Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Standards
2.3. Olive Leaf Extracts (OLEs) Preparation
2.4. Polyphenol Oxidase (PPO) Activity Assay
2.5. Total Phenolic Content and Radical Scavenging Activity
2.6. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.7. HPLC-DAD and HPLC-ESI-MS Analyses
2.8. Statistical Analysis
3. Results
3.1. Effect of Different Extraction Media and Extraction Conditions on the TPC Content and Antiradical Capacity (DPPH Assay) of OLEs
3.2. Effect of Different Extraction Mixtures and Conditions on PPO Activity
3.3. Effect of Different Extraction Mixtures and Conditions on Antiradical Activity (ORAC Assay) of OLEs
3.4. Identification and Quantification of the Main Polyphenols Present in the Extracts through HPLC-DAD/ESI-MS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sofo, A.; Manfreda, S.; Fiorentino, M.; Dichio, B.; Xiloyannis, C. The olive tree: A paradigm for drought tolerance in Mediterranean climates. Hydrol. Earth Syst. Sci. 2008, 12, 293–301. [Google Scholar] [CrossRef]
- Soni, M.G.; Burdock, G.A.; Christian, M.S.; Bitler, C.M.; Crea, R. Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol. 2006, 44, 903–915. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Espin, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Agric. Food Chem. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Gòmez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of Cultivar and Ripening on Minor Components in Spanish Olive Fruits and their corresponding Virgin Olive Oils. Food Res. Int. 2008, 41, 433–440. [Google Scholar] [CrossRef]
- Ragazzi, E.; Veronese, G.; Guiotto, A. Demetil oleuropeina, nuovo glucoside isolato da olive mature. Ann. Chim. 1973, 63, 13–20. [Google Scholar]
- Ryan, D.; Robards, K. Phenolic compounds in olives. Analyst 1998, 123, 31R–44R. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- Goulas, V.; Exarchou, V.; Troganis, A.N.; Psomiadou, E.; Fotsis, T.; Briasoulis, E. Phytochemicals in olive-leaf extracts and their antiproliferative activity against cancer and endothelial cells. Mol. Nutr. Food Res. 2009, 53, 600–608. [Google Scholar] [CrossRef]
- Japon-Lujan, R.; de Castro, M.D.L. Superheated liquid extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1136, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and Antioxidant Activity of Olive Leaf Extracts from Greek Olive Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376. [Google Scholar] [CrossRef]
- Altıok, E.; Baycın, D.; Bayraktar, O.; Ulku, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef]
- Savournin, C.; Baghdikian, B.; Elias, R.; Dargouth-Kesraoui, F.; Boukef, K.; Balansard, G. Rapid high-performance liquid chromatography analysis for the quantitative determination of oleuropein Olea europaea leaves. J. Agric. Food Chem. 2001, 49, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Antolovich, M.; Prenzler, P.; Robards, K.; Lavee, S. Biotransformations of phenolic compounds in Olea europaea. L. Sci. Hortic. 2002, 92, 147–176. [Google Scholar] [CrossRef]
- Niaounakis, M.; Halvadakis, C.P. Olive Processing Waste Management: Literature Review and Patent Survey, 2nd ed.; Elsevier Waste Management Series; Elsevier: Amsterdam, The Netherlands, 2006; Volume 5. [Google Scholar]
- Abaza, L.; Taamalli, A.; Nsir, H.; Zarrouk, M. Olive Tree (Olea europaea L.) Leaves: Importance and Advances in the Analysis of Phenolic Compounds. Antioxidants 2015, 4, 682–698. [Google Scholar] [CrossRef]
- Taamalli, A.; Arràez-Roman, D.; Zarrouk, M.; Segura-Carretero, A.; Fernandez-Gutièrrez, A. The occurrence and bioactivity of polyphenols in Tunisian olive products and by-product: A review. J. Food Sci. 2012, 77, R83–R92. [Google Scholar] [CrossRef]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-ethanolic mixtures for the recovery of phenolic from Mediterranean plant materials. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Afaneh, I.; Yateem, H.; Al-Rimawi, F. Effect of Olive Leaves Drying on the Content of Oleuropein. Am. J. Anal. Chem. 2015, 6, 246–252. [Google Scholar] [CrossRef]
- Heemken, O.P.; Theobald, N.; Wenclawiak, B.W. Comparison of ASE and SFE with Soxhlet, sonication, and methanolic saponification extractions for the determination of organic micropollutants in marine particulate matter. Anal. Chem. 1997, 69, 2171–2180. [Google Scholar] [CrossRef]
- Ansari, M.; Kazemipour, M.; Fathi, S. Development of a simple green extraction procedure and HPLC method for determination of oleuropein in olive leaf extract applied to a multisource comparative study. J. Iran. Chem. Soc. 2011, 8, 38–47. [Google Scholar] [CrossRef]
- Procopio, A.; Alcaro, S.; Nardi, M.; Oliverio, M.; Ortuso, F.; Sacchetta, P.; Pieragostino, D.; Sindona, G. Synthesis, biological evaluation, and molecular modeling of oleuropein and its semisynthetic derivatives as cyclooxygenase inhibitors. J. Agric. Food Chem. 2009, 57, 11161–11167. [Google Scholar] [CrossRef]
- Paladino, S.; Zuritz, C. Antioxidant grape seed (Vitis vinifera L.) extracts: Efficiency of different solvents on the extraction process. Rev. Fac. Cienc. Agrar. 2011, 43, 187–199. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Yildirim, A.; Mavi, A.; Oktay, M.; Kara, A.A.; Algur, O.F.; Bilaloggu, V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argentea Desf Ex DC), sage and black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000, 48, 5030–5034. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, F.; Blanco, S.; Peinado, M.A.; Peragon, J. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. ‘Picual’ trees during fruit ripening. Tree Physiol. 2008, 28, 45–54. [Google Scholar] [CrossRef]
- Palmeri, R.; Monteleone, J.I.; Spagna, G.; Restuccia, C.; Raffaele, M.; Vanella, L.; Li Volti, G.; Barbagallo, I. Olive leaf extract from sicilian cultivar reduced lipid accumulation by inducing thermogenic pathway during adipogenesis. Front. Pharmacol. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Gambacorta, G.; Faccia, M.; Previtali, M.A.; Pati, S.; La Notte, E.; Baino, A. Effect of olive maturation and stoning on quality indices and antioxidant content of extra virgin olive oil (cv. Coratina) during storage. J. Food Sci. 2010, 75, C229–C235. [Google Scholar] [CrossRef]
- Siracusa, L.; Patanè, C.; Avola, G.; Ruberto, G. Polyphenols as chemotaxonomic markers in Italian “long storage” tomato genotypes. J. Agric. Food Chem. 2012, 60, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Romero-Segura, C.; García-Rodríguez, R.; Sánchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The role of olive β-glucosidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2012, 1, 191–196. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Investigation of Australian olive mill waste for recovery of biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920. [Google Scholar] [CrossRef]
- Rahmanian, N.; Mahdi Jafari, S.; Wani, T. Bioactive profile, dehydration, extraction and application of the bioactive components of olive Leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. Phenol Metabolism in the Leaves of the Olive Tree (Olea europaea L.) cv. Picual, Verdial, Arbequina, and Frantoio during Ripening. J. Agric. Food Chem. 2010, 58, 12440–12448. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Sanusi, J.; Kanthimathi, M.S. Temperature and time of steeping affect the antioxidant properties of white, green and black tea infusions. J. Food Sci. 2016, 81, H246–H254. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Benta, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [PubMed]
- Ahmad-Qasem, M.H.; Ahmad-Qasem, B.H.; Barrajón-Catalán, E.; Micol, V.; Carcel, J.A.; García-Pérez, J.V. Drying and storage of olive leaf extracts. Influence on polyphenols stability. Ind. Crops Prod. 2016, 79, 232–239. [Google Scholar] [CrossRef]
- Chism, G.W.; Haard, N.F. Characteristics of edible plant tissues. In Food Chemistry; Fennema, O.R., Ed.; Dekker: New York, NY, USA, 1996; pp. 943–1011. [Google Scholar]
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crops Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Konno, K.; Hirayama, C.; Yasui, H.; Nakamura, M. Enzymatic activation of oleuropein: A protein crosslinker used as a chemical defense in the privet tree. Proc. Natl. Acad. Sci. USA 1999, 96, 9159–9164. [Google Scholar] [CrossRef]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Briante, R.; Patumi, M.; Terenziani, S.; Bismuto, E.; Febbraio, F.; Nucci, R. Olea europea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002, 50, 4934–4940. [Google Scholar] [CrossRef] [PubMed]
- Xynos, N.; Papaefstathiou, G.; Psychis, M.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Development of a green extraction procedure with super/subcritical fluids to produce extracts enriched in oleuropein from olive leaves. J. Supercrit. Fluids 2012, 67, 89–93. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; De Luca, P.F.; Monaco, P.; Fiorentino, A. Polyphenol characterization and antioxidant evaluation of Olea europaea varieties cultivated in Cilento National Park (Italy). Food Res. Int. 2012, 46, 294–303. [Google Scholar] [CrossRef]

| Extraction Mixture | Temperature (°C) | Time (min) |
|---|---|---|
| MeOH:H2O:HCl (70:29.9:0.1) | 25 | 30 |
| EtOH:H2O:HCl (70:29.9:0.1) | 25 | 30 |
| MeOH:H2O (70:30) | 25 | 30 |
| EtOH:H2O (70:30) | 25 | 30 |
| EtOH:H2O (50:50) | 25 | 30 |
| H2O:Citric acid (98.1:1.9) | 60 | 30 and 60 |
| H2O | 60 | 30 and 60 |
| H2O | 90 | 30 and 60 |
| Extraction Mixture/Solvent | Temperature (°C) | Time (min) | T.P. 1 (mg/g) 2 | DPPH (TEAC mM) 3 |
|---|---|---|---|---|
| MeOH:H2O:HCl (OLEM; 70:29.9:0.1) | 25 | 30 | 45.41 ± 0.47d 4 | 2.45 ± 0.01c |
| EtOH:H2O:HCl (OLEE; 70:29.9:0.1) | 25 | 30 | 46.29 ± 0.49e | 2.46 ± 0.02c |
| MeOH:H2O (OLEM; 70:30) | 25 | 30 | 46.83 ± 0.12e | 2.84 ± 0.01d |
| EtOH:H2O (OLEE; 70:30) | 25 | 30 | 47.75 ± 0.52e | 2.83 ± 0.02d |
| EtOH:H2O (OLEE; 50:50) | 25 | 30 | 46.29 ± 0.07e | 2.80 ± 0.03d |
| H2O:Citric acid (OLEA; 98.1:1.9) | 60 | 30 | 30.45 ± 0.16a | 2.19 ± 0.08ab |
| H2O:Citric acid (OLEA; 98.1:1.9) | 60 | 60 | 31.51 ± 0.06b | 2.20 ± 0.01ab |
| H2O (OLEA) | 60 | 30 | 30.44 ± 0.34a | 2.16 ± 0.01a |
| H2O (OLEA) | 60 | 60 | 31.12 ± 0.22ab | 2.25 ± 0.01b |
| H2O (OLEA) | 90 | 30 | 40.31 ± 0.08c | 2.77 ± 0.06d |
| H2O (OLEA) | 90 | 60 | 40.01 ± 0.76c | 2.76 ± 0.04d |
| Extraction Mixture/Solvent | PPO Activity (U/g) |
|---|---|
| MeOH:H2O:HCl.(70:29.9:0.1) | 1.76 × 10−3 ± 0.03 |
| EtOH:H2O:HCl (70:29.9:0.1) | 1.41 × 10−3 ± 0.03 |
| MeOH:H2O (70:30) | 2.61 × 10−3 ± 0.05 |
| EtOH:H2O (70:30) | 1.98 × 10−3 ± 0.02 |
| EtOH:H2O (50:50) | 4.31 × 10−3 ± 0.02 |
| H2O:Citric acid (98.1:1.9) | 1.30 × 10−3 ± 0.03 |
| H2O:Citric acid (98.1:1.9) | 2.00 × 10−3 ± 0.03 |
| H2O (60 °C; 30′) | 7.74 × 10−3 ± 0.03 |
| H2O (60 °C; 60′) | 7.18 × 10−3 ± 0.02 |
| H2O (90 °C; 30′) | 2.45 × 10−3 ± 0.02 |
| H2O (90 °C; 60′) | 1.59 × 10−3 ± 0.05 |
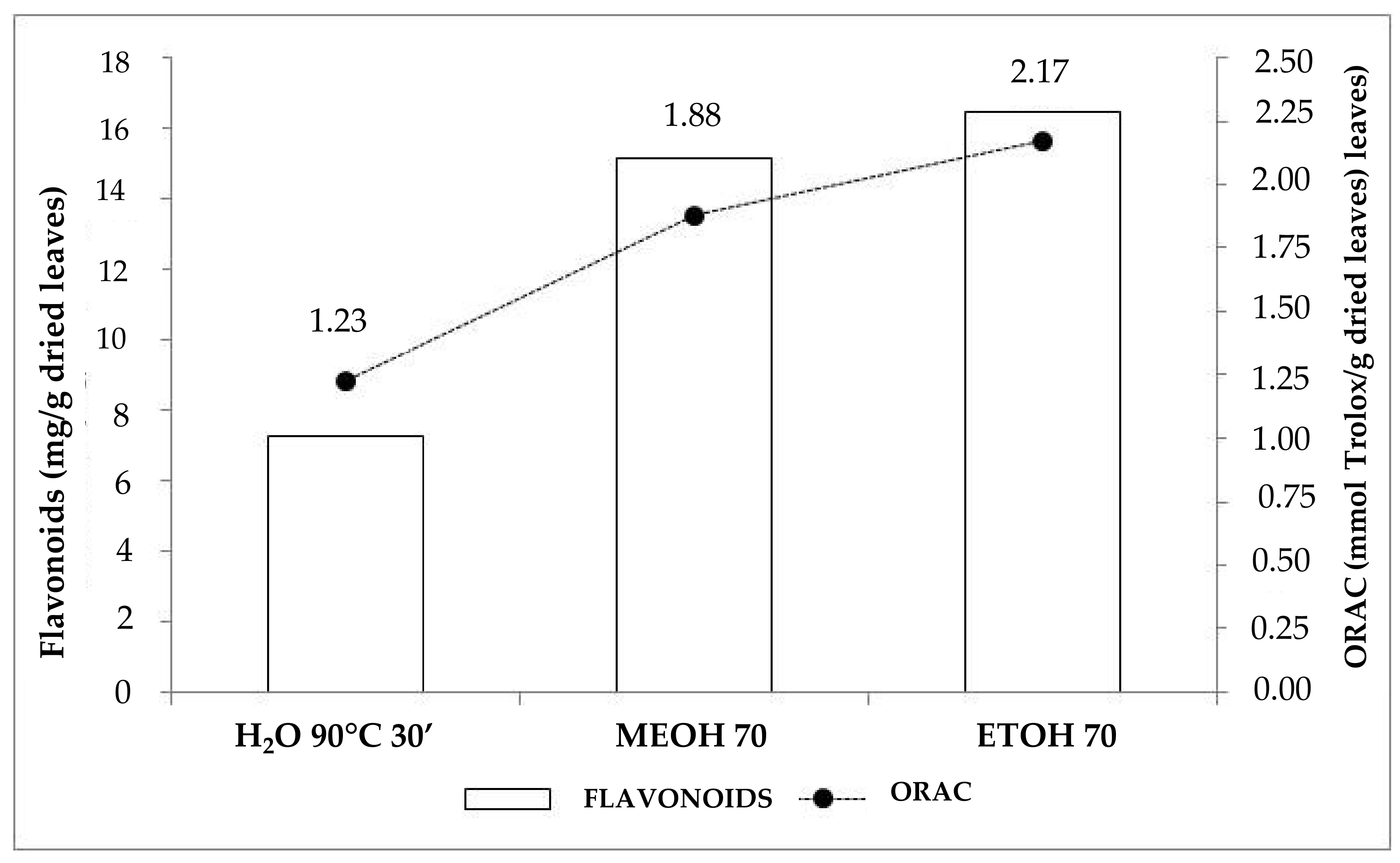
| Extraction Medium, Conditions | T.P. (mg/g) 1 | DPPH (% Inhibition) | ORAC (mmol TE/g) 2 |
|---|---|---|---|
| H2O, 90°C, 30′ | 40.31 a 3 | 88.90 | 1.23 a |
| MeOH:H2O (70:30), 25°C, 30′ | 46.83 b | 91.20 | 1.88 b |
| EtOH:H2O (70:30), 25°C, 30′ | 47.75 b | 90.85 | 2.17 b |
| Peak | Compound | OLEA | OLEM | OLEE |
|---|---|---|---|---|
| mg/g a | mg/g a | mg/g a | ||
| 1 | hydroxytyrosol glucoside | 14.97b b | 5.137a b | 5.018a b |
| 2 | hydroxytyrosol | 1.769b b | 0.138a b | 0.143a b |
| 3 | dihydroxyphenylacetic acid (DOPAC) | 0.489b b | 0.042a b | 0.031a b |
| 4 | chlorogenic acid | 0.167 | n.d.c | n.d. |
| 5 | caffeic acid | 0.157b b | 0.104a b | 0.099a b |
| 6 | verbascoside | 5.313c b | 2.465a b | 2.669b b |
| 7 | p-coumaric acid | 0.006 | n.d. c | n.d. c |
| 8 | rutin | 1.245a b | 1.539b b | 1.893c b |
| 9 | ferulic acid | 0.066b b | 0.007a b | 0.009a b |
| 10 | luteolin 7-O-glucoside | 4.039a b | 8.120b b | 8.937c b |
| 11 | oleuropein | 46.25c b | 39.40b b | 36.35a b |
| 12 | apigenin 7-O-glucoside | 1.947a b | 5.157b b | 5.370c b |
| 13 | ligstroside | 9.684b b | 7.200a b | 7.568a b |
| 14 | oleuropein aglycone | n.d. c | 0.070 | 0.072 |
| 15 | luteolin | n.d. c | 0.281 | 0.237 |
| 16 | apigenin | n.d. c | 0.026 | 0.023 |
| total | 86.102 | 69.68 | 68.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteleone, J.I.; Sperlinga, E.; Siracusa, L.; Spagna, G.; Parafati, L.; Todaro, A.; Palmeri, R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy 2021, 11, 465. https://doi.org/10.3390/agronomy11030465
Monteleone JI, Sperlinga E, Siracusa L, Spagna G, Parafati L, Todaro A, Palmeri R. Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy. 2021; 11(3):465. https://doi.org/10.3390/agronomy11030465
Chicago/Turabian StyleMonteleone, Julieta Ines, Elisa Sperlinga, Laura Siracusa, Giovanni Spagna, Lucia Parafati, Aldo Todaro, and Rosa Palmeri. 2021. "Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves" Agronomy 11, no. 3: 465. https://doi.org/10.3390/agronomy11030465
APA StyleMonteleone, J. I., Sperlinga, E., Siracusa, L., Spagna, G., Parafati, L., Todaro, A., & Palmeri, R. (2021). Water as a Solvent of Election for Obtaining Oleuropein-Rich Extracts from Olive (Olea europaea) Leaves. Agronomy, 11(3), 465. https://doi.org/10.3390/agronomy11030465








