156–162. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Kucukoduk, M. Protective roles of exogenously applied gallic acid in Oryza sativa subjected to salt and osmotic stresses: Effects on the total antioxidant capacity. Plant Growth Regul. 2015, 75, 219–234. [Google Scholar] [CrossRef]
- Rudolphi-Skórska, E.; Sieprawska, A. Adaptation of wheat cells to short-term ozone stress: The impact of α-tocopherol and gallic acid on natural and model membranes. Acta Physiol. Plant. 2016, 38, 85. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. Improvement of cold stress resistance via free radical scavenging ability and promoted water status and photosynthetic capacity of gallic acid in soybean leaves. J. Soil Sci. Plant Nutr. 2017, 17, 366–384. [Google Scholar] [CrossRef][Green Version]
- Haug, A.; Jensen, A. Seasonal variations in the chemical composition of Alaria esculenta, Laminaria saccharina, Laminaria hyperborea and Laminaria digitata from northern Norway; Norsk Institutt for Tang- og Tareforskning: Oslo, Norway, 1954; ser. A. no. 4. [Google Scholar]
- Wu, Y.R.; Lin, Y.C.; Chuang, H. Laminarin modulates the chloroplast antioxidant system to enhance abiotic stress tolerance partially through the regulation of the defensin-like gene expression. Plant Sci. 2016, 247, 83–92. [Google Scholar] [CrossRef]
- Kusaykin, M.I.; Silchenko, A.S.; Zakharenko, A.M.; Zvyagintseva, T.N. Fucoidanases. Glycobiology 2015, 3–12. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from brown seaweeds: An update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef]
- Soto, M.J.; Urbanowicz, B.R.; Hahn, M.G. Plant fucosyltransferases and the emerging biological importance of fucosylated plant structures. Crit. Rev. Plant Sci. 2019, 38, 327–338. [Google Scholar] [CrossRef]
- Zhang, L.; Paasch, B.C.; Chen, J.; Day, B.; He, S.Y. An important role of l-fucose biosynthesis and protein fucosylation genes in Arabidopsis immunity. New Phytol. 2019, 222, 981–994. [Google Scholar] [CrossRef]
- Stevenson, C.C.; Harrington, G.N. The impact of supplemental carbon sources on Arabidopsis thaliana growth, chlorophyll content and anthocyanin accumulation. Plant Growth Regul. 2009, 59, 255–271. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Saa, S.; Rio, O.D.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Briat, J.; Lobreaux, S. Iron transport and storage in plants. Trends Plant Sci. 1997, 2, 187–193. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.I.C.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Marschner, H. Functions of mineral nutrients: Micronutrients. In Mineral Nutrition of Higher Plants; Marschner, H., Ed.; Academic Press: Cambridge, MA, USA, 1995; pp. 313–324. [Google Scholar]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Roussos, P.A.; Denaxa, N.K.; Damvakaris, T. Strawberry fruit quality attributes after application of plant growth stimulating compounds. Sci. Hortic. 2009, 119, 138–146. [Google Scholar] [CrossRef]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithivira, B. Regulation of phytohormone biosynthesis and accumulation in arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Rodríguez-Larramendi, L.A.; González-Ramírez, M.; Gómez-Rincón, M.A.; Guevara-Hernández, F.; Salas-Marina, M.; Gordillo-Curiel, A. Effects of salicylic acid on the germination and initial growth of bean seedlings (Phaseolus vulgaris L.). Rev. Fac. Agron. 2017, 34, 253–269. [Google Scholar]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic effects of 21 plant secondary metabolites on Arabidopsis thaliana germination and root growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Promotive effects of alginate-derived oligosaccharide on maize seed germination. J. Appl. Phycol. 2004, 16, 73–76. [Google Scholar] [CrossRef]
- Iwasaki, K.I.; Matsubara, Y. Purification of alginate oligosaccharides with root growth-promoting activity toward lettuce. Biosci. Biotechnol. Biochem. 2000, 64, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Iwamoto, Y.; Kitamura, Y.; Oda, T.; Muramatsu, T. Root growth-promoting activity of unsaturated oligomeric uronates from alginate on carrot and rice plants. Biosci. Biotechnol. Biochem. 2003, 67, 2022–2025. [Google Scholar] [CrossRef]
- Johnson, T.R.; Kane, M.E. Differential germination and developmental responses of Bletia purpurea (orchidaceae) to mannitol and sorbitol in the presence of sucrose and fructose. J. Plant Nutr. 2013, 36, 702–716. [Google Scholar] [CrossRef]
- Vítová, L.; Stodůlková, E.; Bartoníčková, A.; Lipavská, H. Mannitol utilisation by celery (Apium graveolens) plants grown under different conditions in vitro. Plant Sci. 2002, 163, 907–916. [Google Scholar] [CrossRef]
- Yvin, J.-C.; Levasseur, F.; Amin-Gendy, C.; Thanh, K.-T.; Patier, P.; Rochas, C.; Lienar, Y.J.; Cloarec, B. Laminarin as a Seed Germination and Plant Growth Accelerator. U.S. Patent 5,750,472, 6 July 1993. [Google Scholar]
- Yücel, N.C.; Heybet, E.H. Salicylic acid and calcium treatments improves wheat vigor, lipids and phenolics under high salinity. Acta Chim. Slov. 2016, 63, 738–746. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic acid-regulated antioxidant mechanisms and gene expression enhance rosemary performance under saline conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef] [PubMed]
- Youssef, R.A.; El-Azab, M.E.; Mahdy, H.A.A.; Essa, E.M.; Mohammed, K.A.S. Effect of salicylic acid on growth, yield, nutritional status and physiological properties of sunflower plant under salinity stress. Int. J. Pharm. Phytopharm. Res. 2017, 7, 54–58. [Google Scholar]
- Ma, L.J.; Li, X.M.; Bu, N.; Li, N. An alginate-derived oligosaccharide enhanced wheat tolerance to cadmium stress. Plant Growth Regul. 2010, 62, 71–76. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.H.; Yin, H.; Wang, W.X.; Zhao, X.M.; Du, Y.G. Alginate oligosaccharides enhanced Triticum aestivum L. tolerance to drought stress. Plant Physiol. Biochem. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Kaya, C.; Sonmez, O.; Aydemir, S.; Ashraf, M.; Dikilitas, M. Exogenous application of mannitol and thiourea regulates plant growth and oxidative stress responses in salt-stressed maize (Zea mays L.). J. Plant Interact. 2013, 8, 234–241. [Google Scholar] [CrossRef]
- Chaney, R.L.; Chen, Y.; Green, C.E.; Holden, M.J.; Bell, P.F.; Luster, D.G.; Angle, J.S. Root hairs on chlorotic tomatoes are an effect of chlorosis rather than part of the adaptive Fe-stress-response. J. Plant Nutr. 1992, 15, 1857–1875. [Google Scholar] [CrossRef]
- Römheld, V.; Marschner, H. Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Physiol. Plant. 1981, 53, 354–360. [Google Scholar] [CrossRef]
- Pinton, R.; Cesco, S.; de Nobili, M.; Santi, S.; Varanini, Z. Water- and pyrophosphate-extractable humic substances fractions as a source of iron for Fe-deficient cucumber plants. Biol. Fertil. Soils 1997, 26, 23–27. [Google Scholar] [CrossRef]
- Landsberg, E.C. Hormonal regulation of iron-stress response in sunflower roots: A morphological and cytological investigation. Protoplasma 1996, 194, 69–80. [Google Scholar] [CrossRef]
- Mugnai, S.; Azzarello, E.; Pandolfi, C.; Salamagne, S.; Briand, X.; Mancuso, S. Enhancement of ammonium and potassium root influxes by the application of marine bioactive substances positively affects Vitis vinifera plant growth. J. Appl. Phycol. 2008, 20, 177–182. [Google Scholar] [CrossRef]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithivira, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G.; Jenkins, T.; Liu, Y.W. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1996, 8, 535–543. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Hernandez-Apaolaza, L.; Lucena, J.J. Effect of several commercial seaweed extracts in the mitigation of iron chlorosis of tomato plants (Solanum lycopersicum L.). Plant Growth Regul. 2018, 86, 401–411. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, R.; Pandey, R. Exogenous application of rutin and gallic acid regulate antioxidants and alleviate reactive oxygen generation in Oryza sativa L. Physiol. Mol. Biol. Plants 2017, 23, 301–309. [Google Scholar] [CrossRef]
- Jie, W.; Kaiyun, W.; Qian, Z.; Yukun, Z.; Lili, J. Inhibition of laminarin against TMV and effect on protective enzymes in tobacco. Acta Phytophylacica Sin. 2011, 32, 532–538. [Google Scholar]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef]
- Sun, B.; Jing, Y.; Chen, K.; Song, L.; Chen, F.; Zhang, L. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J. Plant Physiol. 2007, 164, 536–543. [Google Scholar] [CrossRef]
- Sun, C.; Wu, T.; Zhai, L.; Li, D.; Zhang, X.; Xu, X.; Ma, H.; Wang, Y.; Han, Z. Reactive oxygen species function to mediate the fe deficiency response in an fe-efficient apple genotype: An early response mechanism for enhancing reactive oxygen production. Front. Plant Sci. 2016, 7, 1726. [Google Scholar] [CrossRef] [PubMed]
- Iturbe-Ormaetxe, I.; Moran, J.F.; Arrese-Igor, C.; Gogorcena, Y.; Klucas, R.V.; Becana, M. Activated oxygen and antioxidant defences in iron-deficient pea plants. Plant. Cell Environ. 1995, 18, 421–429. [Google Scholar] [CrossRef]
- Molassiotis, A.; Tanou, G.; Diamantidis, G.; Patakas, A.; Therios, I. Effects of 4-month Fe deficiency exposure on Fe reduction mechanism, photosynthetic gas exchange, chlorophyll fluorescence and antioxidant defense in two peach rootstocks differing in Fe deficiency tolerance. J. Plant Physiol. 2006, 163, 176–185. [Google Scholar] [CrossRef]
- M’sehli, W.; Houmani, H.; Donnini, S.; Zocchi, G.; Abdelly, C.; Gharsalli, M. Iron deficiency tolerance at leaf level in Medicago ciliaris plants. Am. J. Plant Sci. 2014, 5, 2541–2553. [Google Scholar] [CrossRef]
- Fike, J.H.; Allen, V.G.; Schmidt, R.E.; Zhang, X.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Coelho, R.W.; Wester, D.B. Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J. Anim. Sci. 2001, 79, 1011–1021. [Google Scholar] [CrossRef]
- Zhang, X. Influence of Plant Growth Regulators on Turfgrass Growth, Antioxidant Status, and Drought Tolerance. Ph.D. Thesis, Va. Tech, Blacksburg, Virginia, 1997. [Google Scholar]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; el Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J. Appl. Phycol. 2015, 27, 1689–1698. [Google Scholar] [CrossRef]
- Song, Y.; Dong, Y.; Kong, J.; Tian, X.; Bai, X.; Xu, L. Effects of root addition and foliar application of nitric oxide and salicylic acid in alleviating iron deficiency induced chlorosis of peanut seedlings. J. Plant Nutr. 2017, 40, 63–81. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed glycine max L. J. Soil Sci. Plant Nutr. 2016, 16, 662–676. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Tari, I.; Páldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Plant. 2002, 115, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Cui, J.M.; Li, G.X.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plant. 2016, 60, 139–147. [Google Scholar] [CrossRef]
| Concentration (mg/L) | |||
|---|---|---|---|
| Organic Compound | ×1/10 | ×1 | ×10 |
| SA | 4.7 | 47 | 470 |
| GA | 4.7 | 47 | 470 |
| SoA | 9.0 | 90 | 900 |
| MA | 5.8 | 58 | 580 |
| LA | 0.070 | 0.70 | 7.0 |
| FU | 3.1 | 31 | 310 |
| Germination (Number of Seeds) | ||||
|---|---|---|---|---|
| Organic Compound | C | ×1/10 | ×1 | ×10 |
| SA | 0 | 1 | 0 | 0 |
| GA | 0 | 6 | 4 | 1 |
| SoA | 0 | 2 | 0 | 4 |
| MA | 0 | 2 | 4 | 4 |
| LA | 0 | 2 | 1 | 3 |
| FU | 0 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Gil, S.; Allende-Montalbán, R.; Hernández-Apaolaza, L.; Lucena, J.J. Application of Seaweed Organic Components Increases Tolerance to Fe Deficiency in Tomato Plants. Agronomy 2021, 11, 507. https://doi.org/10.3390/agronomy11030507
Carrasco-Gil S, Allende-Montalbán R, Hernández-Apaolaza L, Lucena JJ. Application of Seaweed Organic Components Increases Tolerance to Fe Deficiency in Tomato Plants. Agronomy. 2021; 11(3):507. https://doi.org/10.3390/agronomy11030507
Chicago/Turabian StyleCarrasco-Gil, Sandra, Raúl Allende-Montalbán, Lourdes Hernández-Apaolaza, and Juan J. Lucena. 2021. "Application of Seaweed Organic Components Increases Tolerance to Fe Deficiency in Tomato Plants" Agronomy 11, no. 3: 507. https://doi.org/10.3390/agronomy11030507
APA StyleCarrasco-Gil, S., Allende-Montalbán, R., Hernández-Apaolaza, L., & Lucena, J. J. (2021). Application of Seaweed Organic Components Increases Tolerance to Fe Deficiency in Tomato Plants. Agronomy, 11(3), 507. https://doi.org/10.3390/agronomy11030507







