Abstract
To evaluate if the combined foliar application of nitrogen (N) and zinc (Zn) in broccoli improves the efficiency of the single Zn biofortification, five treatments were tested: (i) control; (ii) one Zn application at the beginning of flowering (0.5M1); (iii) combined 0.5M1 with N application (0.5M1N); (iv) two Zn applications, one at the beginning of the flowering and other after two weeks (0.25 + 0.25) and (v) combined 0.25 + 0.25 with N (0.25N + 0.25N). The Parthenon cultivar showed a good concentration of Ca, Fe, Mg, and Zn, with good bioavailability and glucosinolates, mainly in the florets, and high antioxidant activity and total phenols, mainly in the leaves, showing their potential not only as regards to human consumption, but also for the use of their by-products. All the studied treatments increased floret growth (19%), antioxidant capacity and total phenol content, not affecting Ca, Fe, and Mg concentrations. Glucosinolate content was mostly independent from the studied treatments, decreasing slightly in terms of glucoiberin and the Zn concentration in the florets increased in >50 mg Zn kg−1 in the split treatments and ~40 mg Zn kg−1 when the application was unique, with excellent bioavailability, measured as PA:Zn ratio. Therefore, 0.25 + 0.25 and 0.25N + 0.25N applications have been confirmed as the applications that improve more both growth and accumulation of Zn and biocompounds in broccoli.
1. Introduction
In the last few years, species of the Brassica genus have become a key part of the human diet, owing to their richness in nutrients and bioactive compounds, and are among the ten most economically important vegetables [1]. Within the genus, broccoli (Brassica oleracea var. italica L.) has been shown to be an excellent dietary source of phytochemicals, such as glucosinolates, polyphenols, antioxidants, and essential dietary nutrients (Ca, Mg, Na, K, Fe, and Zn) [1,2,3]. Their intake is related to lower risks of developing certain types of cancer, degenerative and cardiovascular diseases, and immune dysfunction [4,5,6]. This richness, though, depends on numerous factors, such as variety, maturation, growing conditions, plant fraction, soil type, storage conditions after harvest, industrial processing, cooking, and the availability of plant nutrients [4].
Fertilization is another factor that can affect the crop’s growth and composition. Accordingly, in this study, agronomic biofortification with Zn, which involves increasing this nutrient through the use of Zn-rich fertilizers, is studied. Zinc is an essential mineral for plants and animals, including humans. In plants, Zn is used for DNA replication, protein synthesis, catalytic activity, energy transfer reactions, and chlorophyll formation [7,8]. In humans, its deficiency is associated with severe health complications, including hindered physical growth, and learning ability, neurological disorders, DNA damage, and cancer development, causing death in extreme cases [9,10]. Although Zn deficiency is considered a risk factor for disease or death around the world [11], its deficiency is estimated in at least one-third of the world’s population, and not only in developing countries but in developed ones too. One of the main causes of this is the intake of food crops grown in low-phytoavailability Zn areas, Zn-deficient soils being most widespread in agricultural lands around the world [12,13,14], such as in the southwestern Iberian Peninsula [15]. In fact, in Spain, many studies have found intakes below two thirds of 15 mg Zn kg−1 (the recommended dietary allowance (RDA)), mainly among people over 50 years old [9,16,17].
Previous studies have shown that agronomic biofortification through foliar application increases total Zn concentrations in the edible fractions of broccoli, in both deficient and adequate Zn-containing soils [2,3,18,19,20], finding increases in the yield, as well as the sulforaphane and glucosinolate contents, in broccoli following the foliar application of ZnSO4. However, information regarding the combined application of Zn with nitrogen (N), which is perhaps the major agricultural practice in crop production, has not been practically studied, despite Zn being needed to accomplish various essential processes including the metabolism of N [7,21]. Previous studies have shown the synergic effect of N on Zn biofortification efficiency in cereals [14], increasing not only plant growth but also Zn concentration, being therefore, interesting establish if the combined application could increase the Zn biofortification efficiency. However, the effects on other fodder crops, or on polyphenols, glucosinolates and antioxidant capacity, are not yet known. Therefore, the aim of the present study was to evaluate the effect of different combinations of Zn and N foliar applications on the yield and main phytochemicals in broccoli including mineral bioavailability, polyphenols, glucosinolates, and antioxidant capacity in the different broccoli fractions.
2. Materials and Methods
2.1. Soil Preparation for Pot Experiment
Zinc-deficient sandy soil was collected from the area of the Tierra de Barros region in western Spain (38°88′ N, 7°04′ W; 186 m above sea level). The soil was air-dried and sieved to < 5 mm. Four subsamples of the sieved soil were analyzed, showing pH 6.5 ± 0.1 (mean ± standard error) (10 g soil:25 mL deionized H2O), and the extractable Zn in the soil was 0.35 ± 0.03 mg kg−1, which was extracted with DTPA (diethylenetriamine penta acetic acid) [22] and determined by inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Fisher Scientific iCAPQ, Bremen, Germany). A certified soil reference material for quality assurance, as well as blanks, were included in each batch of samples. All results are reported on a dry weight basis. To ensure Zn was the only nutrient limiting growth, the following basal nutrients (in mg kg−1) were added to the soil as solutions and mixed thoroughly: 767 KH2PO4; 1189 K2SO4; 341 MgSO4·7H2O; 809 NH4NO3; 1278 CaCl2·2H2O; 85 MnSO4·H2O; 17 CuSO4·5H2O; 4.3 CoSO4·7H2O; 1.7 Na2MoO4·2H2O; and 6.0 H3BO3. Extra quantities of 95.2 mg NH4NO3 kg−1 were applied every three weeks to avoid N deficiencies.
2.2. Experimental Setup
The experiment was arranged in a completely randomized design with four replications. The Zn treatments comprised the following: (i) the foliar application of distilled water spray (control); (ii) the foliar application at 0.5% (w/v) of ZnSO4·7H2O at the beginning of flowering (0.5M1); (iii) a combined 0.5 M1 treatment with 27% (w/v) N diluted in 0.4% calcium ammonium nitrate (CAN) at the beginning of flowering (0.5M1N); (iv) the foliar application of 0.25% (w/v) ZnSO4·7H2O both at the beginning of the flowering and after two weeks (0.25 + 0.25); and (v) the combined 0.25 + 0.25 treatment with 27% N diluted in 0.4% (w/v) CAN at the beginning of flowering and after two weeks (0.25N + 0.25N). The volume of sprayed solution was 15 mL, sufficient to wet the whole plant.
Seeds of broccoli (Brassica oleracea var. italica L.) cv. Parthenon were surface-sterilized and sown in a seedbed containing commercial substrate. After four weeks, the plants were transplanted into 30 cm high and 30 cm wide free-draining pots containing 8.5 kg soil (one plant per pot). During the experiment, soil moisture was maintained around 60% of the water holding capacity by watering the plants every two days with deionized water. The plants grew between 1December 2018 and 27 March 2019 in a naturally lit glasshouse located at the School of Agronomy Engineering, University of Extremadura, Badajoz, Spain (38°89′ N, 6°97′ W; 186 m above sea level).
2.3. Plant Analysis
The plants were harvested at maturity, 12 weeks after transplanting. The aerial fractions were cut just above the soil surface and were washed with deionized water, and their heights were measured. Florets, stems, and leaves were separated, and the height, maximum diameter (D) and minimum diameter (d) of each floret was measured. Finally, the florets were separated from the stems and leaves, weighted, and air-dried at 60 °C for 72 h in a forced-air cabinet until a constant weight was achieved. The total Zn, Ca, Fe, and Mg concentrations were determined in each fraction by ICP-MS (as described above) after the plant material was digested in a heated mixture of concentrated nitric and perchloric acids, following the methodology described by Gomez-Coronado et al. [14]. Two operational blanks and two samples of certified reference material (CRM; tomato leaf SRM 1573a NIST, Gaithersburg, MD, USA) were included in each digestion run. The digested was determined by ICP-MS being the Zn-specific recovery from CRMs of 95%. To estimate the bioavailability of Zn, Ca, Fe, and Mg, phytic acid (PA) was measured in the stems, leaves, and florets as described by Reason et al. [23] using a PA-total phosphorus assay kit (Megazyme, County Wicklow, Ireland). Molar ratios of phytic acid to Zn, Ca, Fe, and Mg were estimated using a 65% P conversion ratio, and by subsequently dividing by the respective Zn, Ca, Fe, and Mg concentrations.
2.4. Total Phenolic Compounds
Phenolic compounds were extracted as described by Casquete et al. [24]. Briefly, 10 g of each sample was homogenized with 60 mL of solvent (80% aqueous ethanol, containing 1% concentrated HCl), followed by mixing for 1 h in the absence of light at room temperature (25 °C) and filtering. This process was repeated twice. Excess ethanol was removed by heating at 37 °C in a rotary evaporator in a vacuum, and assessed for the final known volume. The total phenolic content was measured spectrophotometrically (λ 760 nm) using Folin–Ciocalteu reagent, and the results were expressed as equivalent of gallic acid (mg GAE 100 g−1 of dry plant material).
2.5. Determination of the Total Antioxidant Activity
Total antioxidant activity was determined by DPPH and ABTS assays. The ability to eliminate the free radical of 2,2-diphenyl-1-picrylhydrazil (DPPH) was assessed following the method of Teixeira et al. [25]. The absorbance was read spectrophotometrically at 515 nm after 30 minutes in the dark, using Trolox (6-hydroxy-2,5,7,8-tetramethylcromano-2-carboxylic acid) as the external standard. The ABTS radical scavenging test (2,2’-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) was carried out as described by Cano et al. [26]. Briefly, 40 μL was taken from the extract to be determined and reacted with 2 mL of ABTS. Previously, a calibration line had been drawn up using Trolox as the standard. The initial absorbance value at λ 730 nm was then compared with the absorbance obtained after 20 min of reaction. The results were expressed as mg Trolox 100 g−1 D.M.
2.6. Glucosinolates
The samples (0.5 g) were boiled in 10 mL of distilled water for 5 min and then filtered through a 0.25 μm filter. The extracts were reconstituted to a final volume of 50 mL. The separations were carried out in an automated Agilent 7100 EC-DAD system (Agilent, Waldbronn, Germany). The used separation buffer was 50 mM SDS in 25 mM tetraborate, at pH 9, according to the method reported by Lee et al. [27]. Uncoated fused silica capillaries of 75 μm inner diameter and 57 cm total length (50 cm detector opening) were used (Supelco, Tecknocroma, Barcelona, Spain). The separation voltage was 263 V/cm (15 kV) at 25 °C and the wavelength was 230 nm. Samples were injected under pressure (0.5 psi) for 6 seconds. The glucosinolate spectra were monitored from 190 to 600 nm (λmax at 230 nm), and the peaks were identified using corrected migration times and spectra.
2.7. Statistical Analysis
While the plant determinations were subjected to one-way ANOVA for each of the Zn treatments (control, 0.5M1, 0.5M1N, 0.25 + 0.25, 0.25N + 0.25N), the mineral concentrations, the molar ratios of phytic acid and different minerals, the total antioxidant activity, the total phenol content, and the glucosinolates were subjected to two-way ANOVA, also examining the broccoli fractions (stem, leaves, and florets) for their interaction with Zn treatment in the model. When significant differences were found, the means were compared using Fisher’s protected least significant difference (LSD) test at p < 0.05. All analyses were performed using Statistix v. 8.10 for Windows 10 (Analytical Software, Tallahassee, USA).
3. Results and Discussion
3.1. Effect of Zn and N Treatments on Broccoli Growth
All the measured weights, i.e., plant, floret, and stem weight, were significantly affected by the applied treatment (Table 1). In fact, the floret (as well as the plant weight), the most important parameter from the farmers’ point of view, increased significantly under all the studied treatments. The application of 0.5% (w/v) ZnSO4·7H2O alone caused a significant increase of 19.2% in the florets compared to the control (11% increase in plant weight). This increase was higher than those found by Slosar et al. [10] following the foliar application of 750 g ha−1. The increases were even greater in 0.5M1N, 0.25 + 0.25, and 0.25N + 0.25N, with increases of 41.5%, 33.8%, and 29.2%, respectively, (25.6%, 20.6%, and 22.2%, respectively, for plant weight) compared with the non-fertilized ones, with a slightly higher, although not significant, floret heights and floret diameters (D and d) measured in those treatments. These major weights could be explained on the one hand, for a better plant utilization when ZnSO4 is split into two applications, because its high solubility [28], and on the other hand, because of the positive and synergistic effect of N with Zn already found by Gomez-Coronado [14]. Rivera-Martin et al. [19] reported less increase with applications like the 0.25 + 0.25 treatment, but the Green Top cultivar growth seems to be more independent of the low bioavailable Zn soil content. This increase is very interesting, as it will allow farmers to cover the extra costs involved in implementing a biofortification program.

Table 1.
Broccoli growth characteristics under different agronomic Zn biofortification treatments (plant and floret heights and weights, maximum (D), and minimum (d) diameters), and leaves and stem weights mean ± standard error of the mean; F-values are derived following a one-way analysis of variance for Zn treatments.
3.2. Phytochemicals in the Different Broccoli Fractions
While the total Ca measured was significantly higher (more than twofold) in the leaves than in the stem and florets, the total Fe content in florets was 1.44- and 1.71-fold higher than in the leaves and stems, respectively. The total Mg was also significantly higher in the florets than in the stems (5.3%), and was significantly higher than in the leaves, by 45.0%. The total Zn was significantly higher in both florets and leaves (~80.7 mg kg−1) than stem (15.8 mg kg−1) (Table 2). Liu et al. [29] also detected higher concentrations of Fe, Mg, and Zn in the florets, and of Ca in the leaves. Kaluzewicz et al. [30] found ranges in the florets between 2.2 and 4.4 g Ca kg−1, 29 and 48.9 mg Fe kg−1, 1.87 and 2.95 g Mg kg−1, and 43.3 and 65.4 mg Zn kg−1 in ten broccoli cultivars. Recently, Rivera-Martin et al. [19] analyzed stems and leaves together, finding higher values of Ca (12.0 g Ca kg−1) but lower values of Fe (25.0 mg Fe kg−1), and in the florets, they found 2.4 g Ca kg−1 and 40 mg Fe kg−1. Regarding Mg, the values found were similar for stem but were lower in the florets (1.0 g Mg kg−1), and regarding Zn, the values in the stems and leaves were higher, at 47.6 mg Zn kg−1, but were lower in florets and leaves, at 39.3 mg Zn kg−1.

Table 2.
Total broccoli Ca, Fe, Mg, Zn, phytic acid (PA), PA:mineral molar ratios, ABTS, DPPH, total phenols, glucoraphanin, glucoiberin, other glucosides and total glucosinolates under the different broccoli fractions (mean ± standard error of the mean; F-values derived following a one-way analysis of variance for the broccoli fraction).
Nutrient bioavailability is directly related to the presence of the so-called phytate (myo-inositol-1,2,3,4,5,6-hexakis dihydrogen phosphate), which is a strong chelator of minerals such as Zn, Ca, Fe, and Mg, and its anti-nutritional effect is caused by human digestion’s inability to degrade it [31]. The nutrient bioavailability was generally greater in the florets, and they therefore had lower bioavailability, whereas the leaves had lower values (Table 2). Rivera-Martin et al. [19] found similar values for PA; in the florets, they found lower PA:Ca, PA:Fe, and PA:Mg ratios, but the phytate:Zn ratio was about two times higher. Once again, the strong effect of the cultivar can explain this, as Kaluzewicz et al. [30] pointed out. In any case, all the molar ratios, except for PA:Zn, were lower than their respective thresholds of 0.24 for PA:Ca [32], 10 for PA:Fe [33], and 0.2 for PA:Mg [34], and they therefore had very good bioavailability. In the case of PA:Zn, only the stem exceeded the threshold of 15 established by Gibson [35].
The antioxidant capacity measured by both ABTS and DPPH analysis displayed higher values in the leaves than in the stems and florets (with significant higher DPPH in the floret than in the stem). In agreement with Casquete et al. [24], the higher values found for ABTS by DPPH may indicate a relative difference in the ability of the antioxidant compounds in the extracts to quench aqueous peroxyl radicals [36]. The total phenols followed the sequence leaves > stem > floret, with respective reductions of 15.8 and 27.4% for the latter two compared to the leaves. Liu et al. [29] also found the highest antioxidant activities and total phenolic concentrations in the leaves—more than 45% and 2.3 %, respectively, higher than in stems and florets. However, the levels of glucosinolates were generally higher in florets; on average, they were more than four times more abundant than in the stem and leaves, while glucoiberin was about three times more abundant and the other found glucosides were 4.8 times more abundant (Table 2). Liu et al. [29] also found higher concentrations (~3.9-fold) in florets, glucoraphanin being the major glucosinolate in all the broccoli tissues, representing 32.5% of total glucosinolates in the florets, which was similar to that in the leaves (27.5%) but lower than that in the stems (48.9%). More recently, Li et al. [37] found a wide range when characterizing glucosinolates in 80 broccoli genotypes, and glucoraphanin represented 29% of the total glucosinolate content.
All these data highlight both the nutritional richness of all broccoli fractions, with a very high bioavailability, and the considerable quantity of health-promoting compounds, particularly in the florets. Regardless, the leaves and stems, which make up 40% of the total biomass, should be taken into account to increase the utility of broccoli by-products.
3.3. Effect of Zn and N Treatments on Broccoli Nutritional Composition
The studied treatments and fraction–treatment interaction only significantly affected the total Zn concentration and the PA:Zn molar ratio (Figure 1).
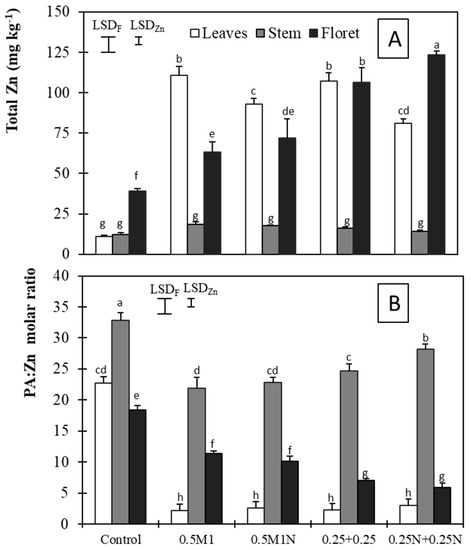
Figure 1.
Total Zn (mg kg−1) (A) and PA:Zn molar ratio (B) ± standard error of the mean. Vertical bars represent LSD (p < 0.05) for comparison. LSDF: same broccoli fraction; LSDZn: same Zn treatment. Different letters represent a significant difference at the p < 0.05 level for each broccoli fraction and Zn treatment.
In the non-fertilized pots, the Zn concentrations (10.8, 12.3, and 39.1 mg Zn kg−1 in leaves, stem, and floret, respectively) were higher than those found by Slosar et al. [18] and Rivera-Martin et al. [19] but were lower than those obtained by Kaluzewicz et al. [30] and Liu et al. [29]. This pointed out the high influence of the cultivar on mineral accumulation. The described application over two instances promoted Zn accumulation in all the broccoli fractions, independently of whether this was combined with N or not. Thus, it is more efficient to divide the same amount of ZnSO4 split in two, from the floret appearance, than to apply once only. The average rise was 3.6-fold compared to the control, i.e., more than 50 mg Zn kg−1, representing an increase with a measurable biological impact on human health [10]; the increase was ~40 mg Zn kg−1 when the application was unique (i.e., 0.5M1 and 0.5M1N). These increases were higher than those measured by Rivera-Martin et al. [19] with two applications of 0.25 (w/v) ZnSO4, but in another cultivar maybe less efficient. The expected synergistic effect of the Zn and N combination on Zn accumulation was only found in florets following the 0.25N + 0.25N treatment, which reached a total concentration of 123.4 mg kg−1 (Figure 1A). Zhao et al. [38] and Erenoglu et al. [39] attributed an increased accumulation of Zn since the supply of enough amounts of N increases the grain protein contents, which are positively correlated with grain Zn contents. Regarding bioavailability, measured as PA:Zn, the stems showed the worst bioavailability, with ratios always above the threshold of 15. The leaves and florets exceeded the limit only in the control treatment, decreasing significantly in all the treatments, and more markedly in the leaves than in the florets, where both the 0.25 + 0.25 and 0.25N + 0.25N treatments produced the best bioavailabilities (Figure 1B). Therefore, the efficiency of Zn biofortification in broccoli is high, not only because of the good accumulation but also given the good Zn bioavailability. It should not be forgotten that the application of Zn did not cause a decrease in any of the minerals studied, or affect their availability.
3.4. Effect of Zn and N Treatments on Broccoli Antioxidant Capacity and Total Phenol Content
The antioxidant capacity in the non-fertilized pots, measured through ABTS and DPPH assays, were 87.1, 51.2, and 71.0 mg Trolox 100 g−1, respectively, in the leaves, stem, and florets, under ABTS, and were respectively 23.9, 11.3, and 9.9 mg Trolox 100 g−1 under DPPH (Figure 2A,B). The treatments with 0.5 M1N and 0.5 M1 under ABTS, and all the treatments, particularly 0.25 + 0.25 under DPPH, significantly increased the antioxidant activity compared to the control. Slosar et al. [18] found slight but significant decreases in the antioxidant activity in broccoli, of between 3.7 and 7.0%, because of the Zn foliar application. Regarding total phenol content, again, all the treatments increased it significantly, in the sequence 0.25N + 0.25N > 0.25 + 0.25 = 0.5M1 > 0.5M1N > control, with a maximum difference of 62.3%. The higher contents, because of the studied foliar Zn and/or N applications, contradict the results of Slosar et al. [10], who reported an average decrease between 11% and 35% due to the foliar Zn application, but agree with Salama et al. [40] for maize. The positive effect of both splitting the application and combining it with N is therefore confirmed.
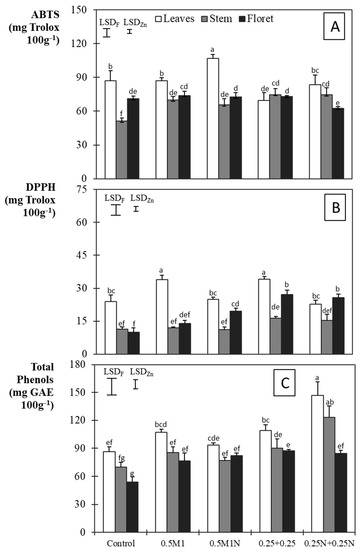
Figure 2.
ABTS (A), DPPH (B), and total phenols (C) ± standard error of the mean. Vertical bars represent LSD (p < 0.05) for comparison. LSDF: same broccoli fraction; LSDZn: same Zn treatment. Different letters represent a significant difference at the p < 0.05 level for each broccoli fraction and Zn treatment.
3.5. Effect of Zn and N Treatments on Broccoli Glucosinolates
In the non-fertilized pots, the values in the florets of glucoraphanin, glucoiberin, other glucosides, and total glucosinolates were 10.85, 1.87, 14.7, and 27.42 mg kg−1, respectively. Only glucoiberin was statistically affected by Zn, with the average result achieved by the 0.25 + 0.25 treatment being 0.70 mg kg−1 (this being the treatment with the lowest content), and the 0.5 M1 treatment achieving 0.89 mg kg−1, this being the highest, but without significant differences from the other treatments. However, the interaction (Figure 3) stands out in florets, the decrease caused by all the studied treatments being in the leaves 0.5M1, the unique treatment giving statistically different results from the control. The rest of the fractions were not affected by any of the treatments, a result, contrary to that of Coolong and Randle [41], who found a direct correlation between Zn application and the content of glucoraphanin.
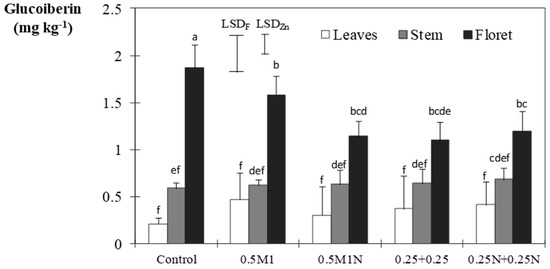
Figure 3.
Glucoiberin ± standard error of the mean. Vertical bars represent LSD (p < 0.05) for comparison. LSDF: same broccoli fraction; LSDZn: same Zn treatment. Different letters represent a significant difference at the p < 0.05 level for each broccoli fraction and Zn treatment.
4. Conclusions
The present study showed that the Parthenon cultivar is a healthy food, with florets rich in Fe, Mg, and Zn, with high bioavailability due to their respective low phytate molar ratios and good total glucosinolate contents. It had a high efficiency for agronomic biofortification with Zn, with the 0.25 + 0.25 treatment, and especially the 0.25 N + 0.25 N treatment (giving rise to the synergistic effect of N on Zn) standing out in terms of both plant and floret growth, and both causing an accumulation of up to 50 mg Zn kg−1 in the floret, as well as a higher total phenol content and without impaired any of the glucosinolates, being therefore, the recommended treatments.
Author Contributions
All authors contributed to the study in general, with the following specific contributions: Conceptualization, D.R.-M., A.M., R.V. and M.J.P.; Data curation, A.M.; Formal analysis, A.M., R.V. and M.J.P.; Funding acquisition, A.M., R.V. and M.J.P.; Investigation, A.R.-M., D.R.-M. and M.J.P.; Methodology, A.R.-M., D.R.-M., R.V. and M.J.P.; Project administration, R.V. and M.J.P.; Resources, A.R.-M., D.R.-M., A.M. and M.J.P.; Supervision, R.V. and M.J.P.; Validation, D.R.-M., A.M., R.V. and M.J.P.; Visualization, R.V. and M.J.P.; Writing—original draft, A.R.-M., R.V. and M.J.P.; Writing—review & editing, D.R.-M., A.M., R.V. and M.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Ministry of Economy and Infrastructures (Economy, Science and Digital Agenda) of the Regional Government of Extremadura and by the European Regional Development Fund (FEDER) and by the Extremadura University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Teodoro Garcia-White for his invaluable help with all the laboratory work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Francisco, M.; Tortosa, M.; Martínez-Ballesta, M.C.; Velasco, P.; Garcia-Viguera, C. Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann. Appl. Biol. 2017, 170, 270–285. [Google Scholar] [CrossRef]
- Yang, R.; Guo, L.; Jin, X.; Shen, C.; Zhou, Y.; Gu, Z. Enhancement of glucosinolate and sulforaphane formation of broccoli sprouts by zinc sulphate via its stress effect. J. Funct. Foods 2015, 13, 345–349. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Mahmoud, A.W.M.; El-Sawy, M.B.I.; Parmar, A. Pre-harvest foliar application of mineral nutrients to retard chlorophyll degradation and preserve bio-active compounds in broccoli. Agronomy 2019, 9, 711. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Landete, J.M. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit. Rev. Food Sci. Nutr. 2012, 52, 936–948. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Cakmak, I. Agronomic approaches in biofortification of food crops with micronutrients. In Proceedings of the International Plant Nutrient Colloquium XVI, Davis, CA, USA, 26–30 August 2009. [Google Scholar]
- Gurmani, A.R.; Khan, S.U.; Andaleep, R.; Waseem, K.; Khan, A. Soil application of zinc improves growth and yield of tomato. Int. J. Agric. Biol. 2012, 14, 91–96. [Google Scholar]
- Sánchez, C.; López-Jurado, M.; Planells, E.; Llopis, J.; Aranda, P. Assessment of iron and zinc intake and related biochemical parameters in an adult Mediterranean population from southern Spain: Influence of lifestyle factors. J. Nutr. Biochem. 2009, 20, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisov, Z.; Erdem, H.; Yazici, A.; Gokmen, O.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef]
- WHO/FAO. Global Health Risks, Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009; pp. 1–70. [Google Scholar]
- Alloway, B. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Hussain, M.; Asghar, H.; Akhtar, M.J.; Arshad, M. Impact of Phosphate Solubilizing Bacteria on Growth and Yield of Maize. Soil Environ. 2013, 32, 71–78. [Google Scholar]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2020, 10, 39. [Google Scholar] [CrossRef]
- Gomez-Coronado, F.; Poblaciones, M.J.; Almeida, A.S.; Cakmak, I. Combined zinc and nitrogen fertilization in different bread wheat genotypes grown under mediterranean conditions. Cereal Res. Commun. 2017, 45, 154–165. [Google Scholar] [CrossRef][Green Version]
- Terrés, C.; Navarro, M.; Martín-Lagos, F.; Giménez, R.; López, H.; López, M.C. Zinc levels in foods from southeastern Spain: Relationship to daily dietary intake. Food Addit. Contam. 2001, 8, 687–695. [Google Scholar] [CrossRef]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Serra-Majem, L.; Szponar, L.; Tetens, I. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef]
- Slosár, M.; Mezeyova, I.; Hegedúsova, A.; Andrejiová, A.; Kovácik, P.; Losak, T.; Kopta, T. Effect of zinc fertilisation on yield and selected qualitative parameters of broccoli. Plant Soil Environ. 2017, 63, 282–287. [Google Scholar]
- Rivera-Martin, A.; Broadley, M.; Poblaciones, M.J. Soil and foliar zinc application to biofortify broccoli (Brassica oleracea var. italica L.): Effects on the zinc concentration and bioavailability. Plant Soil Environ. 2020, 66, 113–118. [Google Scholar] [CrossRef]
- Aziz, M.Z.; Yaseen, M.; Abbas, T.; Naveed, M.; Adnan Mustafa, A.; Hamid, Y.; Saeed, Q.; Ming-gang, X. Foliar application of micronutrients enhances crop stand, yield and the biofortification essential for human health of different wheat cultivars. J. Integr. Agric. 2019, 18, 1369–1378. [Google Scholar] [CrossRef]
- Umar, W.; Ayub, M.A.; Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and Phosphorus Use Efficiency in Agroecosystems. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Reason, D.A.; Watts, M.J.; Devez, A.; Broadley, M.R. Quantification of Phytic Acid in Grains; BGS Open Report, OR/15/070; Natural Environment Research Council: Keyworth, Nottingham, UK, 2015; p. 18. [Google Scholar]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Cordoba, M.G.; Texeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Teixeira, D.M.; Canelas, V.C.; Martins do Canto, A.; Teixeira, J.M.G.; Dias, C.B. HPLC-DAD quantification of phenolic compounds contributing to the antioxidant activity of Maclura pomifera, Ficus carica and Ficus elastica extracts. Anal. Lett. 2009, 42, 2986–3003. [Google Scholar] [CrossRef]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Zeb, H.; Hussain, A.; Naveed, M.; Ditta, A.; Ahmad, S.; Jamshaid, M.U.; Ahmad, H.T.; Hussain, B.; Aziz, R.; Haider, M.S. Compost enriched with ZnO and Zn-solubilizing bacteria improves yield and Zn-fortification in flooded rice. Ital. J. Agron. 2018, 13, 310–316. [Google Scholar] [CrossRef]
- Lee, I.; Boyce, M.C.; Breadmore, M.C. Quantitative determination of glucoraphanin in Brassica vegetables by micellar electrokinetic capillary chromatography. Anal. Chim. Acta 2010, 663, 105–108. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Cumming, J.R.; Ku, K.M. Comparative phytonutrient analysis of broccoli by-products: The potentials for broccoli by-product utilization. Molecules 2018, 23, 900. [Google Scholar] [CrossRef]
- Kaluzewicz, A.; Bosiacki, M.; Fraszczak, B. Mineral composition and the content of phenolic compounds of ten broccoli cultivars. J. Elem. 2016, 21, 53–65. [Google Scholar]
- Dahdouh, S.; Grande, F.; Nájera Espinosa, S.; Fialon, M.; Vincent, A.; Gibson, R.; King, J.; Rittenschober, D.; Charrondiere, U.R. Global Food Composition Database for Phytate; Version 1.0.; FAO/IZiNCG PhyFoodComp1.0.; FAO/INFOODS Databases: Rome, Italy, 2018. [Google Scholar]
- Morris, E.R.; Ellis, R. Usefulness of the dietary phytic acid/zinc molar ratio as an index of zinc bioavailability to rats and humans. Biol. Trace Elem. Res. 1989, 19, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Martin, C.J. Interactions of Mg(II); Co(II); Ni(II); and Zn(II) with phytic acid. A calorimetric study. J. Inorg. Biochem. 1988, 32, 259–268. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. The role of diet and host-related factors in nutrient bioavailability and thus in nutrient-based dietary requirement estimates. Food Nutr. Bull. 2007, 28, 77–100. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprako, U.; Crosby, K.; Cisneros, L.; Hawkins, B.D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, S.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Ly, H.; Wang, Y.; Xu, D. Characterization of glucosinolates in 80 broccoli genotypes and different organs using UHPLC-Triple-TOF-MS method. Food Chem. 2021, 334, 1257519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root to shoot translocation and remobilization of zinc (65Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Salama, Z.A.; Gaafar, A.A.; Fouly, M.M. Genotypic variations in phenolic, flavonoids and their antioxidant activities in maize plants treated with Zn (II) EDTA grown in Salinized Media. Agric. Sci. 2015, 6, 397–405. [Google Scholar]
- Coolong, T.W.; Randle, W.M. Zinc availability in hydroponic culture influences glucosinolate concentrations in Brassica rapa. Hortscience 2004, 39, 84–86. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).